Abstract
Chronic obstructive pulmonary disease (COPD) is the third-leading cause of death in the United States. Despite clinical practice guidelines endorsed by national organizations, the management of COPD deviates from guideline recommendations. Patients with COPD are frequently underdiagnosed and misdiagnosed, due in large part to the lack of spirometry testing. When diagnosed, about one third of patients are not receiving any COPD-related drug therapy. Factors that contribute to suboptimal management include provider, patient, and system factors. Physician factors such as understanding and attitude toward the disease, and awareness of guidelines, may affect appropriate management of COPD. Patient factors include medication non-adherence, understanding of the disease, severity of their symptoms, and access to medications. System factors such as insurance coverage may limit aspects of COPD care. To overcome clinical inertia, a multifaceted approach is required. Provider and patient education, the use of health informatics, changes in provider work-flow and the recent development of performance measures, such as the use of spirometry in patients with COPD, can improve the delivery of recommended care for COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is now the third-leading cause of death in the United States (Citation1). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) (Citation2) and the American Thoracic Society/European Respiratory Society (ATS/ERS) (Citation3) guidelines have been published to improve the diagnosis and management of COPD. In actual practice, management of patients with COPD deviates from guideline recommendations for both routine and acute management (Citation4, 5). These gaps should be considered in the context of clinical inertia, which is broadly defined as “recognition of the problem, but failure to act” (Citation6). Clinical inertia includes patients’ nonadherence to prescribed treatment, therapeutic inertia (providers fail to initiate medications or to intensify treatment), and inappropriate therapy (Citation7).
In cardiovascular disease, some studies link clinical inertia to as much as 80% of cardiovascular events (Citation8). Clinical inertia related to the management of COPD has been less commonly researched, and little is known about physicians’ knowledge and practices (Citation9). This review seeks to understand the current management of stable COPD in the United States, the prevalence of and risks for clinical inertia as well as potential interventions to improve the quality of care for patients with COPD.
Methods
Key search terms linking clinical inertia with COPD were suggested by two independent reviewers after reading literature that defines clinical inertia (Citation6, 7) and examining indexed search terms in Medline. The search terms used were ‘Pulmonary Disease, Chronic Obstructive [Majr]’ plus ‘medication adherence’, ‘clinical inertia’, ‘diagnosis’, ‘guidelines’, ‘medical management’, ‘physician standard(s)’, ‘practice pattern(s)’, ‘patterns of care’, ‘physician performance’, ‘practice guideline(s)’, ‘therapy’ or ‘drug therapy’. The limits for these searches included English language, studies in humans and publication dates of January 1, 2000 to April 1, 2010. Non-U.S. articles (i.e., those with a non-U.S. address in affiliation) and those that only discussed acute management of COPD (e.g., oxygen, antibiotics or steroid therapy for acute exacerbations, or appropriate use of mechanical ventilation) were excluded. Two reviewers independently evaluated the articles to develop a final list of articles for inclusion. If the reviewers disagreed, a third independent reviewer made the determination.
Results
Current Care Model for COPD
About 64% of patients receive COPD care from primary care providers (PCPs) while the remaining 27% and 9% of patients are seen by pulmonary specialists and other healthcare providers, respectively (Citation10). It is estimated that the average PCP who manages 2,000 patients will have 76 patients with COPD under his/her care (Citation11).
Clinical Inertia in COPD
Underdiagnosis and Misdiagnosis: In 2000, only 10 million adults in the U.S. were diagnosed with COPD even though NHAHES III estimates were that 24 million adults had impaired lung function (Citation12). The most commonly studied reason for underdiagnosis is the lack of spirometry testing (Citation13, 14). Spirometry is the gold standard for diagnosing COPD (Citation2). The lack of a spirometry machine and physician unfamiliarity contribute to underutilization of spirometry and underdiagnosis. A survey of primary care practices found that 66% of offices owned a spirometer, 38% of physicians were unfamiliar with the test, and 34% lacked training to perform and interpret the test (Citation15). Among smaller physician offices, even when spirometry was accessible, it was underused (Citation16).
