Abstract
Background: Endogenous opioids are naturally occurring peptides released by the brain in response to noxious stimuli. Although these naturally occurring peptides modulate pain, it is unknown whether endogenous opioids affect the perception of breathlessness associated with a specific respiratory challenge. The hypothesis is that intravenous administration of naloxone, used to block opioid signaling and inhibit neural pathways, will increase ratings of breathlessness during resistive load breathing (RLB) in patients with chronic obstructive pulmonary disease (COPD). Methods: Fourteen patients with COPD (age, 64 ± 9 years) inspired through resistances during practice sessions to identify an individualized target load that caused ratings of intensity and/or unpleasantness of breathlessness ≥ 50 mm on a 100 mm visual analog scale. At two intervention visits, serum beta-endorphins were measured, naloxone (10 mg/25 ml) or normal saline (25 ml) was administered intravenously, and patients rated the two dimensions of breathlessness each minute during RLB. Results: Patient ratings of intensity (p = 0.0004) and unpleasantness (p = 0.024) of breathlessness were higher with naloxone compared with normal saline. Eleven patients (79%) reported that it was easier to breathe during RLB with normal saline (p = 0.025). RLB led to significant increases in serum beta-endorphin immunoreactivity and decreases in inspiratory capacity. There were no significant differences in physiological responses between interventions. Conclusions: Endogenous opioids modulate the intensity and the unpleasantness of breathlessness in patients with COPD. Differences in breathlessness ratings between interventions were clinically relevant based on the patients’ global assessment.
INTRODUCTION
Endogenous opioids are released by the brain in response to various stimuli, including pain, stress, surgery, anesthesia, acupuncture, and exercise. These naturally occurring peptides modulate subjective experiences, such as pain, pleasantness of food, and sexual pleasure (Citation1–3). In 1985, Santiago and Edelman (Citation4) proposed that endogenous opioids were elaborated to relieve breathing discomfort that developed in response to a respiratory challenge. However, Kirsch and colleagues (Citation5) found no difference in ratings of breathlessness during incremental cycle ergometry between naloxone and normal saline in 6 patients with COPD. We recently demonstrated the role of endogenous opioids in modifying dyspnea during treadmill exercise in 17 patients with COPD, who reported higher ratings of breathlessness after intravenous administration of naloxone, a receptor antagonist that blocks opioid signaling, compared with normal saline (Citation6).
Although exercise testing is used widely to evaluate symptoms and to quantify exercise performance, it requires complex interactions involving the respiratory, cardiovascular, musculoskeletal, and neurological systems. The exact stimulus for the release of endorphins during exercise is unknown. For example, endorphin levels increase in healthy individuals who perform exercise, but do not experience breathing difficulty (Citation7, 8). Thoren (Citation9) proposed that rhythmic exercise can activate the opioid system by triggering discharge from mechanosensitive afferent nerves arising from the contracting leg muscles. In our previous study, it is unknown whether endogenous opioid release was due to respiratory distress or related to musculoskeletal factors during high-intensity exercise (Citation6).
The two related hypotheses of our study were that: 1) resistive load breathing (RLB), as a intense respiratory stimulus, would cause release of beta (ß)-endorphins in peripheral blood; and 2) administration of naloxone would increase ratings of breathlessness by patients with COPD during RLB. In contrast to previous investigators (Citation10, 11), we identified an individualized target inspiratory load during practice sessions to provoke each patient to report substantial breathlessness. Based on previous studies in healthy individuals (Citation12) and in goats (Citation13), we anticipated that targeted RLB for at least 10 minutes would be a sufficient respiratory challenge to cause release of ß-endorphins. Patients were instructed to rate both the intensity and unpleasantness of breathlessness during RLB in order to examine the effects of opioid blockade on both the sensory and affective dimensions of dyspnea (Citation14, 15).
METHODS
Subjects
Patients with a diagnosis of COPD (Citation16) were recruited from out-patient clinics and provided informed written consent. Each patient reported breathing difficulty with activities of daily living. The protocol was approved by the Committee for the Protection of Human Subjects at Dartmouth College. Inclusion criteria were: ≥ 10 pack-year history of smoking; and post-bronchodilator forced expiratory volume in one second (FEV1) 30 – 80% predicted. Exclusion criteria were any condition that might interfere with study procedures and current use of a narcotic medication.
