Abstract
Current guidelines recommend inhalation therapy as the preferred route of drug administration for treating chronic obstructive pulmonary disease (COPD). Previous systematic reviews in COPD patients found similar clinical outcomes for drugs delivered by handheld inhalers - pressurized metered-dose inhalers (pMDIs), dry powder inhalers (DPIs) - and nebulizers, provided the devices were used correctly. However, in routine clinical practice critical errors in using handheld inhalers are highly prevalent and frequently result in inadequate symptom relief. In comparison with pMDIs and DPIs, effective drug delivery with conventional pneumatic nebulizers requires less intensive patient training. Moreover, by design, newer nebulizers are more portable and more efficient than traditional jet nebulizers. The current body of evidence regarding nebulizer use for maintenance therapy in patients with moderate-to-severe COPD, including use during exacerbations, suggests that the efficacy of long-term nebulizer therapy is similar, and in some respects superior, to that with pMDI/DPIs. Therefore, despite several known drawbacks associated with nebulized therapy, we recommend that maintenance therapy with nebulizers should be employed in elderly patients, those with severe disease and frequent exacerbations, and those with physical and/or cognitive limitations. Likewise, financial concerns and individual preferences that lead to better compliance may favor nebulized therapy over other inhalers. For some patients, using both nebulizers and pMDI/DPI may provide the best combination of efficacy and convenience. The impact of maintenance nebulizer treatment on other relevant clinical outcomes in patients with COPD, especially the progressive decline in lung function and frequency of exacerbations, needs further investigation.
Introduction
National and international guidelines recommend inhalation therapy as the preferred route of drug administration to treat asthma (Citation1,2) and chronic obstructive pulmonary disease (COPD) (Citation3–5) based on several advantages of this delivery route over oral or parenteral treatment (e.g., faster onset of action, lower dose requirement, reduced systemic side effects) (Citation6). Several years ago, systematic reviews of the published literature concluded that drugs delivered by any of the 3 commonly prescribed inhalation devices – pressurized metered-dose inhalers (pMDIs) with or without spacers, dry powder inhalers (DPIs), and nebulizers—had similar efficacies in patients with asthma and COPD provided they were used appropriately (Citation7–9).
However, in clinical practice, it is commonly observed that patients with a faulty inhalation technique get less symptom relief from handheld inhalers than patients who use the devices appropriately (Citation10,11). Many patients, especially elderly patients with COPD, are unable to use their pMDIs and DPIs in an optimal manner (Citation10–12). Furthermore, some drug formulations are not available for specific devices (e.g., there is no approved pMDI formulation of long-acting beta-agonists in the United States), thereby limiting the choice of particular drug/device combinations. In recent years, several new devices and drug formulations have entered the marketplace, and it is relevant to explore the merits of various drug/device combinations for specific patient populations. The recently published ERS/ISAM Task Force Report comprehensively reviews the characteristics of various aerosol delivery devices and discusses their appropriate use in clinical practice (Citation13).
A growing body of evidence supports using nebulizers for maintenance therapy of respiratory disease in an outpatient setting, despite limitations that might negatively affect ambulatory use (e.g., reduced portability, increased time for drug administration, variability of drug output, and need for cleaning after each use) (Citation14). The primary aim of this article is to explore the role of nebulizers for the treatment of patients with stable COPD and during exacerbations of the disease. Issues concerning the choice of aerosol delivery devices in patients receiving noninvasive or invasive mechanical ventilation are described elsewhere (Citation15).
Selection of Articles for Review
The topic was divided into specific subsections, which were assigned to at least two authors each. Articles were selected for inclusion based on systematic reviews of the literature according to each subsection. Database searches (1996–current) of MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews, limited to English language, used multiple primary topic headers combined with appropriate terms for each section of the paper (e.g., COPD + aerosols or COPD + inhaled therapy). The authors also identified relevant papers from reference lists of published articles and their own reference libraries. All relevant articles were included without any selection bias.
Aerosol Drug Delivery Systems
A variety of inhaled drugs are available for the treatment of COPD, including several long- and short-acting bronchodilators, anticholinergics, corticosteroids, and combination products. Although the choice of pharmacologic agent is unquestionably important, selecting an appropriate aerosol delivery device is also critical for successful therapy. Patients with COPD and their physicians need to consider the unique features of various inhalers that make the delivery of aerosol formulations more suitable to the ventilatory nuances imposed by the disease. For example, delivery systems that separate generation and inhalation of the aerosol (e.g., pMDI + spacer) might be more useful for elderly patients with COPD (Citation6, Citation9).
Three categories of aerosol drug delivery systems (ADDSs) are currently used to deliver the above medications to patients with COPD: pMDIs, DPIs, and nebulizers. The number of different ADDS — each with different characteristics, requiring different inhalation techniques — can be confusing for both patient and clinician. Using multiple ADDDs – a common practice in patients with more severe disease – can add to the confusion, resulting in decreased adherence with therapy (Citation16,17). Thus, for optimal clinical benefit, it is important to carefully and appropriately match the delivery system to the patient, and, whenever possible, to simplify the number and type of devices employed.
Pressurized Metered-dose Inhalers (pMDIs)
The pMDI remains the most commonly used handheld aerosol delivery device, despite the increasing use of DPIs and other handheld inhalers (Citation6). Most of the newer pMDIs formulated with hydrofluoroalkane (HFA) propellants provide an aerosol with a lower forward jet velocity than older chlorofluorocarbon pMDIs (Citation6,Citation9,Citation18,19). The decreased flow velocity makes it easier for patients who may have difficulty with the coordination required to inhale the medication. The issues of taking a slow rather than a rapid inhalation, whether to inhale from residual volume or functional residual capacity, and the length of breath-hold at end-inspiration are common to both solution and suspension HFA pMDIs (Citation6,Citation11). Priming and shaking the canister beforehand may be required for reproducible dosing, and patients should be reminded to review the package insert for specific instructions (Citation6).
For patients who have difficulty with coordination, the use of a valved spacer with the pMDI should be encouraged. This allows a slower-moving and smaller-particle-sized aerosol to be formed, extending penetration into the lungs and potentially enhancing the response to therapy (Citation6,Citation9,Citation18,19). Even with an HFA-pMDI, 50% or more of the aerosol may be deposited in the oropharyngeal region. This can be reduced, but not eliminated, with the use of a valved spacer. For some patients, a facemask may be easier to interface with the pMDI and spacer than a mouthpiece (Citation6). Using a non-electrostatic spacer may increase the amount of drug available at the mouth compared to plastic spacers. The aerosol remains in suspension within non-electrostatic spacers for several seconds longer than within plastic spacers, allowing the patient to briefly delay inhaling the drug (Citation6,Citation20,Citation21).
