Abstract
Desmosine and isodesmosine are products of elastin breakdown which are candidate biomarkers to measure lung destruction in COPD. Data exist on the burden of desmosines in urine and plasma in COPD but long-term changes have never been investigated. We determined the changes of desmosine levels over 14 months in urine and plasma of patients with type ZZ alpha-1-antitryspsin deficiency-related COPD. Urines and plasma for determination of desmosines were collected from 11 ex-smokers with moderate/severe emphysema at monthly intervals for 14 months. Spirometry and gas transfer were assessed at baseline and 6-month intervals. At baseline and month 14, eleven healthy partners of patients volunteered to give a blood sample for detection of desmosines. Desmosines were determined by capillary electrophoresis combined with laser-induced fluorescence. Urine and plasma desmosines were significantly increased after 14 months in patients (p = 0.027 and p = 0.0005, respectively). Plasma desmosines of healthy partners at baseline were 4-fold lower than from patients and not significantly different from values at month 14. Only a significant decline in lung gas transfer occurred in patients (p = 0.015). The variability of desmosines was higher in urine than in plasma (coefficient of variation 0.17 and 0.087, respectively). As longitudinal desmosine changes likely reflect the elevated elastic fiber turnover associated with the progression of lung damage and destruction in COPD, they appear to be a suitable marker for application in long-term studies. Plasma desmosines were more stable long-term biomarkers than desmosines in urine.
INTRODUCTION
Elastin is a fundamental component of the extracellular matrix composing the lung alveolar architecture, where it contributes to the maintenance of the structural integrity and to lung mechanics (Citation1). Pulmonary emphysema is characterized by degradation of elastin and other components of the alveolar structure caused by elastolityc enzymes, among which neutrophil elastase (NE) has a prominent role (Citation2). Alpha-1-antitrypsin (α1-AT) is the main anti-protease inactivating NE, therefore individuals with α1-AT deficiency are particularly prone to the development of chronic obstructive pulmonary disease (COPD), with emphysema as the main component (Citation3).
Desmosine (DES)- and isodesmosine (IDES)-containing peptides are products of elastolytic breakdown that have been proposed as candidate biomarkers for lung destruction in COPD (Citation4). DES and IDES are two tetrafunctional pyridinium ring-containing amino acids associated only with mature cross-linked elastin and are therefore useful for discriminating peptides derived from elastin breakdown from precursor elastin peptides (Citation5). Due to these features, DES and IDES have been studied as markers of increased lung elastic fibers’ turnover in emphysema; thus far elevated DES levels have been detected in the urine of patients with COPD with and without α1-AT deficiency as compared to healthy non-smoking subjects (Citation6, 7).
Some evidence indicates higher DES excretion in the urine of α1-ATD-related COPD as well as in adult cystic fibrosis and in subjects with diffuse bronchiectasis, as compared to stable, non α1-ATD-related COPD (Citation8, 9). Advances in detection techniques, including liquid chromatography tandem mass spectrometry (LC-MS/MS) and high-performance capillary electrophoresis with laser-induced fluorescence (CE-LIF), have recently allowed detection of DES and IDES at very low concentration in body fluids other than urine, including plasma and sputum, with the advantage that the measurements do not have to be corrected for the kidney function (Citation10).
A recent review identified longitudinal studies on well-defined COPD phenotypes as the next step needed to evaluate the usefulness of DES as surrogate end-point/biomarkers (Citation10). Indeed no data exist on long-term changes of DES and/or IDES excretion and their relationship with the progression of the disease. In α1-ATD-related emphysema, two studies aimed at evaluating DES changes in urine after treatment with augmentation therapy (Citation11, 12). The longer of these two studies lasted 24 weeks and showed a persistent decrease of urinary DES that was not statistically significant (Citation12). Because of the lack of a placebo group in these studies, it was not possible to evaluate DES changes due to the natural progression of the disease along time.
As the natural history of COPD is that of an irreversible progression of lung damage, we hypothesized that DES/IDES level should increase along time in patients affected by α1-ATD-related COPD, where the elastolytic burden is particularly elevated. Based on this hypothesis we measured at monthly intervals for 14 months DES/IDES levels in urine and plasma in a group of α1-ATD patients, to evaluate the possibility of using them as long-term biomarkers of lung damage.
