Abstract
Dyspnea is deemed to result from an imbalance between ventilatory demand and capacity. The single-breath diffusing capacity for carbon monoxide (DLCO) is often the best correlate to dyspnea in COPD. We hypothesized that DLCO contributes to the assessment of ventilatory demand, which is linked to physiological dead space /tidal volume (VD/VT) ratio. An additional objective was to assess the validity of non-invasive measurement of transcutaneous PCO2 allowing the calculation of this ratio. Forty-two subjects (median [range] age: 66 [43–80] years; 12 females) suffering mainly from moderate-to-severe COPD (GOLD stage 2 or 3: n = 36) underwent pulmonary function and incremental exercise tests while taking their regular COPD treatment. DLCO% predicted correlated with both resting and peak physiological VD/VT ratios (r = −0.55, p = 0.0015 and r = −0.40, p = 0.032; respectively). The peak physiological VD/VT ratio contributed to increase ventilation (increased ventilatory demand), to increase dynamic hyperinflation and to impair oxygenation on exercise. Indirect (MRC score) and direct (peak Borg score/% predicted V˙O2) exertional dyspnea assessments were correlated and demonstrated significant relationships with DLCO% predicted and physiological VD/VT at peak exercise, respectively. The non-invasive measurement of transcutaneous PCO2 both at rest and on exercise was validated by Bland-Altman analyses. In conclusion, DLCO constitutes and indirect assessment of ventilatory demand, which is linked to exertional dyspnea in COPD patients. The assessment of this demand can also be non invasively obtained on exercise using transcutaneous PCO2 measurement.
Keywords :
Introduction
Dyspnea is deemed to result from an imbalance between ventilatory demand and capacity. Nevertheless, first-line assessment of the dyspneic complaint usually relies on spirometry and static lung volume measurements, evaluating ventilatory capacity only, as recommended by ATS consensus on dyspnea (Citation1). We recently showed that only severe to very severe defects of FEV1 or FVC can reliably predict exertional dyspnea (Medical Research Council [MRC] score >2) in chronic obstructive pulmonary disease (COPD) patients (manuscript submitted), as previously suggested (Citation2, 3). Consequently, other means of assessment are warranted to explain exertional dyspnea. COPD encompasses a group of disorders characterised by the presence of incompletely reversible airflow obstruction with overlapping subsets of different phenotypes including chronic bronchitis and/or emphysema (Citation4, 5).
The airflow limitation is caused by a mixture of small airways disease and parenchyma destruction, the relative contribution of which varies from person to person, and may differently affect exertional dyspnea. Among resting pulmonary function tests, the single-breath diffusing capacity for carbon monoxyde (DLCO) is often the best correlate to dyspnea in COPD (Citation2, 3). One may hypothesize that DLCO contributes to the indirect assessment of ventilatory demand. The ventilatory demand relies on CO2 production (V˙CO2), PaCO2 set point and wasted ventilation (physiological VD/VT ratio). The latter index can be related to alveolar dead space increase due to vascular injury characterizing emphysema. DLCO is obtained by multiplying DLCO/VA (∼KCO) with VA. Vascular injury related to emphysema is associated with a decrease in KCO, which would be indicative of high ventilation-perfusion areas (∼alveolar dead space).
The usual mean to assess ventilatory demand is to perform an exercise test allowing V˙CO2, PaCO2 and physiological VD/VT ratio measurements, which is time-consuming. As a consequence, the validation of DLCO measurement at rest as a marker of ventilatory demand deserved to be done. The first aim of this cross-sectional physiological study was to compare traditional markers of ventilatory demand obtained during an exercise test and DLCO parameters (DLCO, KCO and VA/TLC) obtained at rest. The second aim was to evaluate the functional consequences of the increase in ventilatory demand, including exertional dyspnea. To these ends, correlative analyses were made between these functional parameters, and subsequently with exertional dyspnea. Since the measurement of PaCO2 is mandatory for the assessment of ventilatory demand, an additional aim was to assess the validity of non invasive measurement of transcutaneous PCO2.