Given their history, errors in inhalation technique for pMDIs have been well documented. Hand-breath coordination, a slow inspiratory flow rate (IFR), and breath-hold are critical steps for effective pMDI drug delivery (Citation22,23). Patients with COPD have a higher incidence of errors in pMDI use (26%) than patients with asthma (13%) (Citation10). However, in a more recent report differences in error rates for handling various inhalers between patients with asthma and COPD were not significant after adjustment for age, device, and level of instruction (Citation11). Actuating a pMDI may be more difficult for patients with arthritis of the hands. Improper spacer technique also may lead to dosing errors (Citation24). Both pausing before inhaling from a holding chamber/spacer and multiple actuations into a chamber/spacer – not uncommon patient practices – can significantly reduce drug availability (Citation25).
Dry powder inhalers (DPIs)
As breath-actuated devices, DPIs eliminate the majority of problems associated with coordinating device actuation and inhalation that occur with pMDIs. However, these inhalers require the patient to generate a higher inspiratory flow rate to readily dispense the powder than pMDIs (Citation1,Citation19,Citation26). Use of DPIs with increased airflow resistance may mean greater difficulty for some patients with severe COPD who cannot achieve an adequate IFR (Citation19). The resistance of the DPI can vary 10-fold (0.02–0.20 cmH2O1/2/L/min), depending on design (Citation6). Quinet and colleagues demonstrated in a geriatric population that lower than optimal inspiratory pressure and peak IFRs as well as decline in cognitive function limit the use of DPIs (Citation27).
Inhalation at a less than optimal IFR for the specific DPI increases oropharyngeal deposition of the inhaled powder (Citation6). Failure to hold the device correctly and improper dose and inhaler preparation also contribute to high error rates in some patients. Exhalation into the device prior to inhalation can be a serious problem with some DPIs, and even the exhalation-induced increase in humidity around the inhalation channel can affect product quality and dosing efficiency over time (Citation6). These issues are complicated by the proliferation of DPIs with different designs and dose-loading engineering (Citation10,Citation28).
Nebulizers
Nebulizers are an alternate method to pMDIs and DPIs for providing aerosol therapy, provided the drug is available in liquid form. They are the most user-friendly of the inhaler devices for patients to use and are frequently prescribed de facto for patients with COPD (Citation1). Developments over the past 10 years have made higher-efficiency nebulizers and computer-controlled delivery devices available for the management of patients with COPD (Citation6).Conventional pneumatic nebulizers are inexpensive, but require a compressor or pressurized gas to operate. Additionally, lung deposition is variable and frequently less than that with the newer high-efficiency models due to drug wastage during the expiratory phase of the breathing cycle (Citation6). presents the features of the different types of nebulizers currently used.
Table 1 Features of different nebulizers6
One of the primary advantages of nebulizers in COPD is the minimal coordination and effort required during inhalation compared to pMDIs and DPIs. The aerosol is continuously produced, and the patient can sit comfortably while taking medication, using tidal-volume breathing. If the patient requires a facemask, the design should provide a tight, but comfortable seal around the cheeks to avoid aerosol leakage into the environment and also to prevent/ reduce aerosol exposure to the eyes, an important consideration with anti-cholinergic therapy (Citation6).
The most common complaints from patients reflect shortcomings of the device itself: conventional jet nebulizers are bulky and require an external power source (compressed gas, batteries or electricity). They require more preparation to set-up and take an average of 10–15 minutes to deliver the complete dose, and then must be cleaned. Other issues relate to the interaction of device and drug formulation. Formulation properties such as viscosity and surface tension can influence the production of the aerosol; for example, combining 2 medications from different vials may save time for the patient, but also may affect nebulizer performance, possibly delivering different quantities of the drugs used (Citation6). Hygroscopic drugs can alter the final particle-size distribution of the aerosol, although this is less of a problem with nebulizers than pMDIs/DPIs. The efficiency of drug delivery with jet nebulizers can be improved by employing breath-enhanced or breath-actuated devices designed to minimize drug wastage during exhalation (Citation29,30).
Newer designs of nebulizers, based on vibrating mesh or vibrating plate technologies or micro-pump engineering are highly efficient (). Portable and handheld, some are small enough to fit in a pocket, and are usually battery-operated. Delivery efficiencies to the lung periphery are upwards of 60% of the nominal reservoir drug dose, with minimal loss of drug due to residual volume. These devices also deliver the required dose within a much shorter treatment time than conventional jet nebulizers – usually, over minutes (Citation6). Vibrating mesh nebulizers can effectively nebulize solutions, suspensions, liposomal formulations, and proteins (Citation6). The major drawback for the patient is the need to completely disassemble the devices for cleaning after each use, which is more challenging than cleaning the more commonly used jet nebulizers. Another nebulizer design (the Respimat® SoftMist™Inhaler Boehringer-Ingelheim, Ingelheim, Germany) provides a metered dose of liquid aerosol at a low velocity when a button on the device is pressed. Treatment, consisting of 1–2 actuations, translates into a markedly reduced treatment time for the patient. Currently, the Respimat is marketed in several countries for delivery of a combination of ipratropium bromide and fenoterol hydrobromide (Berodual®Respimat) or tiotropium solution (Spiriva®Respimat).
Figure 1. Examples of marketed nebulizers that incorporate newer technologies. The eFlow (PARI, Midlothian, VA), MicroAir (Omron, Vernon Hills, IL,), I - Neb (Phillips Healthcare, Murrysville, PA) and Aeroneb (Aerogen, Galloway, IRE) incorporate VM/VAP aerosol generators. The I-neb and Akita Control System (Activaero,GER) employ Adaptive Aerosol Delivery technology as the patient/device interface for delivering and monitoring aerosol treatments. TheI-Neb and eFlow are formulation-specific for iloprost and cayston, respectively, and as such are not part of current COPD treatment paradigms. The Respimat (Boehringer-Ingelheim, Ingelheim GER) is a high efficiency soft-mist inhaler that employs a precise dosimetric system with multi-dose capability. All of these devices are approved for use in the United States. Photos courtesy of Myrna Dolovich, P.Eng.

Other improvements in nebulizer delivery systems include the use of control units that interface with the nebulizer, either a pneumatic design or one of the new technologies (e.g., the Akita® Jet Inhalation System, Activaero GmbH, Germany; the i-NEB AAD System, Philips Healthcare, Andover, MA, as shown in ) (Citation6). These control units monitor the patient's breathing pattern and control the time at which the drug is introduced into the inspiratory phase of the breathing cycle. They also track when treatment is initiated and the amount of drug inhaled, thereby improving the efficiency of delivery and adherence to therapy (Citation6).
