Abstract
Amoxicillin is a widely used antibiotic in COPD. Little is known about the transfer of amoxicillin into sputum of COPD patients. The objective was to investigate the relationship between the concentration of amoxicillin in sputum in hospitalized COPD patients and length of hospitalization. To be effective against bacterial pathogens, the amoxicillin concentration in target tissues should be higher than the Minimal Inhibiting Concentration (MIC) of 2 mg/l. Therefore, this was also used as the cut-off value for the amoxicillin concentration in sputum, as a marker for lung tissue concentration. Fifty-two COPD in-patients with an exacerbation, treated with amoxicillin clavulanic acid, were included in this cohort study. Of these patients 7 also had pneumonia. Patients were divided in patients with an amoxicillin sputum concentration ≥ 2 mg/l and < 2 mg/l. Furthermore, inflammation markers in sputum and serum and clinical parameters were obtained. Of the 33 patients with usable sputum, 11 had a concentration in sputum ≥ 2 mg/l. The mean length of hospitalization for patients with concentrations below the MIC90 to common respiratory pathogens was 11.0 days, while for patients with concentrations at or above the MIC90 this was 7.0 days (p = 0.005). COPD patients admitted for an acute exacerbation of COPD, with a sputum concentration of amoxicillin ≥ 2 mg/l had a markedly reduced length of hospitalization compared to patients with a concentration < 2 mg/l. It is worthwhile testing whether individualized treatment based on sputum amoxicillin concentrations of patients during hospitalization for acute exacerbations can effectively reduce hospital stay.
INTRODUCTION
Patients with COPD are prone to exacerbations and these are an important cause of morbidity and mortality. The management of exacerbations is usually empirical and includes oral corticosteroids combined with broad spectrum antibiotics, such as amoxicillin clavulanic acid, to treat presumed bacterial infection (Citation1). However, evidence of the efficacy of adding antibiotics is debatable, since some controlled studies showed a clear benefit, whereas others did not (Citation2–4).
Theoretically, to be effective, the amoxicillin concentration in target tissues should reach the Minimal Inhibiting Concentration of 90% (MIC90) for potential pathogenic micro-organisms (PPM) such as S. pneumoniae, H. influenzae and M. catarrhalis (Citation5, 6). As these micro-organisms involved are often located in the bronchial lumen, levels of antimicrobial agents in sputum may be the relevant predictor of efficacy (Citation7).
Little is known about the transfer of amoxicillin clavulanic acid into sputum of COPD patients. Due to the instability of clavulanic acid, levels in sputum and serum are difficult to measure. The concentration of amoxicillin reached in sputum may differ markedly from the concentration in serum due to various factors such as the diffusion of amoxicillin into the airways (Citation5, Citation8, Citation9).
Theoretically, steady state sputum concentrations should be reached after three days of oral or intravenous treatment. In non-COPD patients and in healthy subjects the concentration of amoxicillin in sputum ranged from 0.05 to 0.54 mg/l with a median of 0.11 mg/l (3 times daily oral 500/125 mg amoxicillin clavulanic acid)(Citation10). In contrast, peak amoxicillin levels in serum were excellent, with a mean of 8.7 (±6.0) mg/l. Other studies using higher doses of amoxicillin (with or without clavulanic acid) showed concentrations of amoxicillin in sputum between 0.23 and 4.4 mg/l (Citation6, Citation11–15) with serum concentrations between 3.87 and 45.2 mg/l depending on for example dosing and timing of blood samples.
Since some clinical studies have suggested that with regard to clinical efficacy of amoxicillin, sputum concentration is a better predictor of efficacy than serum concentration (Citation5, Citation7), the objective of the present study was to investigate the relationship between the concentration of amoxicillin in sputum in hospitalized COPD patients and length of hospitalization.
MATERIAL AND METHODS
Study subjects
Patients hospitalized from January 2006 through February 2007 for an exacerbation of COPD at the in-patient pulmonary department of Medisch Spectrum Twente in Enschede, the Netherlands, were recruited. The recruitment criteria were: 1) a clinical diagnosis of COPD, as defined by GOLD criteria (Citation16), Citation2) admitted with signs and symptoms of an exacerbation of COPD, defined as an acute negative change from the baseline, reported by the patient, in dyspnoea and/or sputum volume and/or colour of sputum (yellowish or greenish sputum) and/or cough, which warranted additional treatment of prednisolone with or without antibiotics by a physician. Patients with pneumonia were not excluded from the study. Pneumonia was defined as an acute respiratory tract illness associated with radiographic shadowing on a chest radiograph which was neither pre-existing nor of any other cause. Chest radiographs were judged independently by two pulmonary physicians. In case of discrepancy, the judgment of a radiologist was used for consensus, 3) age 40 years or over, 4) current or former smoker.