MATERIALS AND METHODS
Study population
Eleven patients with moderate-to-severe emphysema were recruited among those regularly visiting the dedicated α1-ATD outpatient clinic at Leiden University Medical Centre (LUMC) and registered in the Dutch chapter of the Alpha1 International Registry (Citation13). Selection criteria were: a) severe α1-ATD (Pi*ZZ or Pi*Null phenotype; b) emphysema confirmed by CT scan; c) TLCO and KCO less than 80% of the predicted value; d) ex-smoker, who has stopped smoking since at least 6 months; e) clinically stable for at least one month prior to the study; f) no changes in inhaled medications, no oral steroid courses in the month before the study.
Patients were excluded when CT scan showed the presence of bronchiectasis. In addition, 11 healthy partners of patients volunteered to give a blood sample at the baseline visit and at month 14. They did not perform lung function tests and CT scans of the chest, nor were they requested to collect 24 h urine samples. The study was approved by the ethics committee of the LUMC and participants provided informed consent.
Study design
The design was that of a longitudinal biomarker study, with collection of urine and plasma samples for DES measurement at monthly intervals for 14 months. At baseline, month 8 and 14, the lung function airflow limitation parameters (FEV1, FEV1/FVC) and gas transfer (Kco) were collected.
Desmosines measurement
From each patient, urine and blood were always collected in the morning, at the same time ±1 hrs. The partner of each patient only donated a blood sample. Collected urine samples were anonymized, frozen and stored at −80°C. The blood samples were centrifuged (3000 rpm per 15 min) after collection and plasma was anonymized, frozen and stored at –80°C. In this way all samples could be analyzed in the same session, avoiding possible time-effects. To avoid sample order-effects, random samples were taken to determine desmosines concentration.
Aliquots (250 μl) of urine/deproteinized plasma were put into pyrex tubes, evaporated to dryness in vacuo and hydrolyzed by refluxing with 500 μl of twice-distilled constant boiling HCl at 106°C for 24 hr. The hydrolyzed samples were dried under a nitrogen stream, the residue washed four times with de-ionized water and neutralized with 0.5 M Na2CO3 pH 8.7. After derivatizing with Fluoresceine Isothiocyanate (FITC), DES/IDES were detected by applying capillary electrophoresis coupled with laser induced fluorescence (CE-LIF) detection, as previously described (Citation14). Due to its very good sensitivity, this method allowed to detect amounts of desmosines as low as 5.26 μg/L−1 avoiding time-consuming pre-treatment procedures, including concentration, of the urine samples.
Since the cited CE-LIF method does not allow discrimination between DES and IDES, the results shown in this manuscript are the sum of the two isomers. The lower limit of detection of the method was 5 nM (equivalent to 0.1 fmol on column), with a within and between-batch coefficient of variation of 2.5% and 3.5%, respectively. Urinary DES/IDES were expressed as μg/g creatinine and plasma DES/IDES as μg/L. All measurements (creatinine in urine and DES/IDES in urine and plasma) were performed in triplicate and in random order. The values reported are the mean of three independent determinations.
Table 1 Clinical characteristics of the patients (n = 11) participating in the study.
Statistical analysis
Statistical analysis was performed using the software package SPSS 15.0 for Windows and SAS. Summary measures were represented as means (SE) or medians (range) as appropriate. Paired T-test on log-transformed data was used to test the differences between DES/IDES values at baseline, 6 months and at the end of the study. In addition, DES/IDES results of all time points were used for the SAS analysis of repeated measurements: after log transformation of the data, slopes were analysed in a linear mixed effect model. Paired t-test and a mixed model for repeated measurements were also used for analyzing the changes of FEV1 and Kco at M2, M8 and M14. The calculation of the coefficient of variation was used to determine the variability of urine and plasma DES/IDES during the measurement period.
RESULTS
Clinical characteristics
All patients had moderate-to-severe emphysema, as shown from lung function values in . Most patients had stopped smoking since more than 10 years (median 12 years, range 26); onset of the disease had been at a median age of 40.5 (range 31). All patients had lung emphysema documented by CT scan, with panlobular emphysema, prevalent in the lower fields, and absence of bronchiectasis and of any systemic or organ diseases other than emphysema. Nine were males and two were females. The clinical characteristics are shown in .