For nebulizers with higher efficiencies and increased total (drug) output, the amount of drug loaded into the nebulizer may need to be adjusted to provide equivalent dosing to the lungs and avoid over-dosing. This information should be provided by the manufacturer based on sound bioequivalence studies. For example, a 2- to 4-fold decrease in ipratropium was needed for comparable bronchodilator response using an Aeroneb prototype compared to the Pari LC plus (Citation31). Likewise, in bench models of noninvasive positive pressure ventilation, the fine particle dose delivered with a vibrating mesh nebulizer was 2- to 4-times higher compared to a jet nebulizer (Citation32).
Considerations when choosing an aerosol drug delivery system for patients with COPD
How the patient uses the selected device and adheres to treatment may be influenced by personal preference, convenience, facility of use, economic factors, and the availability of competent caregiver assistance (Citation11,Citation33). Adherence, unlike compliance, represents an active choice on the part of the patient to follow prescribed therapy. Adherence failures may be “intentional” – also representing an active choice; however, for aerosol therapy, most failures are “unintentional” and related to incorrect device technique. Although all 3 types of devices require patient instruction and review to master technique, this is especially important for the handheld devices, for which regular monitoring of patient technique is needed to ensure optimal drug delivery.
Errors in using handheld inhalers are commonly observed in clinical practice. In one review, over 94% of 120 patients with asthma and COPD committed at least one error related to inappropriate technique, despite claiming to know how to use inhalation devices (Citation33). In another review, 2,288 records of handheld inhaler technique among patients with asthma and COPD were examined (Citation11).Critical mistakes were found among users of all the inhalers, with significant associations between inhaler misuse and older age (p = 0.008), less education (p = 0.001), and lack of instruction (p < 0.001). Misuse was associated with increased risk of urgent care and decreased disease control (Citation11).
Information and instructions for use for a number of the aerosol devices and inhalers available for therapy are given on the educational websites of the American Association for Respiratory Care (Citation34) and the American College of Chest Physicians (Citation35) as well as in each product's package insert.
Proper breathing technique and repeated instruction can be problematic for older patients with COPD as well as for patients with recognized physical and/or cognitive limitations (Citation36). Variable dosing may arise from poor technique that is not recognized by the patient (or caregiver) or, with recognition that the inhalation maneuver was suboptimal, from potential added puffs taken in compensation. Although many patients with COPD can use pMDIs (with or without a spacer) or DPIs for maintenance therapy, certain populations will benefit from administration by nebulizer ().
Table 2 Clinical scenarios where maintenance nebulizer therapy is preferred in patients with COPD*
In elderly patients and those with severe COPD and disabling dyspnea, delivering the total dose over several breaths by nebulizer may be more effective than delivery during a single breath by DPI or pMDI. In 2008, market analyses indicated that approximately 45% of patients with COPD had a nebulizer, and 69% of those patients used their nebulizer on a regular basis (Citation37). Based on estimated prevalence of COPD in the United States, there are several million patients who use nebulizers on a regular basis (Citation38).For these patients, using nebulized treatment may ensure adequate dosing and, possibly, slower progression of the disease as suggested in studies with handheld devices in patients who are monitored and capable of using the devices (Citation39). Although it is typically expected that the outcomes are medication-related as opposed to device-related, there are no comparable data on disease progression in patients using nebulizers, and studies are warranted.
The Role of Nebulized Therapy in The Pharmacologic Management of COPD
Current guidelines for the management of COPD (Citation3–5,Citation40,41) present a comprehensive approach based on 1) assessing and monitoring disease, 2) reducing risk factors, 3) managing stable COPD, and 4) preventing and managing exacerbations. Guidance for therapy is based upon four stages of disease lung function abnormalities, classified by severity: mild, moderate, severe, and very severe (). This section presents an overview of the use of nebulized therapy for the day-to-day management of COPD as well as for treatment of exacerbations ().
Figure 2. Pharmacological therapy of COPD by severity classification (stages I – IV). Delivery by nebulizer may be appropriate at any stage according to patient needs and preferences. (Adapted from ATS/ERS41 and GOLD3).
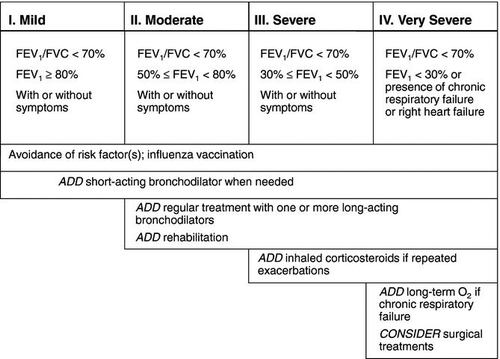
Table 3 Commonly used nebulized medications for patients with COPD*
Nebulized maintenance therapy for COPD
Most patients with early COPD – and even those with mild-to-moderate disease — use handheld inhalers for their regular maintenance therapy. Although the body of evidence clearly shows that all devices provide similar clinical benefit when used correctly, few studies have directly compared nebulizers and inhalers in the management of COPD. summarizes some of the data available on the use of nebulized treatments for the maintenance therapy of COPD in ambulatory patients (Citation42–59).
Table 4 Studies evaluating use of nebulized drugs for maintenance therapy in COPD.
Short-acting bronchodilators
Use of a short-acting bronchodilator agent by nebulization is recommended at all stages of COPD management for relief of acute bronchospasm. Improvement in lung function with a nebulized albuterol-ipratropium (2500/500 mcg) combination product compared to the individual treatments of albuterol or ipratropium was evident in a 6-week, 3-period, crossover study of 863 patients (Citation46). The nebulized combination product resulted in a 24% greater improvement in FEV1compared to albuterol alone and a 37% greater improvement than with ipratropium alone. Similarly, the area under the curve for FEV1 was 30% and 32% greater with the combination than albuterol or ipratropium, respectively (Citation46).
In patients with COPD combination therapy (albuterol + ipratropium) given by nebulizer, morning and night, with midday use of a pMDI, provided adequate control and improvement in quality of life (Citation49). In this 12-week study by Tashkin and coworkers (Citation49), 126 patients received treatment by nebulization (albuterol 2,500 mcg + ipratropium 500 mcg) 4 times-a-day, pMDI (albuterol 90 mcg + ipratropium 18 mcg) 2 puffs 4 times-a-day, or nebulization in the morning and evening with midday use of pMDI. Symptoms were significantly improved (p ≤ 0.05) in both nebulizer groups, but health-related quality of life, measured with the St. George Respiratory Questionnaire Quality of Life Instrument (SGRQ), was better in the nebulizer + pMDI group, possibly reflecting enhanced symptom relief provided by the nebulizer and the convenience of pMDI treatment when away from home during the day (Citation49).