Material and Methods
Study design
This was a cross-sectional study in which informed consent was obtained from all subjects, and ethical approval was received from the Institutional Review Board of the French learned society for respiratory medicine –Société de Pneumologie de Langue Française (CEPRO 2010–011). Only COPD patients were included since our aim was to perform correlative analyses between physiological indices. On the visit, all subjects completed the Medical Research Council (MRC) scale, and had physiological evaluations. Treatments as bronchodilators were not withheld before testing because indirect dyspnea assessment by MRC was obtained while the patients were on their regular treatment. Part of this study was designed to specifically assess new indices of dyspnea on exercise and has already been published (Citation6).
Subjects
All COPD patients were current or past smokers (>15 Pack-Year) and had COPD according to GOLD-2007 criteria (Citation7). Patients were not included if they had (Citation1) other unstable medical conditions that could cause or contribute to breathlessness (i.e., metabolic, cardiovascular, or other respiratory diseases) or (Citation2) other disorders that could interfere with exercise testing or (Citation3) disproportionate pulmonary hypertension (resting pulmonary artery pressure >35 mmHg in their medical record).
Procedures
Spirometry, constant-volume body plethysmography, and single-breath DLCO were performed in accordance with recommended techniques (Citation8–10) using an automated pulmonary function testing system (MasterScreenBody, Jaeger, CareFusion). Symptom-limited incremental exercise testing was conducted on an electronically braked cycle ergometer using the Vmax Cardiopulmonary Exercise Testing System (Sensor Medics, Yorba Linda, CA) according to recommended guidelines (Citation11) as previously described (Citation6, Citation12). After a 2-minute warm-up period, workload increased by 5–15 W/minute using a ramp protocol until subjects stopped due to symptom limitation. Ventilation and gas exchange measurements were made throughout the test using the breath-by-breath computerized system. Subjects rated the magnitude of their perceived breathing and leg discomfort at rest, every minute during exercise, and at peak exercise by pointing to the 10-point Borg scale (Category scale with Ratio properties: CR10 (Citation13)). Upon exercise cessation, subjects were asked to verbalize their main reason for stopping exercise. Changes in end-expiratory lung volume were estimated from IC measurements at rest, at the end of each 2-minute increment of exercise, and at peak exercise (Citation12).
Ventilation was compared with the maximal ventilatory capacity (MVC), which was estimated by multiplying the measured FEV1 by 35 (Citation14). Slopes of V˙O2/Work rate, fH/V˙O2, V˙E /V˙O2, V˙E /V˙CO2 and anaerobic threshold (ventilatory threshold, using the V slope method) were calculated. At rest and immediately before the end of exercise arterial sampling was performed allowing to calculate physiological VD/VT ratio (Citation15). PaCO2 was also monitored continuously using transcutaneous measurement (TOSCA 500, Radiometer, Copenhagen, Denmark). Measurements were standardized as percentages of predicted normal values (Citation16–18). Since both healthy and diseased patients usually stop their exercise while exhibiting similar levels of dyspnea (Borg score) (Citation19), we calculated exertional dyspnea as peak Borg score divided by percentage of predicted V˙O2 that describes dyspneic complaint related to maximal performance.
Statistical analysis
We planed to enroll at least 40 patients to allow correlative analyses with normally distributed variables. Continuous variables were expressed as mean ± SD, except when stated. Correlative analyses were performed using Pearson's correlations. Statistical significance was defined by a P value ≤ 0.05. All analyses were performed using the Statview 4 package (SAS institute, Grenoble, France).
Results
The clinical and baseline physiological characteristics of the COPD patients are described in , and the results of their exercise tests are shown in .