Table 2 Urine and plasma DES values at baseline, 6 months and 1 year
Urine and plasma DES/IDES levels
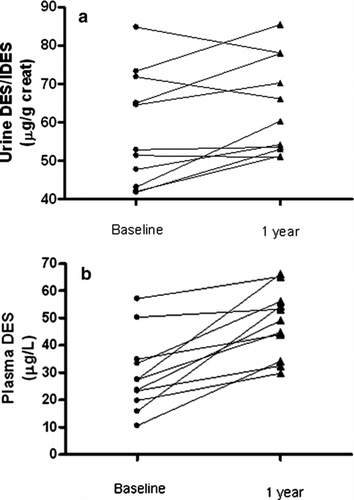
At the beginning of the study the median levels of DES/IDES in urine were 52.9 μg/g creatinine (min-max 41.8–84.8) and 27.5 μg/L (min-max 10.5–57.1) in plasma. The rolling means of urine and plasma desmosines at baseline (M1+M2), at 6 months (M7+M8) and at the end of the study (M13+M14) are shown in . Urine and plasma DES/IDES were significantly increased at the end of the study as compared to baseline when tested by a paired t-test ( and ). The linear mixed effects model, using all time points, confirmed that urine and plasma DES/IDES increased significantly during the study period. No changes in urine or plasma DES/IDES were detected at 6 months. The mean level of plasma DES/IDES of healthy partners at the baseline visit was 5.7 μg/L (min-max 4.81-6.47 μg/L). At month 14, the mean difference from the baseline visit was −0.18 μg/L (min-max −0.5 to +0.25 μg/L, p > 0.05).
Variability of urinary and plasma DES/IDES
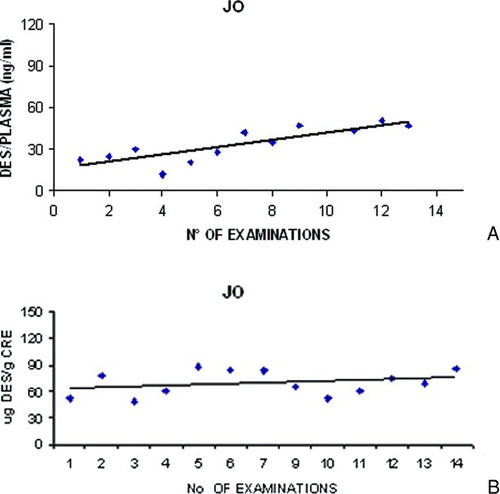
The variability of urine DES/IDES in the study period, measured by the coefficient of variation, was higher than the variability of plasma DES/IDES. (Urine = 0.17; plasma = 0.087). shows all time-point measurements of urine and plasma DES/IDES in one patient chosen at random among all available.
Progression of lung function parameters
During the 14-month observation period, FEV1 decreased but the decrease was not statistically significant (). On the other hand, changes in Kco were detected between the beginning and the end of the study (p = 0.015) and these correlated with change in plasma DES/IDES (r = 0.65, p = 0.01). The results of lung function measurements at M2, M8 and M14 are shown in .
DISCUSSION
In the present work we showed that urine and plasma levels of DES/IDES are significantly increased over a more than 1-year observation period in patients with α1-ATD-related COPD, with plasma DES/IDES showing a lower variability than urine DES/IDES. No such changes occurred in plasma of healthy partners.
COPD biomarkers should have the following characteristics (Citation15, 16): a) central to the pathophysiological processes of the disease; b) true surrogate end-point; c) stable, varying only with events related with progression of the disease; d) able to predict progression; e) cost-effective; f) sensitive to effective intervention factors.
DES/IDES are unique to mature elastin, which is degraded during the inflammatory processes taking place in COPD as effect of smoking, infections and other noxious agents. As such, DES and IDES are biomarkers directly related to the pathophysiological processes of COPD. In cross-sectional studies, patients with COPD and other destructive lung disease excrete more urinary DES and IDES than normal controls (Citation10), and higher DES values have been found in smokers with rapid lung function decline as compared to smokers with slow lung function decline (Citation17). Together with these findings, the significant increase over time in our study supports the role of DES/IDES as biomarkers of ongoing structural lung destruction associated with the natural course of COPD in alpha-1-antitrypsin deficiency.
No lung-specific DES and/or IDES isoforms have ever been described, therefore whenever DES/IDES are measured, the possibility of other sites of origin such as skin and cardiovascular system, compartments that contain elastin, and undergo changes in COPD, cannot be excluded (Citation18). However, the specific association of elevated DES/IDES excretion with COPD and the recent detection of DES in sputum of COPD and cystic fibrosis patients suggest that the site of origin of the degraded elastin is most likely uniquely the lung (Citation8, Citation19). In addition, subjects with α1-ATD-related COPD are characterized by low incidence of comorbidity, mainly due to the younger age than that of non-α1-ATD patients, and any organ and systemic disease other than COPD was an exclusion criterion in the present study.