Long-acting bronchodilators
As disease severity increases, a long-acting bronchodilator agent (LABD) is recommended as routine, maintenance therapy (Citation3,Citation41). The only LABD currently available in nebulized form is formoterol, as the racemic mixture, formoterol fumarate inhalation solution (FFIS) at a strength of 20 mcg/2 ml, or as the enantiomeric formulation, arformoterol at a strength of 15 mcg/2 ml (Citation60,61).Studies have demonstrated the effectiveness of both products in the regular treatment of patients with COPD with or without adding tiotropium bromide DPI (Citation50–58). Tiotropium, a long-acting anti-cholinergic, is not available in a nebulized formulation in the United States but is available by micronebulizer (Respimat®) in Europe (Citation6,Citation26).
The FFIS 20 mcg/2 ml administered twice daily over a period of 12 weeks demonstrated a rapid onset of action associated with increased FEV1 and improvements in symptoms and quality-of-life scores (Citation53). The persistence of improvement over time showed no loss of efficacy or tachyphylaxis and was comparable to formoterol (12 mcg) given by DPI twice daily. In this study, there were no significant differences in the efficacy of either treatment with regard to lung function or symptoms; however, only the patients using FFIS showed statistically significant improvements in quality of life (QOL) (Citation53). A 12-month, open-label trial comparing the two preparations found comparable retention and adherence rates, with no between-group differences in adverse events, laboratory values or cardiovascular events (Citation51).
Nebulized arformoterol 15-mcg twice-a-day has been studied in several trials, although head-to-head comparisons with FFIS have not been conducted. In a placebo-controlled, 12-week, double-blind, randomized study of 717 patients with COPD 3 doses of arformoterol (15 mcg bid, 25 mcg bid, 50 mcg qd) were compared to salmeterol pMDI, 42 mcg twice-a-day (Citation50). The FDA-approved dose of 15 mcg arformoterol twice-a-day provided sustained and significant improvement in FEV1 over the 12-week study, with a mean change in peak FEV1 at study end of 252 mL (21.2%) compared to 190 mL (15.1%) for salmeterol (p < 0.05 vs. arformoterol) and 170 mL (13.6%) for placebo (p < 0.01 vs. arformoterol).
When compared to placebo, improvements in other parameters – SQRQ, rescue albuterol, supplemental ipratropium – were similar between the treatments; although changes in the transition dyspnea index (TDI) only reached statistical significance with arformoterol. Perhaps the greatest difference between the treatments was the median time to response: between 3 and 14 minutes for arformoterol compared to 132 minutes for salmeterol at 12 weeks, and the placebo response at 327 minutes (Citation50).
In a study of 155 COPD patients, Hanania and colleagues evaluated FFIS (20 mcg) twice-a-day as add-on therapy to once daily tiotropium bromide (18 mcg) (Citation54). The group receiving FFIS + tiotropium showed greater improvements in FEV1, FVC, and inspiratory capacity, with efficacy maintained over the entire 6 weeks of the study. The combination group also experienced fewer adverse events: 37% versus 51% for those receiving tiotropium alone. The additional improvement in FEV1 with the combined product was approximately 130 ml compared to tiotropium alone and 410 ml compared to no treatment (Citation54).
Inhaled corticosteroids
Inhaled corticosteroids are recommended with LABD for patients at GOLD (Global Initiative for Chronic Lung Disease) stages III and IV () (Citation3). Recent studies have shown that GOLD stage II patients might also benefit, with specific regard to improvements in quality of life, decreased exacerbations, better lung function, and lower risk of mortality (Citation3). Budesonide is the only corticosteroid currently available in the United States for treatment of COPD in a combination inhaler with formoterol (Symbicort® 160/4.5, AstraZeneca) (Citation62). Data are not available for nebulized budesonide, but based on the authors’ unpublished experiences, FFIS and budesonide can be used twice-a-day with a jet nebulizer. A small 6-month study of regular therapy with nebulized flunisolide (1,000 mcg) in 114 COPD patients showed numerical, but not statistically significant, reductions in exacerbation rates (the primary variable) in addition to improvement in FEV1 (Citation59).
All recommended drug categories for the chronic treatment of COPD are available in nebulized form, though inhaled corticosteroids are not approved as monotherapy for COPD in the United States. Nebulized drugs have been found to be efficacious in patients with COPD for a chronic use strategy and may be most cost-effective with the current Medicare reimbursement system in patients who qualify under Part B coverage.
In summary, the limited data available suggest that when patients use their aerosol devices correctly, maintenance therapies provide similar clinical benefits. Nebulized LABDs alone and in combination with tiotropium may yield slightly better outcomes in some patients (Citation50–58). Based on very limited data, it seems that older, male patients with more severe COPD do better (and prefer) nebulized therapy (Citation44,Citation57). However, as discussed previously, it is likely that other subgroups might also benefit. Whether nebulized delivery in such patients could slow disease progression has not yet been determined.
Inhaled pharmacotherapy during exacerbations of COPD (ECOPD)
The GOLD guidelines define an exacerbation of chronic obstructive pulmonary disease (ECOPD) as an event in the natural course of the disease characterized by a change in the patient's baseline dyspnea, cough and/or sputum that is beyond normal day-to-day variations, is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD (Citation3). An average of 22% of patients with stage II disease, 33% with stage III and 47% with stage IV have two or more ECOPD events in the first year of follow-up. The single best predictor of exacerbations across all GOLD stages appears to be a previous history of exacerbations (Citation63).
Hospitalizations due to ECOPD are significant events in the natural history of COPD as they are associated with a more rapid decline in lung function, worsening quality of life, and increased morbidity and mortality (Citation50,51).Complete recovery from ECOPD may take up to 3 months after hospital discharge (Citation52). Therefore, an optimal therapeutic approach to reducing the incidence and severity of ECOPD can improve long-term health status and may conserve health care resources and costs (Citation53,54).
The symptomatic deterioration associated with ECOPD routinely requires treatment with antibiotics and/or corticosteroids in addition to bronchodilators, and may require hospitalization (Citation3,Citation41,Citation64).For acutely dyspneic patients being treated for an episode of ECOPD, the potential for variability in drug delivery with handheld inhalers may have a negative impact on clinical outcomes (Citation65). Therefore, nebulization may be the preferred method for administering inhaled medications in the acute setting of ECOPD as little effort and coordination are needed on the part of the patient (Citation18,Citation66,Citation67). With the development of newer generations of high-efficiency nebulizers, a more effective delivery of bronchodilators and corticosteroids may potentially change ECOPD outcomes (Citation68).