Patients received care, according to daily practice, by their chest physician, which included oral corticosteroids and amoxicillin clavulanic acid either orally or intravenously starting on the day of admission. Doses for oral administration varied from 3 to 4 times daily amoxicillin (500 mg), dose for intravenous administration was 4 times daily 1000 mg amoxicillin. Some patients received both treatment forms sequentially. One patient received 2 doses of amoxicillin with and 2 doses of amoxicillin without clavulanic acid. This was due to renal insufficiency. The choice for amoxicillin clavulanic was based on a negligible resistance to this drug combination in our region (less than Citation5%). The hospital's medical ethical committee approved the study. All patients provided written informed consent.
Outcome measurements
Total amoxicillin concentrations were measured, by high performance liquid chromatography method using ultraviolet detection at 225 nm, in sputum samples collected on the third day of antibiotic treatment. Sputum samples were first homogenised and liquefied by a mechanical method. Samples were deproteinized with acetonitrile, dried under N2 and reconstituted with water. Amoxicillin was separated from other components using a C18 column and a mobile phase containing ammonium dihydrogen phosphate 0.025 M, adjusted to pH 2.6 with phosphoric acid and acetonitrile 5%.
An alternative mobile phase, consisting of the same concentration ammonium dihydrogen phosphate 0.025 M and pH, but without acetonitrile was used for analysing samples which contained many disturbing components. These samples were mostly (highly purulent) sputum samples. The method showed a mean accuracy of 103% for plasma samples and 92% for sputum samples and the concentration range was 2.0 to 10.0 mg/l for sputum samples and 1.0 to 10.0 mg/l for plasma samples.
The method was validated and is applicable for the quantitative determination of amoxicillin in sputum and plasma samples of (COPD) patients. Patients were divided in patients with an amoxicillin sputum concentration on the third day of antibiotic treatment at or above (≥) or below (<) the MIC90 of PPM being 2 mg/l. This cut-off of 2 mg/l was determined in advance. Amoxicillin concentrations were determined after hospital discharge, so this information could not influence the decision of discharge made by the physicians. The primary outcome measure was length of stay, defined as number of days between day of admission and day of medical discharge.
On the first day of hospital admission a sputum sample was collected to perform a Gram's stain and a semi-quantitative culture. Bacterial infection was considered to be present in case of the presence of ≥1 PPM in excess (≥1 log) of the normal microbiological flora in sputum. Bacterial colonization was declared in case of the presence of ≥1 PPM in equal or less amount compared with the background flora (Citation17,18).
Furthermore, the concentration of IL(interleukin) -6, IL-8, and IL-10, the number of leucocytes and MPO (Myeloperoxidase) were measured in this sputum. To process the sputum, DTT was not used; instead sputum was homogenized by mechanical disruption (Magnalizer®, Roche, The Netherlands) and analysed without adding any agent. CRP (C-reactive protein), IL-6, IL-8 and Il-10 were measured in blood, within one and ten days after hospital admission. In all sputum and blood samples IL-6, IL-8, and IL-10 concentrations were quantified using PeliKine CompactTM human sandwich ELISA kids (Sanquin, CLB, Amsterdam, The Netherlands). MPO enzymatic activity was determined by colorimetric change in absorbance during a reaction with O-Dianisidine dihydrochloride (Sigma-Aldrich©). C-reactive protein (CRP) level in sputum and blood was determined using the NycoCard® CRP Single Test (Clindia Diagnostics©).
Statistical analysis
Prior to the study conduct, we defined a difference of 3 days in length of stay between the patient groups as clinically relevant. Based on this difference, a standard deviation of 3 days, a power of 80% and a type I error probability for a two-sided test of 5%, it was calculated that 34 persons were required to detect this difference. This power calculation was based on the assumption that the ratio of patients with an amoxicillin concentration at or above (≥) to a concentration below (<) the MIC90 of PPM being 2 mg/l, would be 1:1.