Table 1. Clinical characteristics and results of pulmonary function tests
Table 2. Measurements obtained from symptom-limited incremental cycle exercise
Assessment of ventilatory demand
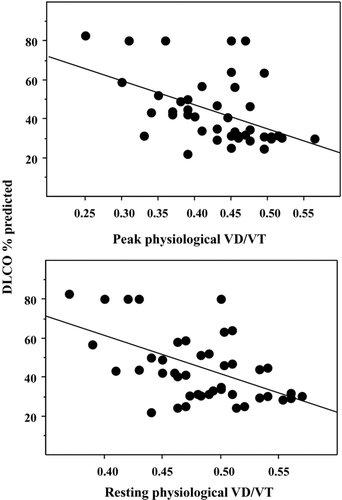
We first analysed the correlates of the primary variables of DLCO, namely VA and KCO. Alveolar volume (VA% predicted) correlated with FVC% predicted (r = 0.47, p = 0.009) and TLC% predicted (VA% predicted = 45 + 0.26 TLC% pred; r = 0.50; p = 0.004). The VA/TLC ratio was clearly indicative of airway obstruction since it correlated with FEV1% predicted, FVC% predicted, FRC% predicted and RV% predicted. The VA/TLC ratio negatively correlated with an index of ventilation-perfusion inequality, namely resting P(a-et)CO2 (r = −0.52, p = 0.012). This ratio also negatively correlated with both resting and peak physiological VD/VT ratios (r = −0.60, p = 0.002 and r = −0.20, p = 0.033; respectively). The KCO correlated with both resting and peak physiological VD/VT ratios (r = −0.52, p = 0.004 and r = −0.43, p = 0.021; respectively). Finally, DLCO% predicted correlated with both resting and peak physiological VD/VT ratios ().
Consequences of increased ventilatory demand
The consequences of increased ventilatory demand on exercise (peak physiological VD/VT ratio as independent variable) were increased ventilation (peak V˙E% MVC: r = 0.32, p = 0.049), increased dynamic hyperinflation (peak IC% predicted: r = −0.45, p = 0.004) and impaired oxygenation (peak PaO2: r = −0.66, p < 0.0001). Indirect (MRC score) and direct (peak Borg score/% predicted V˙O2) exertional dyspnea assessments well correlated (r = 0.53, p = 0.0003). There was a significant negative relationship at rest between DLCO% predicted and MRC score (r2 = 0.18, p = 0.021), while there was a weak positive relationship between peak Borg score/% predicted V˙O2 and physiological VD/VT at peak exercise (r2 = 0.10, p = 0.042).
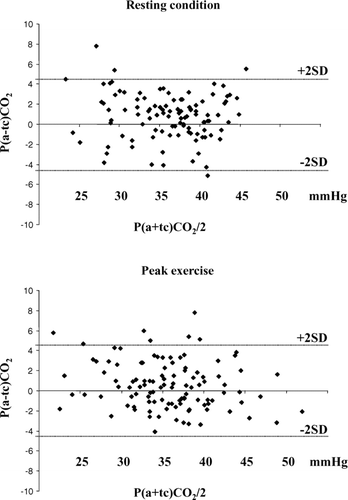
The calculation of physiological VD/VT ratio necessitates an arterial PCO2 measurement, which is invasive and often not systematically performed. To validate the measurement in a larger sample and over a wider range of PaCO2, the validation sample involved all patients evaluated at exercise during the same period of COPD enrolment (n = 120 patients). shows the Bland-Altman analysis between arterial and transcutaneous assessments. A good agreement between arterial and transcutaneous measurements of PCO2 is evidenced, both at rest and at peak exercise.
Figure 2. Bland-Altman analyses describing PCO2 assessment by arterial and transcutaneous evaluations. The upper panel describes the assessment on resting condition and the lower panel the assessment on peak exercise. These couples of measurements have been performed in 120 patients. The lines for bias ± 2SD are shown.
Discussion
The main result of this cross-sectional physiological study is the demonstration that DLCO obtained at rest is linked to indices of ventilatory demand (physiological VD/VT ratio) both at rest and on exercise. Logically, these pulmonary function parameters contributed to exertional dyspnea assessed both indirectly (MRC score) and directly (exercise test). Finally, we show that the physiological VD/VT ratio can be obtained from the non invasive measurement of transcutaneous PCO2.
It has emerged that COPD is a complex disease with multiple clinical manifestations and that COPD subjects cannot be described by only using the severity of airflow limitation. Identification of clinical COPD phenotypes has been described as early as the 1950s, when Dornhorst proposed the distinction between pink puffers (mainly with emphysema) and blue bloaters (mainly with chronic bronchitis) (Citation20). Consequently, pulmonary function testing should allow discriminating these phenotypes that differently impact exertional dyspnea. Dyspnea is usually linked to ventilation (V˙E = VT. fB, tidal volume × breathing frequency) and results from an imbalance between ventilatory demand and capacity that can be summarized by the following equation: V˙E = k. V˙˙CO2/(PaCO2 [1 –VD/VT]) = fB. C. (∆Pm –RV˙ –IV˙˙) where demand relies on CO2 production (V˙CO2), PaCO2 set point and wasted ventilation (physiological VD/VT ratio), while ventilatory capacity is related to compliance of respiratory system (C), resistance of respiratory system (R), inertance of respiratory system (I: considered as negligible, at least in healthy subjects) and muscular effort allowing respiratory motion (∆Pm: pressure drop).