We did not detect any significant change in FEV1 between baseline and the end of this study with a relatively low number of patients. Overall, airflow limitation parameters such as FEV1 reflect macroscopic changes in lung structure, therefore their change is slow, while DES and IDES are thought to represent active lung destruction and are therefore expectedly changing during shorter periods of observation. A recent review raised the hypothesis that FEV1 might not be specific enough for lung destruction to correlate with products of elastin degradation (Citation10). This hypothesis has strong basis, as several authors showed that FEV1 is poorly correlated with parenchymal destruction in COPD assessed by pathology and by CT scan (Citation20, 21). On the other hand, Kco was significantly decreased during the observation period, likely reflecting COPD progression (Citation22). Emphysema is the prevalent component in α1-ATD-related COPD, where the bronchial involvement is limited even in very advanced phases (Citation23).
If the homogeneity of the population of our study (moderate-to severe α1-ATD-related emphysema) is an advantage in interpreting the changes of DES/IDES as biomarker, on the other hand it can limit the generalizability of the results. While elastic breakdown is central to the pathogenesis of both α1-ATD related and non-α1-ATD related COPD, the lack of α1-AT makes the lungs particularly prone to the destruction by neutrophil elastase, configuring a phenotype of COPD where the elastolytic burden is particularly high. To this regard, a recent cross-sectional study found that PiZZ a1-ATD subjects with emphysema had higher DES levels in plasma and sputum than stable or even exacerbated COPD subjects without α1-ATD (Citation8). The subjects of the aforementioned study were under treatment with augmentation therapy so the results would be valuable of confirmation in a non-treated population.
Significantly, the variability of DES/IDES was lower in plasma than in urine. In general, a good correlation has been described between urine and plasma desmosines but no study investigated long-term variability of the two types of measurement (24). Current measurement methods of plasma desmosines are based on radioimmunoassay techniques, using antibodies to elastin peptides with different specificity and sensitivity, and the standardization and quantification of desmosines can largely vary accordingly. Although more laborious, the CE-LIF method used in our study is more sensitive and accurate than other proposed procedures; as such it provides a very reliable measure of DES/IDES plasma level (Citation25).
On the other hand, even though the methodology for DES/IDES determination in urine is very well established, measurement in this biological fluid always requires correction for creatinine values. The dependence of urine DES/IDES measurement from possible fluctuations in kidney function can probably explain the slightly higher variability of this parameter along time.
In conclusion, increased values of DES/IDES were shown for the first time during a long-term observation of 14 months in patients with α1-ATD-related COPD. The results of the present study suggest that DES/IDES might be useful biomarkers for the measurement of lung destruction associated with the progression of COPD, with plasma DES/IDES showing long-term higher stability than urine DES/IDES.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Chrzanowski P, Keller S, Cerreta J, Mandl I, Turino GM. Elastin content of normal and emphysematous lung parenchyma. Am J Med 1980; 69:351–359.
- Damiano VV, Tsang A, Kucich U, Abrams WR, Rosenbloom J, Kimbel P, Fallahnejad, M, Weinbaum G. Immunolocalization of elastase in human emphysematous lungs. J Clin Invest 1986; 78:482–493.
- Hubbard RC, Brantly ML, Sellers SE, Mitchell ME, Crystal RG. Anti-neutrophil-elastase defenses of the lower respiratory tract in alpha 1-antitrypsin deficiency directly augmented with an aerosol of alpha 1-antitrypsin. Ann Intern Med 1989; 111:206–212.
- Rosenbloom J. Biochemical/immunologic markers of emphysema. Ann NY Acad Sci 1991; 624:7–12.
- Foster JA, Curtiss SW. The regulation of lung elastin synthesis. Am J Physiol 1990; 259:L13–L23.
- Stone PJ, Gottlieb DJ, O'Connor GT. Elastin and collagen degradation products in urine of smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 151:952–959.
- Viglio S, Iadarola P, Lupi A, Trisolini R, Tinelli C, Balbi B, Grassi V, Worlitzsch D, Döring G, Meloni F, Meyer KC, Dowson L, Hill SL, Stockley RA, Luisetti M. MEKC of desmosine and isodesmosine in urine of chronic destructive lung disease patients. Eur Respir J 2000; 15:1039–1045.