In the Veterans Health Association (VHA) setting, spirometry use for those who were newly diagnosed with COPD was 3.3 times higher among pulmonologists than generalists (Citation17). Similarly, a lack of spirometers and pulmonary specialists was believed to contribute to up to a threefold difference in spirometry utilization among different geographical locations (Citation18).
Statistics regarding the use of spirometry differ depending on study methodology and practice setting (Citation10, Citation19, Citation20). In a university-based family medicine clinic, 58.5% of patients had pulmonary function testing (Citation19). Han and colleagues examined data from several health plans, including commercial, Medicare, and Medicaid, and found that only 32% of patients newly diagnosed with COPD had spirometry performed within 2 years before or 6 months after diagnosis (Citation20). The most dismal statistic was inferred from insurance claims data where only 16% of patients with COPD had spirometry testing (Citation10).
Although recommended for diagnosis, the impact of spirometry on clinical outcomes in COPD has not been well studied. A retrospective study of patients with newly diagnosed COPD found those who had received spirometry were more likely to have inhaled steroids and bronchodilators as part of their medication regimen (Citation18). Another study found that physician knowledge of spirometry results changed management in 48% of patients and resulted in greater than 85% concordance with guidelines (Citation21).
In 2008, the US Preventative Services Task Force advised against using spirometry for routine screening of all patients at risk for COPD because it is not cost-effective (Citation22). Although this recommendation is evidence-based, it only applies to screening healthy adults and not to individuals who present with symptoms; chronic cough, increased sputum production, wheezing, or dyspnea. In order to assist clinicians with determining which patients should receive further evaluation such as spirometry, a recent study investigated the use of the Lung Function Questionnaire (LFQ) as an alternative screening tool for COPD (Citation23, 24). This simple questionnaire assesses the responses to five items (age, smoking, wheeze, dyspnea, and cough) and has a reasonable sensitivity and specificity of 73.2% and 58.2%, respectively, for identifying patients with air-flow obstruction. The LFQ is intended as a screening modality for spirometry and not for the diagnosis of COPD.
Treatment: Based on medical record review from 12 metropolitan communities, among patients with COPD, recommended care occurred in less than half of the processes of history taking, laboratory and lung function assessment and radiologic studies (Citation4). With regard to drug therapy, only 32% of metered-dose inhalers were used with a spacer.
Smoking Cessation: Smoking cessation is the only treatment proven to decrease morbidity and mortality as well as prevent the progression of COPD (Citation2). Although the rates of advice to quit from healthcare providers have increased from 52.9% in 2000 to 61.2% in 2005, disparities across racial and ethnic groups remain (Citation25). A more recent study found a slightly higher percentage, 67.8%, of current smokers with COPD who had received smoking cessation advice or treatment (Citation19).
Vaccinations: Routine vaccination with the influenza and pneumococcal vaccines is also recommended for patients with COPD (Citation2). In a VHA study, only 16.8% of veterans with COPD received the pneumococcal vaccine (Citation26). Influenza vaccination rates were much higher with 67–77% of patients receiving an annual flu shot (Citation27). In a study of self-reported immunization rates, pneumococcal vaccine rates were 26–39% and influenza vaccine rates were 56–71% (Citation28).
Pharmacological Treatment: Inappropriate therapy, undertreatment, and overtreatment all lead to suboptimal management of patients with COPD. Many studies address undertreatment, which is common. Overall, 23–38% of patients identified through medical claims data with COPD are not receiving any COPD-related drug therapy (Citation5, Citation10). The GOLD and ATS/ERS guidelines recommend pharmacological therapy for the treatment of COPD, depending on the stage of the disease () (Citation2, 3).
Figure 1 Management of patients with COPD by Stage (Citation2). *Reproduced with permission from the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. Available from: http://www.goldcopd.org.