The accomplishment of the activity of the respiratory muscles is ventilation and the capacity of these muscles can be expressed as the maximal breathing capacity [MBC], hence V˙E /MBC is crudely related to the activity of the muscles relative to maximal. Interestingly, Sahebjami and Sathianpitayakul showed that in their stepwise multiple regression model with dyspnea scale as the outcome,% DLCO and% MBC combined were the strongest predictors of the severity of dyspnea (30% of variance) in COPD patients (Citation21), which further suggests that DLCO is related to another dimension than ventilatory capacity.
When looking at the “mechanical” equation of dyspnea (see above), it is obvious that COPD can increase physiological dead space and thus ventilatory demand. Nevertheless, the relationship with DLCO measurement is less self-explanatory. A reduction in DLCO can be related to a decrease in VA due to altered ventilation, which is suggested by the correlations evidenced between VA/TLC ratio and indices of airway obstruction. But a decrease in DLCO can also be related to a decrease in KCO that is associated with an increase in physiological dead space. The latter association in COPD patients has previously been suggested using the multiple inert gas elimination technique (Citation22).
Vascular injury related to emphysema is well-known (Citation23, 24), and contributes to increase the physiological VD/VT ratio both at rest and on exercise. Finally, the raised physiological VD/VT ratio increases wasted ventilation on exercise and logically contributes to dynamic hyperinflation, and thus to dyspnea. In summary, we show that DLCO is linked to both ventilatory capacity (VA) and demand (KCO), explaining its main contribution to dyspnea assessment.
Our additional aim was to assess whether the non invasive measurement of transcutaneous PCO2 may be used. As previously demonstrated by other investigators we show that this transcutaneous measurement may reasonably replace arterial puncture for blood gas analyses (Citation25).
Our study suffers from the limitations common to cross-sectional studies because our conclusions are based on correlative analyses, which do not imply causality. The small number of patients does not allow an adequate description all COPD phenotypes. The relationships between DLCO and wasted ventilation, and subsequently with dyspnea, may seem obvious but deserved to be done from a pathophysiological point of view. It highlights that exertional dyspnea cannot often be evaluated based on the only assessment of ventilatory capacity in COPD patients. The many factors contributing to exercise dyspnea in COPD are interdependent, and recent reviews focused primarily on ventilatory mechanical factors such as dynamic hyperinflation that can potentially be manipulated for the patients’ benefit (Citation26, 27).
The contribution of ventilation-perfusion abnormalities indirectly, reflecting pulmonary vascular damage to dyspnea, deserves to be studied since specific vascular therapeutic approaches are currently under development (Citation28–30). The non-invasive assessment of transcutaneous PCO2, allowing the calculation of physiological VD/VT ratio, helps to better characterize the underlying mechanisms of dyspnea in these patients. In absence of PaCO2 measurement, an increased ventilatory demand cannot be related to either ventilation-perfusion abnormalities and/or decreased PaCO2 set point (hyperventilation) (Citation31), which constitutes a plea for systematic PaCO2 assessment.
In conclusion, DLCO constitutes and indirect assessment of ventilatory demand, which is linked to exertional dyspnea in COPD patients. The assessment of this demand can also be non invasively obtained on exercise using transcutaneous PCO2 measurement.
Declaration of Interest
The authors declare no competing interest. The authors are responsible for the content and the writing of this paper.
Acknowledgments
The authors wish to thank Mrs. Le Bihan Françoise, Mrs. Riquelme Martine, and Mr. Bokouabassa Marien for their expert technical assistance.
References
- Anonymous. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med 1999 Jan; 159(1):321–340.
- de Torres JP, Casanova C, Montejo de Garcini A, Gender and respiratory factors associated with dyspnea in chronic obstructive pulmonary disease. Respir Res 2007; (8):18.
- O'Donnell DE, Webb KA. Breathlessness in patients with severe chronic airflow limitation. Physiologic correlations. Chest 1992 Sep; 102(3):824–831.