Short-acting bronchodilators
Substantial evidence shows that both short-acting beta agonists (SABAs) and short-acting anticholinergic agents (SAACs) can increase the FEV1 and FVC by 15 to 30% over a period of 60 to 120 minutes when given during an ECOPD(Citation69), and both induce similar bronchodilation (Citation70). Inhaled SABAs such as albuterol and levalbuterol administered via a nebulizer or a pMDI are the cornerstone of therapy for ECOPD due to their rapid onset of action (Citation71,72). In the absence of a prompt response to SABAs, the addition of a SAAC is recommended. Ipratropium is routinely administered in addition to SABA agents in most patients with ECOPD, and several studies have reported that the combination produces greater bronchodilation than either agent alone (Citation73,74).However, other trials have not found the same positive outcomes after administration of SABA/SAAC combination in patients with ECOPD (Citation75).
Regarding the potential deterioration of gas exchange in patients with ECOPD following administration of SABAs, Khoukaz and Gross (Citation76) and Polverino et al. (Citation77) found that in hospitalized patients with ECOPD, administration of albuterol did not aggravate the already compromised pulmonary gas exchange, and the declines observed were small, transient, and clinically insignificant. Although administration of a single dose of a SABA may increase heart rate by almost 10 beats per minute and reduce serum potassium by 0.36 mEq/L, these findings are relatively rare and do not appear to increase the risk of fatal or nonfatal acute myocardial infarction (Citation78).
Long-acting bronchodilators
As the cornerstone of the pharmacotherapy of patients with stable COPD, use of long-acting beta agonists (LABAs) and long-acting anticholinergic agents (LAACs) is associated with significant reduction in the rate of ECOPD (Citation79–83). Although formoterol is not approved for use as a rescue medication in the United States, its fast onset of action has drawn attention to a potential role as stand-alone medication or in combination with other bronchodilators in the management of ECOPD (Citation84,85). Incorporating BID formoterol into a bronchodilator protocol for hospitalized patients with asthma or COPD reduced the need for respiratory therapist-administered treatments with SABAs (Citation86). Adding once-daily tiotropium to the protocol further reduced utilization of health-care resources including bronchodilator costs and hospital length of stay (Citation87).
Corticosteroids
The administration of systemic corticosteroids is strongly recommended in the treatment of ECOPD in published guidelines (Citation4,Citation40,Citation41). In addition to improving symptoms and lung function, adding a corticosteroid to bronchodilator therapy significantly reduces treatment failure rates and length of hospital stays (Citation88–91). Short courses of oral corticosteroids have been shown to be as efficacious as intravenous corticosteroids for treating most episodes of ECOPD (Citation92), except in severe refractory cases or patients with impaired gastrointestinal absorption. However, the risk of serious adverse effects can outweigh clinical benefits, particularly as the rate of ECOPD increases in parallel with deteriorating lung function. A growing body of evidence favors using a moderate, rather than high dose of systemic corticosteroid (Citation91,Citation93,Citation94).
The risk:benefit issue has led clinicians to explore using inhaled corticosteroids (ICS), which have high anti-inflammatory activity but a substantially lower systemic side-effect profile than oral or parenteral corticosteroids (Citation95–97). Most trials of ICS in patients with COPD have shown some systemic absorption at high doses, but the clinical significance is unclear. Two meta-analyses evaluated the effects of ICS on bone mineral density (BMD) in adult patients with asthma and COPD and found that even after 2–3 years of administration, there was no significant decrease in the BMD (Citation98,99).
Systemic corticosteroids have onset of action within a few hours of administration, reaching a peak effect after approximately 24 hours. ICS may exert their first anti-inflammatory effects within the first hour, and most attain peak effects within a few hours (Citation100). Three studies comparing the efficacy of systemic corticosteroids and high-dose ICS delivered by pMDI in acute asthma exacerbations (Citation100–102) reported similar improvements in symptoms, peak expiratory flow (PEF), FEV1, treatment failure rates, and anti-inflammatory effects. To date, only 4 published studies have evaluated the efficacy of high-dose nebulized corticosteroids in ECOPD () (Citation103–106).
Table 5 Studies on the use of nebulized budesonide (BUD) vs. prednisolone (PRED) for exacerbations of chronic obstructive pulmonary disease
Earlier studies reported nebulized budesonide to be as efficacious as systemic corticosteroid with no adverse events (Citation103,Citation105). Maltais et al. compared the efficacy of nebulized budesonide and systemic corticosteroid for the first 72 hr of hospitalization in 171 patients with nonacidotic ECOPD and found no differences for improved FEV1, hospital duration, and occurrence of adverse events (Citation104). Systemic treatment was associated with a more substantial decrease in PaCO2 and a larger increase in post-bronchodilator FEV1, suggesting that it may be a better option for patients with imminent acute respiratory failure who require ventilatory support or admission to an intensive care unit.
However, a higher dose and/or more frequent administration of ICS may provide the same efficacy as systemic corticosteroid as demonstrated for asthma exacerbations (Citation107). Gunen et al. conducted the first comprehensive study comparing both short- and long-term effects of high-dose nebulized budesonide (1.5 mg qid) and systemic prednisolone (40 mg) (Citation106). They confirmed that high-dose nebulized budesonide was as effective as systemic corticosteroid for the short- and long-term treatment of 159 patients hospitalized with ECOPD, except in very severe cases. Particularly important is the fact that nebulized budesonide has been shown to be chemically stable and physically compatible when mixed for simultaneous administration with other common nebulized medications used to treat patients with ECOPD, including albuterol, levalbuterol, and ipratropium bromide inhalation solutions (Citation108).
Recommendations and Summary
Inhalation therapy is the cornerstone of treatment for patients with asthma and COPD, and pMDIs and DPIs remain the first line choice for maintenance treatment of these disorders. However, elderly patients, patients with severe disease, and those with other physical and/or cognitive limitations frequently cannot optimally use the handheld inhalers; for such patients nebulizers should be employed on a domiciliary basis.
Nebulizers are more forgiving to poor inhalation technique, especially poor coordination, than pMDIs because the aerosol is delivered over several breaths and no special breathing maneuvers are needed. Likewise, the requirement to generate adequate peak inspiratory flows that limits effective DPI use in elderly patients with severe COPD is not a barrier for nebulizer therapy. Some patients may prefer nebulizers because the costs of the equipment and medications are covered by Medicare, unlike pMDIs and DPIs for which separate coverage under Medicare part D is needed. Although there are no convincing data to show superiority of nebulizers for maintenance therapy in patients with COPD, better clinical outcomes could be expected for patients who prefer to use nebulizers over other handheld inhalers as well as those who have difficulty using pMDIs or DPIs. As such, we recommend nebulized delivery of drugs for COPD in the following situations:
Elderly patients, particularly male patients over 65 years of age
Patients with severe disease and frequent exacerbations
Patients who have physical and/or cognitive limitations
For inhalation drug delivery during exacerbations of COPD, especially when higher than routine drug doses are needed
When monetary concerns favor nebulized therapy over other inhalers
Patients who prefer nebulized therapy over other inhalers.