Table 1 Baseline characteristics
When a more conservative ratio of 1:2 was used, 36 patients need to be included. Baseline characteristics are reported as mean with SD or as numbers with corresponding percentages for categorical variables, stratified by patients with an amoxicillin concentration in sputum ≥ 2 mg/l and < 2 mg/l. If variables were not normally distributed, values are reported as median with corresponding range.
First, the crude association between amoxicillin concentration in sputum and time to discharge was analyzed by univariate Cox-regression and since there was no censoring also a t-test was performed to study the difference in mean length of hospitalization. After this, t-tests, in case of normally distributed variables, were performed to identify a subset of independent variables that were associated with the concentration of amoxicillin in sputum. For non-normally distributed variables this was done by Mann-Whitney U-test. Between-group comparisons of nominal or ordinal variables were performed by Chi-square tests or by Fisher's Exact tests as appropriate.
The a priori list of potential confounding variables is displayed in . Only those variables associated with the amoxicillin concentration with a significance at or below p = 0.15 were tested for an association with time to discharge. Variables also associated with time to discharge with a significance at or below p = 0.15 were considered as potential confounders in the relation between amoxicillin concentration in sputum and time to discharge and were entered into multivariate Cox-regression analysis. The cumulative discharge curve was made by means of a Kaplan-Meier graph. Data-analysis was performed with the statistical package SPSS/PC for Windows (version 12.2) (SPSS, Inc., Chicago, IL).
RESULTS
Fifty-two COPD in-patients with an exacerbation, treated with amoxicillin clavulanic acid, were included in the study. Of the 52 patients enrolled only 33 patients produced usable sputum on day 3. The other 19 patients could either not produce enough sputum (n = 16) or the sputum sample could not be analysed due to analytic problems (n = 3). Baseline characteristics, stratified by amoxicillin concentration in sputum are presented in . Apart from the common bacteria (H. influenzae, M. catarrhalis and S. pneumonia), no other pathogens were isolated. Baseline characteristics of the enrolled patients and the 19 patients without usable sputum were comparable (data not shown).
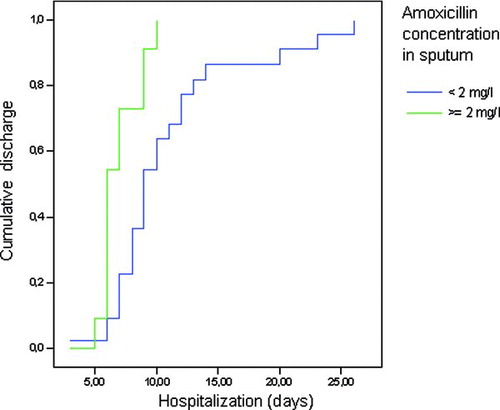
Of the 33 patients with usable sputum, 11 (33%) had a concentration in sputum ≥ 2 mg/l. All isolated organisms had a MIC < 2 mg/L. The mean length of hospitalization for patients with concentrations < 2 mg/l was 11.0 days, while for patients with concentrations ≥ 2 mg/l this was 7.0 days (p = 0.005) (see ). The crude hazard ratio of time to discharge in the patients with concentrations ≥ 2 mg/l as compared to < 2 mg/l was 3.02 (95% CI 1.34 –6.81; p = 0.008).
Of the potential confounding variables displayed in Table 1, only smoking status (ex-smoker versus current smoker) and CRP concentration in blood within 1 day after hospital admission were univariately associated with an amoxicillin concentration at or above and below the MIC90 of 2 mg/l. These two variables were not associated with time to medical discharge, so no adjustment for the relation between amoxicillin concentration and time to discharge was necessary.
To check whether the relationship between amoxicillin concentration and hospital stay differed between patient with an exacerbation with and without pneumonia, we performed a separate analysis of amoxicillin concentration and length of hospitalization for both groups. We observed that within the patients with an exacerbation and pneumonia (n = 7), there was no significant difference in length of hospitalization (1.4 days longer with low amoxicillin (p = 0.2), while in the patients with an exacerbation without pneumonia (n = 26) the difference was increased (4.6 days (95%CI: 1.3–7.8; p = 0.007),
DISCUSSION
Our study demonstrated that COPD patients admitted to the hospital with an acute exacerbation of COPD who have a sputum concentration of amoxicillin ≥ 2 mg/l on the third day of dosing have a markedly reduced length of hospitalization compared to patients with a concentration < 2 mg/l.