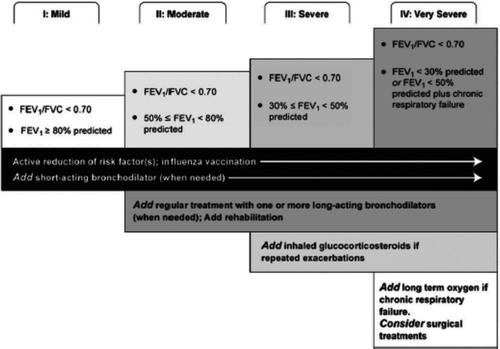
In patients with mild COPD (stage I) short-acting bronchodilators are first-line therapy; while patients with moderate COPD (stage II) are recommended to have one or more long-acting bronchodilators added to their regimen. Patients with severe (stage III) and very severe (stage IV) COPD are appropriate candidates for adding inhaled corticosteroids to their regimen if they report repeated exacerbations. In a study using insurance claim data, up to one third of patients, documented to have 3 or more exacerbations did not receive inhaled corticosteroid, as recommended by GOLD guidelines (Citation5).
In accordance with the guidelines, disease severity does influence whether a patient receives therapy for COPD, with 44%, 72%, and 81%, of patients with mild, moderate and severe COPD, respectively, receiving some treatment (Citation28). However, the level of treatment based on disease severity is frequently suboptimal. In a University based family medicine clinic, only 55% were receiving stage appropriate combination therapy (Citation19). In this study, 22% of patients with mild COPD were being treated with stage I recommended medications (short-acting bronchodilators alone), 5% of moderate stage patients were being treated with stage II recommended combinations (a combination of a short-acting bronchodilator and a long-acting bronchodilator), 28% of patients with severe stage COPD were being treated with stage III recommended combinations (stage 2 combination plus an inhaled steroid), and 13% of patients with very severe stage COPD were being treated with stage IV recommended combinations (all of the prior mentioned medications and oxygen, when appropriate).
Reasons behind clinical inertia
Although there is no conclusive evidence on which factors influence clinical inertia in COPD management, provider factors, patient factors, and system factors may contribute to the problem ().
Table 1. Possible factors that contribute to clinical inertia in COPD*
Provider factors: Only about half of PCPs were aware of COPD treatment guidelines (Citation28, 29) and only 25% used the guidelines to make clinical decisions (Citation30). Internal medicine physicians were more frequently aware of COPD guidelines and had greater confidence in their ability to diagnose and manage COPD than family and general practitioners. However, they also had more patients with COPD in their practice (Citation30).
A survey reported that 87% of PCPs believe that COPD is a self-inflicted disease, while 45% agree that nothing can be done for patients with COPD who continue to smoke (Citation28). Another survey confirmed that some doctors have a “nihilistic” outlook towards smokers; the study found that patients with COPD or asthma who smoked received less pharmacologic therapy than nonsmokers (Citation27). Physicians also have other misconceptions about the disease; some believe that COPD is a disease that mainly affects men, while others believe that medication treatment is not useful (Citation29).
Finally, lack of time during a physician visit may also contribute to clinical inertia in COPD. A survey of 25 small primary care practices concluded that the 15-minute visit was insufficient to address the problems in COPD (Citation16).
Patient Factors: Patient factors such as medication nonadherence, understanding of the disease, perception of symptoms, and access to medications contribute to clinical inertia in the management of COPD. Many patients with COPD underestimate the severity of their disease, and there is a significant disparity between self-perception and the degree of severity indicated by objective scales (Citation36). COPD is under-acknowledged by patients when compared to other disease states such as diabetes or hypertension (Citation35). When surveyed, patients were more aware of their cholesterol level and blood pressure than their pulmonary function test results. Part of this may be related to poor provider-patient communication (Citation39).