For some patients with COPD, the dual use of nebulizers and pMDI/DPI may achieve the best combination of efficacy and convenience. However, to minimize confusion, clinicians should make efforts to employ a single platform for delivery of all inhaled medications. Nebulizers could fulfill this requirement for many patients with COPD.
In summary, for optimal treatment of COPD, consideration of patient characteristics when recommending and prescribing an aerosol delivery device can help to minimize device use errors. The patient's age, cognitive status, visual acuity, manual dexterity and manual strength, ability to coordinate actuation of the inhaler with inhalation of the aerosol released may be as important as disease severity in determining the appropriate approach to delivery of medication. For many patients, using a nebulizer alone or in addition to a pMDI or DPI provides an easy-to-use and cost-effective therapy. Further studies are needed to determine whether this approach will also reduce episodes of exacerbations requiring an Emergency Department visit or hospital admission or diminish the rate of progressive decline in lung function associated with COPD.
Declaration of Interest Statements
Disclosure of potential conflict of interest: Bradley Chipps, MD, has received grants for clinical research from Genentech, AstraZeneca, GlaxoSmithKline, Novartis, Sunovion, and Merck (Schering). He has also received grants for educational activities from Alcon, Genentech, AstraZeneca, GlaxoSmithKline, Novartis, Sunovion, and Merck (Schering). Dr. Chipps serves as an Advisor for consultation to Alcon, Genentech, AstraZeneca, GlaxoSmithKline, Meda, Novartis, Sunovion, Merck (Schering), ISTA, Quintiles, and Dey Pharmaceuticals; and he is on the Speakers’ Bureau of Alcon, Genentech, AstraZeneca, GlaxoSmithKline, Meda, Novartis, Sunovion, Merck (Schering), ISTA, and Dey Pharmaceuticals.
Rajiv Dhand, MD, has received grants for clinical research from GlaxoSmithKline and Novartis; has served as a consultant for Mylan Pharma and Astra-Zeneca and is on the Speakers’ Bureau of GlaxoSmithKline.
Myrna Dolovich, P.Eng., has nothing to disclose.
Judith Farrar, PhD, has nothing to disclose.
Timothy Myers, RRT-NPS, serves on the TiNA Advisory Board and is a consultant for Boehringer Ingelheim.
Ruben Restrepo, MD, RRT has nothing to disclose.
Disclosure of sponsor involvement:
This manuscript was supported in part by an unrestricted educational grant from Dey Pharmaceuticals. The company had no involvement in the development, writing, or review of the manuscript other than providing access to market data analyses on the number of patients with COPD who use nebulizers in the United States. That data would not otherwise have been available to the authors.
References
- Global Initiative for Asthma (GINA), National Heart, Lung and Blood Institute, National Institutes of Health: GINA Report, Global Strategy for Asthma management and prevention, November 2006. Available at http://www.ginasthma.com. Accessed January 2011.
- National Asthma Education and Prevention Program, National Institutes of Health: Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD, National Institutes of Health, 2007. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed January 2011.
- GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, Global initiative for chronic obstructive lung disease (GOLD). 2010 Available at: http://www.goldcopd.org/download.asp?intId = 608. Accessed February 2011.
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis 1987; 136:225–244.
- Canadian Thoracic Society. Canadian Thoracic Society Guidelines for COPD. Available at http://www.copdguidelines.ca. Accessed April 2011.
- Dolovich MB, Dhand R. Aerosol drug delivery: Developments in design and clinical use. Lancet 2011; 377 (No 9770): 1032–1045.
- Ram FS, Brocklebank DM, Muers M, Wright J, Jones PW. Pressurised metered-dose inhalers versus all other hand-held inhalers devices to deliver bronchodilators for chronic obstructive pulmonary disease Cochrane Database Systematic Rev 2002;(1):CD002170.
- Ram FS, Brocklebank DM, White J, Wright JP, Jones P. Pressurised metered dose inhalers versus all other hand-held inhaler devices to deliver beta2-agonist bronchodilators for non-acute asthma. Cochrane Database Systematic Rev 2002;(1):CD002158.
- Dolovich M, Ahrens R, Hess D, Device selection and outcomes of aerosol therapy: evidence-based guidelines. Chest 2005; 127:335–371.
- Melani AS, Zanchetta D, Barbato N, Inhalation technique and variables associated with misuse of conventional metered-dose inhalers and newer dry powder inhalers in experienced adults. Ann Allergy Asthma Immunol 2004; 93:439–446.
- Melani AS, Bonavia M, Cilenti V, Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011; doi:10.1016/j.rmed.2011.01.005.
- Restrepo RD, Alvarez MT, Wittnebel LD, Sorenson H, Wettstein R. Medication adherence issues in patients treated for COPD. Int J COPD 2008; 3(3):371–384.
- Laube BL, Janssens HM, de Jongh FHC, What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011; 37:1308–1331.
- Kesser KC, Geller DE. New aerosol delivery devices for cystic fibrosis. Respir Care. 2009 Jun; 54(6):754–767; discussion 767–768.
- Dhand R. Inhalation therapy in invasive and noninvasive mechanical ventilation. Curr Opin Crit Care 2007; 13:27–38.
- Yu AP, Guerin A, Ponce de Leon D, Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ 2011;14:486–496.
- van der Palen J, Klein JJ, van Herwaarden CL, Zielhuis GA, Seydel ER. Multiple inhalers confuse asthma patients. Eur Respir J 1999; 14:1034–1037.
- Geller DE. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respir Care 2005; 50:1313–21.
- Newman SP. Inhaler treatment options in COPD. Eur Resp Rev 2005; 14:102–108.
- Wildhaber JH, Devadason SG, Eber E, Effect of electrostatic charge, flow, delay and multiple actuations on the in vitro delivery of salbutamol from different small volume spacers for infants. Thorax 1996; 51(10):985–988.
- Lauricella S, Dolovich M. The effects of inhalation delay and spacer pretreatment on HFA-pMDI delivery from several small volume valved holding chambers. J Aerosol Med 2007; 20:202.
- McFadden ER. Improper patient techniques with metered dose inhalers: clinical consequences and solutions to misuse. J Allergy Clin Immunol 1995; 96:278–283.
- Newman SP, Weisz AWB, Talaee N, Clarke SW. Improvement of drug delivery with a breath actuated pressurized aerosol for patients with poor inhaler technique. Thorax 2001; 46:712–16.
- Mitchell JP, Nagel MW. Valved Holding Chambers (VHCs) for use with pressurized metered dose inhalers (pMDIs): a review of causes of inconsistent medication delivery. Prim Care Resp J 2007; 16(4): 7–14.