Host-related, as well as drug-related, factors may influence the penetration of antimicrobial drugs across the blood-bronchus and alveolar-capillary barriers. The most important host-related factor is the integrity of the anatomical barriers which may be damaged by inflammation and mechanical injury (Citation5). In the presence of inflammation, the distribution of antimicrobial agents in tissue compartments may be altered because of an increase in membrane permeability (Citation19,20). Thus, for a drug such as amoxicillin clavulanic acid, a beta-lactam, which does not cross membranes readily, the penetration might increase in the presence of inflammation (Citation5).
In our study the concentration of amoxicillin in sputum was increased in patients with an elevated CRP concentration in blood within 1 day after admission and in ex-smokers. Since CRP is a marker of systemic inflammation, this might explain the higher concentrations of amoxicillin in patients with higher CRP levels due to increased inflammation. Studies exist where patients with a high CRP level were identified. Stolz et al. showed that CRP was significantly higher in patients presenting with an Anthonisen type I exacerbation (Citation21) and two other studies also reported that patients with increased sputum purulence at admission had higher CRP values than those with mucoid sputum (Citation22,23). Pinto-Plata et al. showed that the CRP levels were higher in non-inhaled corticosteroid (ICS) users than in ICS users (Citation24). These results were in agreement with the study of Sin et al. that showed that withdrawal of ICS was associated with an increase in the baseline CRP level, which suggests corticosteroids reduce systemic inflammation. In our study, all patients however, received systemic corticosteroids, so this is not likely the explanation of our findings.
Besides the relationship between CRP and the concentration of amoxicillin in sputum, we also observed that in current smokers the concentration of amoxicillin in sputum was more often under the MIC, suggesting a decreased permeability in current smokers, or that smoking might interfere with the amoxicillin concentration. We are, however, unaware of any study looking at this possible interaction between smoking and antibiotics concentration in sputum.
In patients with an exacerbation that was accompanied by pneumonia we observed a still relevant, but not significant difference in hospital stay. This could be due to the relatively small subgroup sample and should therefore be analysed in a larger study population before drawing any conclusions.
Our study demonstrated that COPD patients admitted to the hospital with an acute exacerbation of COPD, with a sputum concentration of amoxicillin ≥ 2 mg/l had a markedly reduced length of hospitalization by 4 days compared to patients with a concentration < 2 mg/l. This leads to the question whether individual tailoring of amoxicillin dosing to reach at least the MIC90 in sputum will reduce the length of stay in patients hospitalized for a COPD exacerbation, which then leads to a remarkable reduction of costs and the pressure of demand on hospital beds, since the length of stay is the key cost driver (Citation25). In case of individual tailoring patients not able to reach the MIC90 with the standard treatment can be identified by collecting a sputum sample during antibiotic treatment for an exacerbation in which antibiotic concentrations are measured.
The consequence of finding lower concentrations, below the MIC, in patients should be to consider increasing the dose of antibiotics in these patients. Alternatively, other antibiotics that might better penetrate, since we only tested amoxicillin, or routes of administration such as inhalation of antibiotics could be considered like in cystic fibrosis (Citation26). Aerosolized antibiotics have been proven to deliver high concentrations of antibiotics into the airways with low systemic bioavailability, thus reducing toxicity (Citation27).
What should be mentioned is that we used the MIC90 as a cut-off point for the amoxicillin concentration in sputum. The MIC90 however is based on cut points derived from planktonic organisms, while bacteria in biofilms possibly have another MIC90. This does not invalidate the results, but does lend caution to the use of the MIC90 as a marker for individually tailored antibiotic dosing.
Next, we measured inflammation markers within one day of admission; in some cases, patient already started treatment, either in the hospital or sometimes at home, with antibiotics and oral steroids before a blood or sputum sample was taken. This could have affected the measured values of inflammation markers. However, we feel that this possible pre-treatment with oral steroids and/or antibiotics has no effect on the relation between amoxicillin concentration and hospital stay.
Our results need to be confirmed. We suggest doing this by testing the usefulness of a prospective algorithm of antibiotic dosing based on sputum amoxicillin concentrations with the aim to reduce hospitalization length for acute exacerbations of COPD.
DECLARATION OF INTEREST
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Wilson R. Bacterial infection and chronic obstructive pulmonary disease. Eur Respir J 1999 February; 13(2):233–235.