Study design
The study was randomized, cross-over, and double-blind that included 3 – 4 visits (Citation2 – Citation3 days apart). Testing was performed at the same time of day for all visits. At visit 1, patients rated their breathlessness on the self-administered computerized version of the baseline dyspnea index (BDI) (Citation17), performed pulmonary function tests, were familiarized with rating the intensity and unpleasantness of breathlessness, and performed RLB practice sessions to identify a target resistance for each patient that corresponded to mean ratings of ≥ 50 mm for either dimension of breathlessness over 10 minutes.
Interventions
Interventions were naloxone (10 mg/25 mL) and normal saline (25 mL) administered intravenously in random order.
Procedures
Pulmonary function testing (Collins model CPL; Longmont, CO) was performed using standard techniques (Citation18–21). Values were expressed as percentages of predicted normal values (Citation21–24).
Breathlessness was defined for each patient as breathing difficulty. Patients were familiarized with separate 100 mm VAS anchored at the bottom by “No Intensity/Unpleasantness” and at the top by “Greatest Intensity/Unpleasantness” used to rate dimensions of breathlessness. At each visit, patients read instructions that included examples of the two dimensions of breathlessness (Citation14):
Intensity refers to the pure level or magnitude of the sensation. It is like a physical measure, for example, how much do you weigh in p ounds? Intensity does not contain any pleasantness or unpleasantness, like or dislike, or how terrifying the experience is to you.
Unpleasantness describes how much you like or dislike something or feel terrified by it. A high unpleasantness indicates that your breathing feels very bad or terrifying regardless of whether the intensity is high or low.
Investigators ensured that each patient understood the differences between intensity and unpleasantness of breathlessness. During RLB, the patient placed a mark on the vertical VAS (presented in alternating order) each minute.
The resistive circuit consisted of a rigid tube with internal diameter of 3.5 cm and a series of ports. Sheets of filter paper were inserted between the ports to allow the presentation of different resistances, linear at flow rates of 0.5, 1, and 2 liters/sec.
At Visit 1, increasing inspiratory resistances (10, 15, 22, and 27 cm H2O/l/sec) were applied to identify a target resistance that provoked substantial breathlessness. Patients rested for 20 minutes between each RLB trial. If the target resistance was not achieved, patients returned 2 -3 days (Visit 1B) for additional RLB trials (35, 42, and 48 cm H2O/l/sec) as described here.
At intervention visits 2 and 3, the following sequence was followed: spirometry and inspiratory capacity (IC) performed 30 min after inhalation of albuterol (180 μg); 18-gauge catheter placed in an arm vein; after a 30 minute rest, patient breathed quietly through mouthpiece for 5 min; 10 mL of venous blood removed; infusion of naloxone (10 mg/25 mL) or normal saline (25 mL) over 1 minute; patients breathed quietly through mouthpiece for 5 min; targeted resistance was applied and patient breathed for “as long as possible;” patient made ratings of intensity and unpleasantness of breathlessness on cue each minute; RLB terminated when patient stopped voluntarily or at 20 min; 10 mL of venous blood removed; patient performed inspiratory capacity; 10 mL of venous blood removed 30 min end-RLB. A standard method was used for the measurement of IC (Citation25, 26).
After completion of RLB at visit 3, each patient was asked at which visit, 2 or 3,was easier for breathing.
At all visits, expired gas for metabolic parameters and end-tidal carbon dioxide (PETCO2) were analyzed every breath using a calibrated measurement system (MedGraphics Systems, St. Paul, MN). Heart rate (HR) and pulse oximetry (SpO2) were monitored (Nellcor Inc, Hayward, CA).
ß-endorphin immunoreactivity was measured from venous blood withdrawn from a catheter placed in an arm vein by modified ELISA technique (Citation6).
Statistical analysis
All ratings for the intensity and unpleasantness of breathlessness at equivalent times for each patient during RLB were the primary outcomes. For example, if 1 patient provided 6 ratings during 6 minutes of RLB with naloxone and 10 ratings during 10 minutes of RLB with normal saline, then ratings for intensity and unpleasantness through six minutes were used for analysis for that patient (i.e., multiple isotimes). This approach was used with all patients to yield a total of 154 ratings for naloxone and for normal saline.