Following an office visit for COPD, there was disagreement in 38% of physician-patient pairs on disease severity and 50% on the level of patient concern. Physicians did not discuss spirometry results in 31% of visits, smoking cessation in 50% of visits, or counsel patients on inhaler use technique in 78% of visits where an inhaler was initiated. Lastly, 82% of visits did not include a discussion of issues related to quality of life, despite the fact that 84% of patients experienced limitations from COPD.
In a clinical trial scenario, the TORCH (TOwards a Revolution in COPD Health) trial, patients who adhered to medications were significantly less likely to die or be admitted to the hospital for acute exacerbations (Citation40). However, in a real-world evaluation only 52% of patients with COPD received medication treatment for COPD in their last year of life, and many used medication sporadically; with 40% discontinuing medication within 30 days, and 70% discontinuing within 90 days (Citation32). It is recognized that drug adherence needs to be improved in patients with COPD (Citation34).
System Factors: Patients’ access to healthcare services and prescription drugs may also impede proper treatment. A national survey of over 2000 patients with COPD and their providers found that 12% of patients had no insurance coverage and of the patients who did have insurance, 30% believed that their insurance coverage was a barrier to therapy (Citation28). Access to treatment can be considered both a patient and system factor. Although as many as 70% of physicians responded that insurance coverage is not a barrier to treatment for patients, researchers found that insurance may limit all aspects of COPD care (Citation28). Insurance-related limits on access were reported for 38% of prescription drugs, 14% of physician visits, 12% of pulmonary rehabilitation, and 9% of home oxygen therapy. In addition, providers (67% of pulmonologists and 58% of primary care providers) report that insurance reimbursement for management of patients with COPD is inadequate or very unreasonable (Citation28).
Discussion
Possible Solutions for Addressing Clinical Inertia in COPD
Due to the myriad of factors that contribute to clinical inertia, it stands to reason that a multifaceted approach is required. Researchers have tried various methods to improve COPD management by targeting provider, patient, and system factors that contribute to clinical inertia, as well as involving the use of health technology. Unfortunately, not one specific intervention has consistently demonstrated an improvement in COPD management or clinical outcomes.
Changing physician behavior to incorporate guideline based recommendations into daily practice can be difficult. Traditional didactic lectures were ineffective in changing physician behavior (Citation41, 42). In contrast, interactive education that allows for participant activity along with practicing skills has been shown to improve provider behavior (Citation41), as has academic detailing, patient-mediated strategies, and reminders (Citation42).
Although provider and work-flow focused interventions were found to improve provider acceptance of guidelines, work-flow changes appeared to be better accepted by practitioners. Additionally, implementation improved with the number of interventions used. The use of work-flow strategies such as changing the responsibility of non-physicians, the use of computers for reminders and to document services can be combined with provider strategies to achieve a more effective and sustainable result (Citation43). However, this approach may not necessarily be efficient.
One of the work-flow strategies is the use of health informatics, such as electronic reminders or electronic medical records (EMR) (Citation44). Electronic reminders have been shown to change how physicians monitored their patients including an increase in the number of physician contacts, frequency of peak expiratory flow (PEF) measurements and, to a lesser extent, treatment decisions. However, in one study, electronic suggestions concerning drugs and monitoring, delivered to physicians and pharmacists when writing orders or filling prescriptions, did not result in differences in adherence to the care suggestions, quality of life, medication adherence, or health care utilization (Citation45).
Another work-flow strategy involves non-physician allied healthcare members such as nurses and pharmacists. These practitioners can help facilitate what can be accomplished in a short (15 minute) routine follow-up visit (Citation16). Pharmaceutical care provided by clinical pharmacists and pharmacy residents improved clinical and economic outcomes in COPD in a multicenter study (Citation46). Medication Therapy Management (MTM) services, as part of a Medicare part D plan, provided by pharmacists may improve adherence. In 2009, about 13% of Medicare patients were provided with MTM services, and COPD was one of the five most common chronic conditions covered by Medicare part D (Citation47).