- Rau JL. The inhalation of drugs: advantages and problems. Respir Care 2005; 50(3):367–382.
- Hodder R, Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat® Soft Mist™Inhaler. Int J COPD 2009; 4:381–390.
- Quinet P, Young CA, Heritier F. The use of dry powder inhaler devices by elderly patients suffering from chronic obstructive pulmonary disease. Ann Physical Rehab Med 2010; 53(2): 69–76.
- Wieshammer S, Dreyhaupt J. Dry Powder Inhalers: Which Factors Determine the Frequency of Handling Errors? Respiration 2008; 75:18–25.
- Corcoran TE, Dauber JH, Chigier N, Iacono AT. Improving drug delivery from medical nebulizers: the effects of increased nebulizer flow rates and reservoirs. J Aerosol Med 2002; 15;271–282.
- Rau JL, Ari A, Restrepo RD. Performance comparison of nebulizer designs: constant-output, breath-enhanced, and dosimetric. Respir Care 2004; 49:174–179.
- Dhand R, Giri VV, Noth I, Lissin D, Fishman RS, Taylor K. Bronchodilator response to ipratropium delivered via Aerodose inhaler or Pari LC Plus nebulizer in COPD. Am J Respir Crit Care Med 2002; 165:A593.
- Abdelrahim ME, Plant P, Chrystyn H. In-vitro characterization of the nebulized dose during non-invasive ventilation. J Pharmacy Pharmacol 2010; 62:966–972.
- de Moraes Souza ML, Meneghini AC, Ferraz E, Vianna EO, Borges MC. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol 2009; 35(9):824–831.
- American Association of Respiratory Care Aerosol Delivery Guide. Available at http://www.aarc.org/education/aerosol_devices/ aerosol_delivery guide2.pdf. Accessed September 2011.
- American College of Chest Physicians Patient Guides: Patient Instructions for Inhaled Devices. Available at http://www.chestnet.org/accp/patient-guides/patient-instructions-inhaled-devices-english-and-spanish. Accessed September 2011.
- Anderson P. Use of Respimat® Soft Mist™Inhaler in COPD patients. Int J COPD. 2006; 1(3):251–259.
- GfK Market Measures. COPD ATU, Q4 2008;Q 1g.
- COPD e-Newsletter. Available at http://www.nhlbi.nih.gov/health/public/lung/copd./ Accessed April 2011.
- Celli BR, Thomas NE, Anderson JA, Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008; 178:332–338.
- British Thoracic Society. Guidelines for the management of chronic obstructive pulmonary disease. Thorax 1997; 52:S1–S28.
- ATS/ERS Task Force. Standards for the diagnosis and management of patients with COPD. American Thoracic Society and European Respiratory Society 2004. Available at: www.thoracic.org/clinical/copd-guidelines/resources/copddoc.pdf. Accessed February 2011.
- Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. Am J Med 1996; 100 (Suppl 1A): 11–18.
- Friedman M. A multicenter study of nebulized bronchodilator solutions in chronic obstructive pulmonary disease. Am J Med. 1996; 100 (Suppl 1A): 30–39.
- Brophy C, Kastelik JA, Gardiner E, Greenstone MA. Quality of life measurements and bronchodilator responsiveness in prescribing nebulizer therapy in COPD. Chronic Respir Dis 2008; 5:13–18.
- The COMBIVENT Inhalation Solution Study Group. Routine nebulized ipratropium and albuterol together are better than either alone in COPD. Chest 1997; 112:1514–1521.
- Gross N, Tashkin D, Miller R, Oren J, Coleman W, Linberg S. Inhalation by nebulization of albuterol-ipratropium combination (Dey combination) is superior to either agent alone in the treatment of chronic obstructive pulmonary disease. Dey Combination Solution Study Group. Respiration 1998; 65:354–362.
- Levin DC, Little KS, Laughline KR, Addition of anticholinergic solution prolongs bronchodilator effect of ß2 agonists in patients with chronic obstructive pulmonary disease. Am J Med 1996; 100 (Suppl 1A): 40–48.
- Tashkin DP, Bleecker E, Braun S, Results of a multicenter study of nebulized inhalant bronchodilator solutions. Am J Med 1996; 100 (Suppl 1A):62–69.
- Tashkin DP, Klein GL, Colman SS, Zayed H, Schonfeld WH. Comparing COPD treatment: Nebulized, metered dose, inhaler, and concomitant therapy. Am J Med 2007; 120:435–441.
- Baumgartner RA, Hanania NA, Calhoun WJ, Nebulized arformoterol in patients with COPD: a 12-week, multicenter, randomized, double-blind, double-dummy, placebo- and active-controlled trial. Clin Therap 2007; 29:261–278.
- Donohue JF, Hanania NA, Fogarty C, Long-term safety of nebulized formoterol: Results of a twelve-month open label clinical trial. Ther Adv Respir Dis 2008a; 2:199–208.
- Donohue JF, Hanania NA, Sciarappa KA, Arformoterol and salmeterol in the treatment of chronic obstructive pulmonary disease: A one year evaluation of safety and tolerance. Therap Adv Respir Dis 2008b: 2:37–48.
- Gross N, Nelson HS, Lapidus RJ, Efficacy and safety of formoterol fumarate delivered by nebulization to COPD patients. Respir Med 2008; 102:189–197.
- Hanania NA, Boota A, Kerwin E, Tomlinson L, Denis-Mize K. Efficacy and safety of nebulized formoterol as add-on therapy in COPD patients receiving maintenance tiotropium bromide: Results from a 6-week, randomized, placebo-controlled, clinical trial. Drugs 2009; 69:1205–1216.
- Hanania NA, Donohue JF, Nelson H, The safety and efficacy of arformoterol and formoterol in COPD. COPD 2010; 7:17–31.
- Hanrahan JP, Hanania NA, Calhoun WJ, Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD 2008; 5:25–34.
- Sutherland ER, Brazinsky S, Feldman G, Nebulized formoterol effect on bronchodilation and satsfaction in COPD patients compared to QID ipratropium/albuterol MDI. Curr Med Res Opin 2009; 25:653–661.
- Tashkin DP, Littner M, Andrews CP, Concomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trial. Respir Med 2008; 102:479–487.
- Paggiaro PL, Vagaggini B, DiFranco A, Efficacy of nebulized flunisolide combined with salbutamol and ipratropium bromide in stable patients with moderate-to-severe chronic obstructive pulmonary disease. Respiration 2006; 73:603–609.
- BROVANA® (arformoterol tartrate) Inhalation Solution 15 mcg/2mL. Prescribing Information. Sunovion Pharmaceuticals, Inc., Marlborough, MA.
- Perforomist® (formoterol fumarate) Inhalation Solution 20 mcg/2mL Prescribing Information. Dey Pharma LP, Basking Ridge, NJ.