A paired t-test was used to compare 154 ratings of intensity and 154 ratings of unpleasantness between naloxone and normal saline. The repeated measures module of the General Linear Model (v. 18, SPSS) was used to test for differences in ß-endorphin immunoreactivity for the interventions, time periods, and interaction. Paired t-tests were used to examine differences in ß-endorphin immunoreactivity. The binomial test was used to examine patient responses regarding which visit was easier to breathe during RLB. A p value < 0.05 was considered statistically significant.
Based on results of previous studies in patients with COPD, we considered that a sample size of 14 was adequate to provide 80% power to detect a significant difference in breathlessness ratings (alpha = 0.05) (Citation6, Citation27).
RESULTS
Enrollment, allocation, follow-up and analysis of patients are shown in . Descriptive characteristics of the 8 female and 6 male patients are summarized in Eight patients required two visits to identify the target resistance.
Table 1. Descriptive characteristics of the 14 patients
Baseline measures of lung function were similar at Visits 2 and 3. The target inspiratory resistance ranged from 10 – 48 cm H2O/l/sec (31 ± 15 cm H2O/l/sec). Physiological responses at rest and end-RLB are reported in . Compared to rest values, RLB led to increases in inspiratory time (TI) and total respiratory time (TTOT), but decreases in inspiratory capacity (IC) (naloxone: Δ = 260 ml; p = 0.004; normal saline: Δ = 220 ml; p = 0.02). There were no significant changes in other physiological variables at the end of RLB compared to rest conditions. There were no significant differences in any physiological responses at rest (post-intervention) or at end-RLB between naloxone and normal saline.
Table 2. Physiological responses before and at the end of resistive load breathing
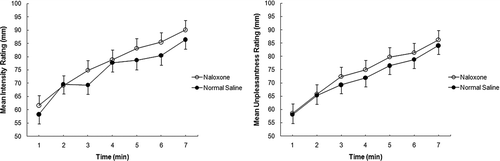
Patients provided a total of 154 ratings for each dimension of breathlessness at equivalent times during RLB. Examination of individual ratings showed no consistent response pattern. Both the intensity (83 ±18 vs. 77 ± 21 mm; p = 0.0004; 95% CI 2.69, 9.38) and the unpleasantness (81 ± 20 vs. 77 ± 19 mm; p = 0.024; 95% CI 0.49, 6.85)of breathlessness were significantly higher with naloxone compared with normal saline (). Eleven of the 14 patients reported that breathing was easier with normal saline than with naloxone (p = 0.025).
Figure 2. Mean and standard error values for ratings of the intensity (Figure 2A) and unpleasantness (Figure 2B) of breathlessness during 7 minutes of resistive load breathing following administration of naloxone (open circles) and normal saline (closed circles). For both conditions, n = 14 for minutes 1 – 4, n = 13 for minutes 5 – 7.Ratings of the intensity and unpleasantness of breathlessness were significantly correlated for naloxone (r = 0.95; p < 0.001) and for normal saline (r = 0.88; p < 0.001) conditions.
Levels of serum ß-endorphin immunoreactivity are shown in . The repeated measures analysis indicated a significant main effect of the intervention medication (p < 0.001), test time (p < 0.001) and the interaction of the intervention medication and test time (p < 0.001). There were significant increases in ß-endorphin levels immediately and 30 minutes after RLB compared to baseline values for each intervention. Paired t-tests indicated that baseline ß-endorphin levels were not significantly different, but that values were significantly higher immediately and 30 minutes after RLB with naloxone compared with normal saline.
Table 3. Levels of ß-endorphin immunoreactivity
Endurance time during RLB was similar with naloxone compared with normal saline (12.5 ± 5.4 vs. 13.9 ± 4.7 min; p = 0.45; 95% CI -4.5, 2.2). In 3 patients, the investigators stopped the RLB trial at 20 minutes with each intervention as established in the study design.
DISCUSSION
The unique findings in this study were: 1) patients with COPD rated both the intensity and the unpleasantness of breathlessness as significantly higher with naloxone compared with normal saline during RLB; 2) RLB was associated with increases in ß-endorphin levels; and 3) RLB was associated with decreases in IC.