Part of the success with involving non-physician healthcare professionals may be related to the time spent teaching patients about their disease, drug therapy, and self-management skills. A systematic review comparing patient education on self-management to usual care in COPD concluded that education was associated with a reduction in hospital admissions. However, it had no effect on emergency department visits, lung function, and exercise capacity (Citation48). In this review, self-management was defined as teaching patients how to carry out activities of daily living optimally with a COPD diagnosis and to prevent or decrease exacerbations by lifestyle changes.
The impact of uni-dimensional approaches on physician behavior or patients’ self-management skills has not been demonstrated in trials. However, a systematic review of multi-dimensional approaches (i.e., disease management programs) has shown improved COPD management and quality of life (Citation49). The components of these disease management programs included patient education, pulmonary rehabilitation, psychosocial support and relaxation techniques. However, none of these programs was able to demonstrate the weighted impact of each component, which means that there may be some cost/resource inefficiencies in applying a broad multidimensional program to all patients when some might respond better to specific aspects or combinations.
Regulatory agencies also have an opportunity to impact care for patients with COPD. Clinical practice guidelines specific to COPD are being incorporated into quality measures for hospitals and ambulatory practices settings. The Centers for Medicare and Medicaid Services (CMS) performance measure is the percentage of adults with a diagnosis of COPD who have a documented spirometry evaluation (Citation50). New in 2010, The National Committee for Quality Assurance's 2010 Healthcare Effectiveness Data and Information Set (HEDIS) measures include spirometry (Citation51). The specific measure reports the rate of spirometry testing to confirm diagnosis in visits for adults with a new diagnosis or newly active COPD. These measures will be available to the public and the use of such measures may represent another strategy to overcome physician barriers to change (Citation52).
The use of performance measures has demonstrated success. One teaching hospital implemented a quality improvement program around COPD management in the ambulatory setting (Citation53). Performance measures included pharmacologic (i.e., appropriate medications depending on the stage of the disease) and non-pharmacologic (smoking cessation, immunizations) quality indicators. Significant improvements were seen after implementation: documented discussion or administration of flu shot and pneumococcal vaccine increased from 24.7% to 62.3% and 22.1% to 59.9%, respectively, and short-acting bronchodilator prescriptions increased from 59.2% to 74.2%.
Areas needed for future research
Clinical inertia in the management of COPD is widespread in current U.S. practice. Research suggests that common obstacles to optimal management include underdiagnosis (lack of spirometry), inadequate non-pharmacological management (smoking cessation counseling, pulmonary rehabilitation, influenza/pneumococcal vaccinations), lack of physician awareness of treatment guidelines, and inappropriate treatment.
Some proposed solutions include:
1. Evaluate methods directed toward physician education and the impact on awareness and use of the GOLD guidelines.
2. Increase COPD screening –Including studies evaluating the use of spirometry vs. the use of short patient questionnaires such as the Lung Function Questionnaire (LFQ) (Citation23, 24) and the COPD Screener from the COPD Alliance (Citation54) and the impact that has on the diagnosis of COPD.
3. Use performance measures such as the Physician Quality Reporting System (Citation55) [formerly known as Physician Quality Reporting Initiative (PQRI)], and the HEDIS 2011 measures assessing the appropriate use of spirometry to confirm the diagnosis of COPD, and the percentage of COPD patients with a COPD exacerbation dispensed appropriate pharmacotherapy including bronchodilators and corticosteroids (Citation51) and evaluate the impact on physician awareness of COPD.
4. Evaluate how health information technology can improve the management of COPD –Collaborate with EMR vendors to include screening templates for COPD and key screening triggers (e.g., history of smoking, history of repeated episodes of bronchitis).
5. Further evaluate the role non-physician allied health providers can play in the management of COPD.
The effectiveness and efficiency of combinations of these interventions require further study. Comparative effectiveness studies involving observational real world and prospective randomized methods may provide insight into which tactics, and which combinations of tactics, may improve the evaluation and continuing care of patients at risk for and with COPD.