- Bafadhel M, Umar I, Gupta S, The role of computed tomography in multi-dimensional phenotyping of chronic obstructive pulmonary disease. Chest 2011 Mar 31; Epub ahead of print.
- Pistolesi M, Camiciottoli G, Paoletti M, Identification of a predominant COPD phenotype in clinical practice. Respir Med 2008 Mar; 102(3):367–376.
- Delclaux C, Chevalier-Bidaud B, Essalhi M, Too rapid increase and too much breathlessness are distinct indices of exertional dyspnea in COPD. Respir Physiol Neurobiol 2011 Jan 22; Epub ahead of print.
- Rabe KF, Hurd S, Anzueto A, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007 Sep 15; 176(6):532–555.
- Miller MR, Hankinson J, Brusasco V, Standardisation of spirometry. Eur Respir J 2005 Aug; 26(2):319–338.
- Macintyre N, Crapo RO, Viegi G, Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005 Oct; 26(4):720–735.
- Wanger J, Clausen JL, Coates A, Standardisation of the measurement of lung volumes. Eur Respir J 2005 Sep; 26(3):511–522.
- Anonymous. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003 Jan 15; 167(2):211–277.
- Callens E, Graba S, Gillet-Juvin K, Measurement of dynamic hyperinflation after a 6-minute walk test in patients with COPD. Chest 2009 Dec; 136(6):1466–1472.
- Borg E, Kaijser L. A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sports 2006 Feb; 16(1):57–69.
- Gandevia B, Hugh-Jones P. Terminology for measurements of ventilatory capacity; a report to the thoracic society. Thorax 1957 Dec; 12(4):290–293.
- Hubert D, Aubourg F, Fauroux B, Exhaled nitric oxide in cystic fibrosis: relationships with airway and lung vascular impairments. Eur Respir J 2009 Jul; 34(1):117–124.
- Quanjer PH, Tammeling GJ, Cotes JE, Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993 Mar; 16:5–40.
- Stanojevic S, Wade A, Stocks J, Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med 2008 Feb 1; 177(3):253–260.
- Wasserman K, Hansen JE, Sue DY, Normal values. Principles of exercice testing and interpretation. Including pathophysiology and clinical applications. Fourth edition. Philadelphia-Baltimore-New York-London-Buenos Aires-Hong Kong-Sydney-Tokyo: L. W. Wilkins; c2005; 160–182.
- Hamilton AL, Killian KJ, Summers E, Symptom intensity and subjective limitation to exercise in patients with cardiorespiratory disorders. Chest 1996 Nov; 110(5):1255–1263.
- Dornhorst AC. Respiratory insufficiency. Lancet 1955 Jun 11; 268(6876):1185–1187.
- Sahebjami H, Sathianpitayakul E. Influence of body weight on the severity of dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000 Mar; 161(3 Pt 1):886–890.
- Rodriguez-Roisin R, Drakulovic M, Rodriguez DA, Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol 2009 Jun; 106(6):1902–1908.
- Barbera JA, Riverola A, Roca J, Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994 Feb; 149(2 Pt 1):423–429.
- Sandek K, Bratel T, Lagerstrand L, Relationship between lung function, ventilation-perfusion inequality and extent of emphysema as assessed by high-resolution computed tomography. Respir Med 2002 Nov; 96(11):934–943.
- Steinacker JM, Liu Y. Transcutaneous pCO2-monitoring during exercise is valid! Int J Sports Med 1994 Nov; 15(8):525–526.
- O'Donnell DE, Banzett RB, Carrieri-Kohlman V, Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc 2007 May; 4(2):145–168.
- O'Donnell DE, Ora J, Webb KA, Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol 2009 May 30; 167(1):116–132.
- Blanco I, Gimeno E, Munoz PA, Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010 Feb 1; 181(3):270–278.
- Humbert M, Simonneau G. Vasodilators in patients with chronic obstructive pulmonary disease and pulmonary hypertension: not ready for prime time! Am J Respir Crit Care Med 2010 Feb 1; 181(3):202–203.
- Peinado VI, Pizarro S, Barbera JA. Pulmonary vascular involvement in COPD. Chest 2008 Oct; 134(4):808–814.
- Ofir D, Laveneziana P, Webb KA, Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008 Mar 15; 177(6):622–629.