Our primary analysis examined 154 ratings of breathlessness at equivalent times (i.e., multiple isotimes) during RLB, which is the same approach as comparing ratings at identical times during exercise (Citation28–30). Although the significantly higher ratings of intensity and unpleasantness of breathlessness with naloxone compared with normal saline were modest, the magnitudes of these differences were similar to those reported with RLB by other investigators (Citation14, Citation31). We believe that the observed differences in VAS ratings are clinically relevant based on the patients’ global assessment of their breathlessness. Eleven of the 14 patients (79%) indicated that their breathing was significantly easier during RLB after administration of normal saline compared with naloxone. It is important to recognize that patient ratings for the different dimensions of breathlessness may be similar, as noted with normal saline condition, even though intensity and unpleasantness represent distinct experiences.
Target resistance loads were selected at practice sessions based on individual patient ratings ≥50 mm on the 100 mm VAS. Thus, the actual range on the VAS used by patients to rate breathlessness during the interventions was ∼ 50 mm. With this consideration, the percent differences were 12% for intensity and 8% for unpleasantness of breathlessness between naloxone and normal saline. These percent changes are consistent with the effects of other interventions on ratings of dyspnea considered clinically important in patients with COPD (Citation6, Citation32, Citation33). It is possible that the higher ratings of the intensity and unpleasantness of breathlessness with naloxone were attenuated, and thus underestimated, as a result of the significantly higher levels of circulating ß-endorphins observed at the end of RLB with naloxone compared with normal saline.
Endogenous opioids are released into the blood from the pituitary gland in response to stress and noxious stimuli (Citation9, Citation34). Our study is the first to demonstrate that ß-endorphins are released into the circulation in patients with COPD in response to RLB. The magnitude of the increment in serum ß-endorphins with targeted RLB is similar to the response observed when patients with COPD perform high-intensity treadmill exercise (Citation6). Endogenous opioids are also released into the cerebrospinal fluid (CSF) and brain from hypothalamic neurons (Citation13, Citation34). Opioid receptors are expressed primarily in the cortex, limbic system, and brain stem and play a central role in nociception and analgesia (Citation35). Although circulating ß-endorphins do not cross the blood-brain barrier, the central effects of endogenously generated opioid peptides can be uncovered by the administration of naloxone, a receptor antagonist that blocks opioid signaling and has its greatest affinity for the μ-receptor.
As we found no differences in any of the physiological responses between interventions, we conclude that endogenous opioids act as neuromodulators of the sensory (intensity) and affective (unpleasantness) dimensions of breathlessness by their effect on receptors within the brain. An alternative possibility is that circulating endogenous opioids relieve dyspnea by activation of opioid receptors (epithelial and/or pulmonary C-fibers) known to present in the respiratory system (Citation36). To our knowledge, the present study is the first to examine the effect of an intervention on different dimensions of breathlessness. Furthermore, the results of our study provide a rationale for the use of exogenous opioid drugs (e.g., morphine) for the treatment of patients with advanced COPD who experience distressful and disabling dyspnea (Citation16, Citation37, Citation38).
As demonstrated previously with RLB, our patients increased TI and TTOT, but showed no changes in expiratory time or pattern of breathing (Citation39). Although we did not measure functional residual capacity in this study, we suspect that the decrements in IC at end-RLB reflect the development of lung hyperinflation. The decreases in IC (Δ = 220 – 260 ml) with RLB in our study are consistent with the development of hyperinflation. Based on these responses, RLB may be analogous, at least in part, to the development of an exacerbation of COPD. Both situations are associated with an acute increase in airway resistance that leads to hyperinflation and breathlessness (Citation40, 41).
One limitation of this study was the modest number of patients. The results were statistically significant with 14 patients and support the hypothesis of the study. However, we can not exclude a type I error. Other investigators have demonstrated significant differences in outcomes with an effective intervention in similar numbers of patients with COPD (Citation27, Citation42). A second limitation was that three patients performed RLB for 20 minutes with both interventions. Our intent was to identify a target resistance load in practice sessions that caused substantial breathlessness and was tolerated for < 20 minutes in order to examine the effects of the two interventions on RLB endurance as a secondary outcome. As noted, we did not achieve this objective in 3 of the 14 patients. The equivalent endurance times (Citation20 minutes) during RLB for three patients likely contributed to the non-significance of the trend for greater endurance time with normal saline compared with naloxone.