Conclusion
COPD is a major health problem in the United States, with clinical inertia contributing to poor real world management of the disease. There are major gaps in present knowledge and further research is warranted to address the various aspects of provider, patient, and system factors that contribute to clinical inertia in order to improve the management and outcomes of patients with COPD.
Declaration of Interest
Catherine Cooke has served as a consultant, received honoraria for speaking, received research grants, owns stock (companies included: Allergan, AstraZeneca, and Novartis, and Pfizer). Anne Fuhlbrigge has served as a consultant, received honoraria for speaking and advisory boards and received unrestricted research grants (companies included: Boehinger Ingelheim, GSK, Merck, and Sunovion). Michelle Sidel and Daniel Belletti are employees of Novartis Pharmaceuticals Corporation. Writing and content is entirely the work of the authors.
References
- Miniño AM, Xu JQ, Kochanek KD. Deaths: Preliminary Data for 2008. National Vital Statistics Reports; vol 59 no 2. Hyattsville, MD: National Center for Health Statistics. 2010. Available at: www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_02.pdf. Accessed September 30, 2011.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (Updated 2010). Available at: http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdf. Accessed September 30, 2011.
- Celli B, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6):932–946.
- Mularski RA, Asch SM, Shrank WH, Kerr EA, Setodji CM, Adams JL, Keesey J, McGlynn EA. The quality of obstructive lung disease care for adults in the United States a measured by adherence to recommended processes. Chest 2006; 130(6):1844–1850.
- Diette GB, Orr P, McCormack MC, Gandy W, Hamar B. Is pharmacologic care of chronic obstructive pulmonary disease consistent with the guidelines? Popul Health Manag 2010; 13(1):21–26.
- Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS. Clinical inertia. Ann Intern Med 2001; 135(9):825–834.
- Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm 2009; 15(8):690–695.
- Safford MM, Shewchuk R, Qu H, Williams JH, Estrada CA, Ovalle F, Allison JJ. Reasons for not intensifying medications: differentiating “clinical inertia” from appropriate care. J Gen Intern Med 2007; 22(12):1648–1655.
- Ramsey SD. Suboptimal medical therapy in COPD: exploring the causes and consequences. Chest 2000;117(2 Suppl):33S–37S.
- Heins-Nesvold J, Carlson A, King-Schultz L, Joslyn KE. Patient identified needs for chronic obstructive pulmonary disease versus billed services for care received. Int J Chron Obstruct Pulmon Dis 2008; 3(3):415–421.
- Nesvold JH, Carlson A, Yawn B, Joslyn K. What are you doing for the 76 patients with COPD in your practice? Minn Med 2008; 91(10):45–46.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance –United States, 1971–2000. MMWR Surveill Summ 2002; 51(6):1–16.
- Yawn B, Mannino D, Littlejohn T, Ruoff G, Emmett A, Raphiou I, Crater G. Prevalence of COPD among symptomatic patients in a primary care setting. Curr Med Res Opin 2009; 25(11):2671–2677.
- Joo MH, Au DH, Lee TA. Use of spirometry in the diagnosis of chronic obstructive pulmonary disease and efforts to improve quality of care. Transl Res 2009; 154(3):103–110.
- Kaminsky DA, Marcy TW, Bachand M, Irvin CG. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care 2005; 50(12):1639–1648.
- Moore PL. Practice management and chronic obstructive pulmonary disease in primary care. Am J Med 2007; 120(8 suppl 1):S23–S27.
- Lee TA, Bartle B, Weiss KB. Spirometry use in clinical practice following diagnosis of COPD. Chest 2006; 129:1509–1515.
- Joo MJ, Lee TA, Au DH, Fitzgibbon ML, Weiss KB. Medication use patterns associated with spirometry in diagnosing COPD. COPD 2008; 5(6):360–368.
- Chavez PC, Shokar NK. Diagnosis and management of chronic obstructive pulmonary disease (COPD) in a primary care clinic. COPD 2009; 6(6):446–451.