- Pulmicort Respules® (budesonide inhalation suspension). Prescribing Information. AstraZeneca LP, Wilmington DE.
- Hurst JR, Vestbo J, Anzueto A, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Calverley PM, Rennard S, Nelson HS, One-year treatment with mometasone furoate in chronic obstructive pulmonary disease. Respir Res 2008; 9(1):73.
- Goodman DE, Israel E, Rosenberg M, The influence of age, diagnosis, and gender on proper use of metered-dose inhalers. Am J Respir Crit Care Med 1994; 150:1256–1261.
- Barta SK, Crawford A, Roberts CM. Survey of patients’ views of domiciliary nebulizer treatment for chronic lung disease. Respir Med 2002; 96:375–381.
- Marcus P. The role of nebulized inhaled corticosteroid therapy in adult patients with asthma and chronic obstructive pulmonary disease. Adv Ther 2005; 22(4):407–418.
- Dhand R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir Care 2002; 47:1406–1416.
- Stoller JK. Clinical practice: acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002; 346:988–994.
- McCrory DC, Brown LE. Anticholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease (review). The Cochrane Library 2003 (issue 1): 1–24.
- Snow V, Lascher S, Mottur-Pilson C, Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134:595–599.
- Bach PB, Brown C, Gelfand SE, McCrory DC. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med 2001; 134:600–620.
- Cydulka RK, Emerman CL. Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med 1995; 25:470–473.
- O’Driscoll, BR, Taylor, RJ, Horsley, MG, Chambers DK, Bernstein A. Nebulised salbutamol with and without ipratropium bromide in acute airflow obstruction. Lancet 1989; 1:1418–1420.
- Karpel, JP. Bronchodilator responses to anticholinergic and beta-adrenergic agents in acute and stable COPD. Chest 1991; 99:871–876.
- Khoukaz G, Gross NJ. Effects of salmeterol on arterial blood gases in patients with stable chronic obstructive pulmonary disease: comparison with albuterol and ipratropium. Am J Respir Crit Care Med 1999; 160:1028–1030.
- Polverino E, Gomez FP, Manrique H, Gas exchange response to short-acting_2-agonists in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2007; 176:350–355.
- Suissa S, Assimes T, Ernst P. Inhaled short acting beta agonist use in COPD and the risk of acute myocardial infarction. Thorax 2003; 58:43–46.
- Stockley RA, Chopra N, Rice L. Addition of salmeterol to existing treatment in patients with COPD: a 12 month study. Thorax 2006; 61:122–28.
- Stockley RA, Whitehead PJ, Williams MK. Improved outcomes in patients with chronic obstructive pulmonary disease treated with salmeterol compared with placebo/usual therapy: results of a meta-analysis. Respir Res 2006b; 7:147.
- Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003; 290:2301–2312.
- Niewoehner DE, Rice K, Cote C, Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med 2005; 143:317–326.
- Mahler DA, Donohue JF, Barbee RA, Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115:957–965.
- Cazzola M, Santus P, Matera MG, A single high dose of formoterol is as effective as the same dose administered in a cumulative manner in patients with acute exacerbation of COPD. Respir Med 2003; 97:458–462.
- Di MF, Verga M, Santus P, Effect of formoterol, tiotropium, and their combination in patients with acute exacerbation of chronic obstructive pulmonary disease: a pilot study. Respir Med 2006; 100:1925–1932.
- Colice GL, Carnathan B, Sung J, Paramore LC. A respiratory therapist-directed protocol for managing inpatients with asthma and COPD incorporating a long-acting bronchodilator. J Asthma 2005; 42:29–34.
- Drescher GS, Carnathan BJ, Imus S, Colice GL. Incorporating tiotropium into a respiratory therapist-directed bronchodilator protocol for managing in-patients with COPD exacerbations decreases bronchodilator costs. Respir Care 2008; 53:1678–1684.
- Quon, BS, Gan, WQ, Sin, DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest 2008; 133:756–766.
- Niewoehner DE, Erbland ML, Deupree RH, Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1999; 340:1941–1947.
- Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999; 354:456–460.
- Lindenauer, PK, Pekow, PS, Lahti MC, Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA 2010; 303:2359–2367.
- de Jong YP, Uil SM, Grotjohan HP, Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest 2007; 132:1741–1747.
- Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Wilkinson TMA, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169: 1298–1303.
- Johansson SA, Andersson KE, Brattsand R, Gruvstad E, Hedner P. Topical and systemic glucocorticoid potencies of budesonide, beclomethasone dipropionate and prednisolone in man. Eur J Respir Dis Suppl 1982; 122:74–82.
- Brogden RN, McTavish D. Budesonide: an updated review of its pharmacological properties and therapeutic efficacy in asthma and rhinitis. Drugs 1992; 44:375–407.
- Walsh LJ, Wong CA, Oborne J, Adverse effects of oral corticosteroids in relation to dose in patients with lung disease. Thorax 2001; 56:279–284.
- Jones A, Fay JK, Burr M, Inhaled corticosteroid effect on bone metabolism in asthma and mild chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; CD003537.
- Richy F, Bousquet J, Ehrlich GE, Inhaled corticosteroids effects on bone in asthmatic and COPD patients: a quantitative systematic review. Osteoporos Int 2003; 14:179–190.
- Belda J, Margarit G, Martinez C, Anti-inflammatory effects of high-dose inhaled fluticasone versus oral prednisone in asthma exacerbations. Eur Respir J 2007; 30:1143–1149.
- Rodrigo GJ. Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. Am J Respir Crit Care Med 2005; 171:1231–1236.
- Levy ML, Stevenson C, Maslen T. Comparison of short courses of oral prednisolone and fluticasone propionate in the treatment of adults with acute exacerbations of asthma in primary care. Thorax 1996; 51:1087–1092.
- Morice AH, Morris D, Lawson-Matthew B. A comparison of nebulized budesonide with oral prednisolone in the treatment of exacerbations of obstructive pulmonary disease. Clin Pharmacol Ther 1996; 60:675–678.
- Maltais F, Ostinelli J, Bourbeau J, Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 165:698–703.
- Mirici A, Akgun M. Comparison of the efficacy of nebulized budesonide with parenteral corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Clin Drug Invest 2003; 23:55–62.
- Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G. The role of nebulized budesonide in the treatment of exacerbations of COPD. Eur Respir J 2007; 29:660–667.
- Rodrigo G, Rodrigo C. Inhaled flunisolide for acute severe asthma. Am J Respir Crit Care Med 2000; 157:698–703.
- McKenzie JE, Cruz-Rivera M. Compatibility of budesonide inhalation suspension with four nebulizing solutions. Ann Pharmacother 2004; 38:967–972.