In conclusion, we have demonstrated for the first time that endogenous opioids modulate breathlessness when patients with COPD are challenged with a specific and intense respiratory stimulus. A novel finding in our study is that individualized and targeted RLB was sufficient to cause release of ß-endorphins into the peripheral blood and presumably into the brain based on the opioid blocking effects of naloxone. These results expand our understanding of the neurobiology associated with the sensory and affective dimensions of breathlessness. Based on current understanding of pleasure and pain, we believe that other neurotransmitters, in addition to endogenous opioids, likely contribute to and/or modify the perception of breathlessness. Further investigations are indicated to explore the neurobiology of breathlessness.
ACKNOWLEDGMENTS
The authors acknowledge funding from the Hitchcock Foundation.
Disclosure of interests: AHG was responsible for design of the study, data collection, statistical analysis, and review of the manuscript. DAM was the principal investigator and responsible for all aspects of the investigation including the final content of the manuscript. LAW was responsible for data collection, statistical analysis, and review of the manuscript. JW was responsible for design of the study, data collection, and review of the manuscript. WJK was responsible for design of the study, measurement of beta-endorphin immunoreactivity, and review of the manuscript. BRK was responsible for design of the study, measurement of beta-endorphin immunoreactivity, and review of the manuscript. JCB was responsible for design of the study, statistical analysis, and review of the manuscript.
AHG and DAM wrote the various drafts of the manuscript.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology 2006; 31:1371–1381.
- Murphy MR, Checkley SA, Seckl JR, Lightman SL. Naloxone inhibits oxytocin release at orgasm in man. J Clin Endocrinol Metab 1990; 71:1056–1058.
- Jarmukli NF, Ahn J, Iranmanesh A, Russell DC. Effect of raised plasma beta endorphin concentrations on peripheral pain and angina thresholds in patients with stable angina. Heart 1999; 82:204–209.
- Santiago TV, Edelman NH. Opioids and breathing. J Appl Physiol 1985; 59:1675–1685.
- Kirsch JL, Muro JR, Stansbury DW, Fischer CE, Monfore R, Light RW. Effect of naloxone on maximal exercise performance and control of ventilation in COPD. Chest 1989; 96:761–766.
- Mahler DA, Murray JA, Waterman LA, Ward J, Kraemer WJ, Zhang X, Baird JC. Endogenous opioids modify dyspnoea during treadmill exercise in patients with COPD. Eur Respir J 2009; 33:771–777.
- Carr DB, Bullen BA, Skrinar GS, Arnold MA, Rosenblatt M, Beitins IZ, Martin JB, McArthur JW. Physical conditioning facilitates the exercise-induced secretion of beta-endorphin and beta-lipotropin in women. N Engl J Med 1981; 305:560–563.
- Gambert SR, Garthwaite TL, Pontzer CH, Cook EE, Tristani FE, Duthie EH, Martinson DR, Hagen TC, McCarty DJ. Running elevates plasma beta-endorphin immunoreactivity and ACTH in untrained human subjects. Proc Soc Exp Biol Med 1981; 168:1–4.
- Thoren P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc 1990; 22:417–428.
- Santiago TV, Remolina C, Scoles V, 3rd, Edelman NH. Endorphins and the control of breathing. Ability of naloxone to restore flow-resistive load compensation in chronic obstructive pulmonary disease. N Engl J Med 1981; 304:1190–1195.
- Simon PM, Pope A, Lahive K, Steinbrook RA, Schwartzstein RM, Weiss JW, Fencl V, Weinberger SE. Naloxone does not alter response to hypercapnia or resistive loading in chronic obstructive pulmonary disease. Am Rev Respir Dis 1989; 139:134–138.
- Vassilakopoulos T, Zakynthinos S, Roussos C. Strenuous resistive breathing induces proinflammatory cytokines and stimulates the HPA axis in humans. Am J Physiol 1999; 277:R1013–109.