- Han MK, Kim MG, Mardon R, Renner P, Sullivan S, Diette GB, Martinez FJ. Spirometry utilization for COPD: how do we measure up? Chest 2007; 132(2):403–409.
- Yawn BP, Enright PL, Lemanskie RF Jr, Israel E, Pace W, Wollan P, Boushey H. Spirometry can be done in family physicians’ offices and alters clinical decisions in the management of asthma and COPD. Chest 2007; 132(4):1162–1168.
- U.S. Preventive Services Task Force. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008; 148(7):529–534.
- Yawn BP, Mapel DW, Mannino DM, Martinez FJ, Donohue JF, Hanania NA, Kosinski M, Rendas-Baum R, Mintz M, Samuels S, Dalal AA; Lung Function Questionnaire Working Group. Development of the Lung Function Questionnaire (LFQ) to identify airflow obstruction. Int J Chron Obstruct Pulmon Dis 2010; 5:1–10.
- Hanania NA, Mannino DM, Yawn BP, Mapel DW, Martinez FJ, Donohue JF, Kosinski M, Rendas-Baum R, Mintz M, Samuels S, Jhingran P, Dalal AA. Predicting risk of airflow obstruction in primary care: Validation of the lung function questionnaire (LFQ). Respir Med 2010; 104(8):1160–1170.
- Cokkinides VE, Halpern MT, Barbeau EM, Ward E, Thun MJ. Racial and ethnic disparities in smoking-cessation interventions: analysis of the 2005 National Health Interview Survey. Am J Prev Med 2008 May; 34(5):404–412.
- Lee TA, Weaver FM, Weiss KB. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. J Gen Intern Med 2007; 22(1):62–67.
- Craig BM, Kraus CK, Chewning BA, Davis JE. Quality of care for older adults with chronic obstructive pulmonary disease and asthma based on comparisons to practice guidelines and smoking status. BMC Health Serv Res 2008; 8:144.
- Barr RG, Celli BR, Martinez FJ, Ries AL, Rennard SI, Reilly JJ Jr, Sciurba FC, Thomashow BM, Wise RA. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med 2005 Dec; 118(12):1415.
- Yawn BP, Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J Chron Obstruct Pulmon Dis 2008; 3(2):311–317.
- Foster JA, Yawn BP, Maziar A, Jenkins T, Rennard SI, Casebeer L. Enhancing COPD management in primary care settings. MedGenMed 2007; 9(3):24.
- Petty TL. Benefits of and barriers to the widespread use of spirometry. Curr Opin Pulm Med 2005; 11(2):115–120.
- Jung E, Pickard AS, Salmon JW, Bartle B, Lee TA. Medication adherence and persistence in the last year of life in COPD patients. Respir Med 2009; 103(4):525–534.
- Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest 2008; 134:38–45.
- Restrepo RD, Alvarez MT, Wittnebel LD, Sorenson H, Wettstein R, Vines DL, Sikkema-Ortiz J, Gardner DD, Wilkins RL. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis 2008; 3(3):371–384.
- Barr RG, Celli BR, Mannino DM. Comorbidities, patient knowledge, and disease management in national sample of patients with COPD. Am J Med 2009; 122(4):348–355.
- Rennard S, Decramer M, Calverly PM, Pride NB, Soriano JB, Vermeire PA, Vestbo J. Impact of COPD in North America and Europe in 2000: subjects’ perspective of confronting COPD international survey. Eur Respir J 2002; 20(4):799–805.
- Heffner JE, Ellis R. The guideline approach to chronic obstructive pulmonary disease: how effective? Respir Care 2003; 48(12):1257–1266.
- Ward MM, Yankey JW, Vaughn TE, BootsMiller BJ, Flach SD, Watrin S, Doebbeling BN. Provider adherence to COPD guidelines: relationship to organizational factors. J Eval Clin Pract 2005; 11(4):379–387.