- Ma S, Lin YY, Turino GM. Measurement of desmosine and isodesmosine by mass spectrometry in COPD. Chest 2007; 131:1363–1371.
- Bode DC, Pagani ED, Cumiskey WR, von Roemeling R, Hamel L, Silver PJ. Comparison of urinary desmosine excretion in patients with chronic obstructive pulmonary disease or cystic fibrosis. Pulm Pharmacol Ther 2000; 13:175–180.
- Luisetti M, Ma S, Iadarola P, Stone PJ, Viglio S, Casado B, Lin YY, Snider GL, Turino GM. Desmosine as biomarker of elastin degradation in COPD: current status and future directions. Eur Respir J 2008; 32:1146–1157.
- Gottlieb DJ, Luisetti M, Stone PJ, Allegra L, Cantey-Kiser JM, Grassi C, Snider GL. Short-term supplementation therapy does not affect elastin degradation in severe1-antitrypsin deficiency. The American–Italian AATD Study Group. Am J Respir Crit Care Med 2000; 162:2069–2072.
- Stoller JK, Rouhani F, Brantly M, Shahin S, Dweik RA, Stocks JM, Clausen J, Campbell E, Norton F. Biochemical efficacy and safety of a new pooled human plasma1-antitrypsin, Respitin. Chest 2002; 122:66–74.
- Stockley RA, Luisetti M, Miravitlles M, Piitulainen E, Fernandez P; Alpha One International Registry (AIR) group. Ongoing research in Europe: Alpha One International Registry (AIR) objectives and development. Eur Respir J 2007; 29:582–586.
- Annovazzi L, Viglio S, Perani E, Luisetti M, Baraniuk J, Casado B, Cetta G, Iadarola P. Capillary electrophoresis with laser-induced fluorescence detection as a novel sensitive approach for the analysis of desmosines in real samples. Electrophoresis 2004; 25:683–691.
- Jones PW, Agusti AG. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J 2006; 27:822–832.
- Stockley RA. Biomarkers in COPD: time for a deep breath. Thorax 2007; 62:657–660.
- Gottlieb DJ, Stone PJ, Sparrow D, Gale ME, Weiss ST, Snider GL, O'Connor GT. Urinary desmosine excretion in smokers with and without rapid decline of lung function: the Normative Aging Study. Am J Respir Crit Care Med 1996; 154:1290–1295.
- Agusti AG. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2:367–370.
- Laguna TA, Wagner BD, Luckey HK, Mann SA, Sagel SD, Regelmann W, Accurso FJ. Sputum desmosine during hospital admission for pulmonary exacerbation in cystic fibrosis. Chest. 2009; 136:1561–1568.
- Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Paré PD, Hogg JC, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000; 162:1102–1108.
- Makita H, Nasuhara Y, Nagai K, Ito Y, Hasegawa M, Betsuyaku T, Onodera Y, Nizawa N, Nishimura M, and the Hokkaido COPD Cohort Study Group. Characterization of phenotypes based on severity of emphysema in chronic obstructive pulmonary disease. Thorax 2007; 62:932–937.
- Baldi S, Miniati M, Bellina CR, Battolla L, Catapano G, Begliomini E, Giustini D, Giuntini C. Relationship between extent of pulmonary emphysema by high-resolution computed tomography and lung elastic recoil in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164:585–589.
- Tomashefski JF, Crystal RG, Wiedemann HP, Mascha E, Stoller JK; Alpha-1 Antitrypsin Deficiency Registry Study Group. The bronchopulmonary pathology of alpha-1 antitrypsin (AAT) deficiency: findings of the Death Review Committee of the national registry for individuals with Severe Deficiency of Alpha-1 Antitrypsin. Hum Pathol 2004; 35:1452–1461.
- Stolk J, Veldhuisen B, Annovazzi L, Zanone C, Versteeg EM, van Kuppevelt TH, Berden JH, Nieuwenhuizen W, Iadarola P, Luisetti M. Short-term variability of biomarkers of proteinase activity in patients with emphysema associated with type Z1-antitrypsin deficiency. Respir Res 2005; 6:47.
- Viglio S, Annovazzi L, Luisetti M, Stolk J, Casado B, Iadarola P. Progress in the methodological strategies for the detection in real samples of desmosine and isodesmosine, two biological markers of elastin degradation. J Sep Sci 2007; 30: 202–213.