- Scardella AT, Parisi RA, Phair DK, Santiago TV, Edelman NH. The role of endogenous opioids in the ventilatory response to acute flow-resistive loads. Am Rev Respir Dis 1986; 133:26–31.
- von Leupoldt A, Dahme B. Differentiation between the sensory and affective dimension of dyspnea during resistive load breathing in normal subjects. Chest 2005; 128:3345–3349.
- Carrieri-Kohlman V, Donesky-Cuenco D, Park SK, Mackin L, Nguyen HQ, Paul SM. Additional evidence for the affective dimension of dyspnea in patients with COPD. Res Nurs Health 2010; 33:4–19.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555.
- Mahler DA, Waterman LA, Ward J, McCusker C, ZuWallack R, Baird JC. Validity and responsiveness of the self-administered computerized versions of the baseline and transition dyspnea indexes. Chest 2007; 132:1283–1290.
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Van Der Grinten CP, Gustafsson P, General considerations for lung function testing. Eur Respir J 2005; 26:153–161.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, Van Der Grinten CP, Gustafsson P, Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Macintyre N, Crapo RO, Viegi G, Johnson DC, Van Der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26:720–735.
- Black LF, Hyatt RE. Maximal respiratory pressures: Normal values and relationship to age and sex. Am Rev Respir Dis 1969; 99:696–702.
- Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis 1971; 103:57–67.
- Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981; 123:659–664.
- Gaensler EA, Smith AA. Attachment for automated single breath diffusing capacity measurement. Chest 1973; 63:136–145.
- O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164:770–777.
- O'Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23:832–840.
- Pepin V, Saey D, Whittom F, LeBlanc P, Maltais F. Walking versus cycling: sensitivity to bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172:1517–1522.
- Ayers ML, Mejia R, Ward J, Lentine T, Mahler DA. Effectiveness of salmeterol versus ipratropium bromide on exertional dyspnoea in COPD. Eur Respir J 2001; 17:1132–1137.
- O'Donnell DE, Sciurba F, Celli B, Mahler DA, Webb KA, Kalberg CJ, Knobil K. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest 2006; 130:647–656.
- Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J 2001; 18:77–84.
- Wan L, Van Diest I, De Peuter S, Bogaerts K, Van Den Bergh O. Repeated breathlessness experiences induced by hypercapnia: differential effects on intensity and unpleasantness. Chest 2009; 135:455–461.
- Oga T, Nishimura K, Tsukino M, Hajiro T, Ikeda A, Izumi T. The effects of oxitropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. A comparison of three different exercise tests. Am J Respir Crit Care Med 2000; 161:1897–1901.
- Brouillard C, Pepin V, Milot J, Lacasse Y, Maltais F. Endurance shuttle walking test: responsiveness to salmeterol in COPD. Eur Respir J 2008; 31:579–584.
- Clement-Jones V, Besser GM. Clinical perspectives in opioid peptides. Br Med Bull 1983; 39:95–100.
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev 2009; 89:1379–1412.
- Zebraski SE, Kochenash SM, Raffa RB. Lung opioid receptors: pharmacology and possible target for nebulized morphine in dyspnea. Life Sci 2000; 66:2221–2231.
- Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri-Kohlman V, Curtis JR, Manning HL, Mularski RA, Campbell M, Carter ER, American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 2010; 137:674–691.
- Varkey B. Opioids for palliation of refractory dyspnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2010; 16:150–154.
- Chonan T, Altose MD, Cherniack NS. Effects of expiratory resistive loading on the sensation of dyspnea. J Appl Physiol 1990; 69:91–95.
- Stevenson NJ, Walker PP, Costello RW, Calverley PM. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172:1510–1516.
- Parker CM, Voduc N, Aaron SD, Webb KA, O'Donnell DE. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J 2005; 26:420–428.
- Ofir D, Laveneziana P, Webb KA, O'Donnell DE. Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol 2007; 102:2217–2226.
- Mahler DA, Ward J, Fierro-Carrion G, Waterman L, Lentine TF, Mejia-Alfaro R, Baird JC. Development of self-administered versions of the modified baseline and transition dyspnea indexes in COPD. J COPD 2004; 1:165–172.