- Nelson M, Hamilton HE. Improving in-office discussion of chronic obstructive pulmonary disease: results and recommendations from an in-office linguistic study in chronic obstructive pulmonary disease. Am J Med 2007; 120(8 Suppl 1):S28–S32.
- Han MK. Medication adherence in COPD: what have we learned? Thorax 2009 Nov; 64(11):922–923.
- Davis D, O'Brien MA, Freemantle N, Wolf FM, Mazmanian P, Taylor-Vaisey A. Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes? JAMA 1999; 282(9):867–874.
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995 6; 274(9):700–705.
- Flanagan ME, Ramanujam R, Doebbeling BN. The effect of provider- and workflow-focused strategies for guideline implementation on provider acceptance. Implement Sci 2009; 4:71.
- Kuilboer MM, van Wijk MA, Mosseveld M, van der Does E, de Jongste JC, Overbeek SE, Ponsioen B, van der Lei J. Computed critiquing integrated into daily clinical practice affects physicians’ behavior- a randomized clinical trial with AsthmaCritic. Methods Inf Med 2006; 45(4):447–454.
- Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou XH, Eckert GJ, Smith FE, Nienaber N, McDonald CJ, Wolinsky FD. Can computer-generated evidence-based care suggestions enhance evidence-based management of asthma and chronic obstructive pulmonary disease? A randomized, controlled trial. Health Serv Res 2005; 40(2):477–497.
- Solomon DK, Portner TS, Bass GE, Gourley DR, Gourley GA, Holt JM, Wicke WR, Braden RL, Eberle TN, Self TH, Lawrence BL. Clinical and economic outcomes in hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash) 1998; 38(5):574–585.
- Rickles NM, Brown TA, McGivney MS, Snyder ME, White KA. Adherence: a review of education, research, practice, and policy in the United States. Pharmacy Practice (Internet) 2010; 8(1):1–17. Available at: http://www.pharmacypractice.org/vol08/pdf/001–017.pdf. Accessed September 30, 2011.
- Effing T, Monninkhof EM, van der Valk PD, van der Palen J, van Herwaarden CL, Partidge MR, Walters EH, Zielhuis GA. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007 Oct 17; (4):CD002990.
- Niesink A, Trappenburg JC, de Weert-van Oene GH, Lammers JW, Verheij TJ, Schrijvers AJ. Systematic review of the effects of chronic disease management on quality-of-life in people with chronic obstructive pulmonary disease. Respir Med 2007; 101(11):2233–2239.
- Centers for MediCare & MediCaid Services. Roadmap for quality measurement in the traditional medicare fee-for-service program. Available at: http://www.cms.gov/QualityInitiativesGenInfo/downloads/QualityMeasurement Roadmap_OEA1–16_508.pdf Accessed September 30, 2011.
- Fallon Community Health Plan. 2011 HEDIS measures. Available at: http://www.fchp.org/providers/resources/hedis-measures.aspx. Accessed September 30, 2011.
- Heffner JE, Mularski RA, Calverly PM. COPD performance measures: missing opportunities for improving care. Chest; Prepublished online March 26, 2010. DOI 10.1378/chest.09–2306. Available at: http://chestjournal.chestpubs.org/content/early/2010/03/24/chest.09–2306. Accessed September 30, 2011.
- Roberts DH, Gilmartin GS, Neeman N, Schulze JE, Cannistraro S, Ngo LH, Aronson MD, Weiss JW. Design and measurement of quality improvement indicators in ambulatory pulmonary care: creating a “culture of quality” in an academic pulmonary division. Chest 2009; 136(4):1134–1140.
- COPD Alliance. COPD Population Screener (COPD-PS). Available at: http://copd.org/sites/default/files/COPD-Screener.pdf. Accessed September 30, 2011.
- Centers for Medicare & Medicaid Services (CMS). Physician Quality Reporting System Overview. Available at: http://www.cms.gov/PQRS/01_Overview.asp#TopOfPage. Accessed September 30, 2011.