- Saint S, Bent S, Vittinghoff E, Grady D. Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysis. JAMA 1995 March 22; 273(12):957–960.
- Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010 January 15; 181(2):150–157.
- Anthonisen NR, Manfreda J, Warren CPW, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204.
- Baldwin DR, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob Agents Chemother 1992 June; 36(6):1176–1180.
- Fraschini F, Scaglione F, Falchi M, Dugnani S, Mezzetti M, Cicchetti F, Alfano G, Pintucci GP. Pharmacokinetics and tissue distribution of amoxicillin plus clavulanic acid after oral administration in man. J Chemother 1990 June; 2(3):171–177.
- Baldwin DR, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob Agents Chemother 1992 June; 36(6):1171–1175.
- Pennington JE. Penetration of antibiotics into respiratory secretions. Rev Infect Dis 1981 January; 3(1):67–73.
- Neu HC. Contribution of beta-lactamases to bacterial resistance and mechanisms to inhibit beta-lactamases. Am J Med 1985 November 29; 79(5B):2–12.
- Wallace RJ, Jr., Steele LC, Brooks DL, Luman JI, Wilson RW, McLarty JW. Amoxicillin-clavulanic acid in the treatment of lower respiratory tract infections caused by beta-lactamase-positive Haemophilus influenzae and Branhamella catarrhalis. Antimicrob Agents Chemother 1985 June; 27(6):912–915.
- Maesen FP, Davies BI, Baur C. Amoxycillin/clavulanate in acute purulent exacerbations of chronic bronchitis. J Antimicrob Chemother 1987 March; 19(3):373–383.
- Havard CW, Fernando A, Brumfitt W, Hamilton-Miller JM. A pilot study of ‘Augmentin’ in lower respiratory tract infections: pharmacokinetic and clinical results. Br J Dis Chest 1982 July; 76(3):255–260.
- Ingold A. Sputum and serum levels of amoxycillin in chronic bronchial infections. Br J Dis Chest 1975 July; 69:211–216.
- Lovering AM, Pycock CJ, Harvey JE, Reeves DS. The pharmacokinetics and sputum penetration of ampicillin and amoxycillin following simultaneous i.v. administration. J Antimicrob Chemother 1990 March; 25(3):385–392.
- Hill SL, Burnett D, Lovering AL, Stockley RA. Use of an enzyme-linked immunosorbent assay to assess penetration of amoxicillin into lung secretions. Antimicrob Agents Chemother 1992 July; 36(7):1545–1552.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001 April; 163(5):1256–1276.
- Isenberg HD. Clinical Microbiology Procedures Handbook. 2004. Herndon, VA: American Society for Microbiology.
- Van Der Valk P, Monninkhof E, Van Der Palen J., Zielhuis G, van Herwaarden C., Hendrix R. Clinical predictors of bacterial involvement in exacerbations of chronic obstructive pulmonary disease. Clin Infect Dis 2004 October 1; 39(7):980–986.
- Braude AC, Cohen RD, Penner JL, Preston MA, Rebuck AS. Pulmonary disposition of moxalactam. Chest 1984 December; 86(6):881–883.
- Bergogne-Berezin E. Penetration of antibiotics into the respiratory tree. J Antimicrob Chemother 1981 September; 8(3):171–174.
- Stolz D, Christ-Crain M, Morgenthaler NG, Leuppi J, Miedinger D, Bingisser R, Muller C, Struck J, Muller B, Tamm M. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest 2007 April; 131(4):1058–1067.
- Stockley RA, O'Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest 2000 June; 117(6):1638–1645.
- Weis N, Almdal T. C-reactive protein—Can it be used as a marker of infection in patients with exacerbation of chronic obstructive pulmonary disease? Eur J Intern Med 2006 March; 17(2):88–91.
- Pinto-Plata VM, Mullerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, Celli BR. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006 January; 61(1):23–28.
- O'Reilly JF, Williams AE, Rice L. Health status impairment and costs associated with COPD exacerbation managed in hospital. Int J Clin Pract 2007 July; 61(7):1112–1120.
- Touw DJ, Brimicombe RW, Hodson ME, Heijerman HG, Bakker W. Inhalation of antibiotics in cystic fibrosis. Eur Respir J 1995 September; 8(9):1594–1604.
- Rubin BK. Other medications for aerosol delivery. Paediatr Respir Rev 2006; 7 Suppl 1:S76–S79.