Abstract
Long-term therapy with systemic corticosteroids is not recommended in the treatment of chronic obstructive pulmonary disease (COPD). However, experience demonstrates that some patients receive low dose therapy. Our objective was to describe the demographic, physiologic and radiologic characteristics of COPD patients treated with chronic systemic corticosteroids. We analyzed COPD subjects with GOLD I-IV disease in the COPDGene® study. Subjects were divided into 2 groups based on whether they reported using chronic oral steroids or not; 1264 subjects were included. Fifty-eight (4.5%) reported chronic systemic corticosteroid use. There were no differences in age, race, co-morbid conditions (other than asthma), or body mass index between the groups. There was a greater proportion of GOLD III (41% vs. 26%) and IV (41% vs. 13%) subjects in the group using chronic systemic corticosteroids. This group used more respiratory medications, required more oxygen (2.31 ± 0.21 vs. 0.59 ± 0.05 L/min; p < 0.0001), and walked less distance (245.4 ± 17.4 vs. 367.2 ± 3.9 meters; p < 0.0001). They reported more total (1.7 ± 0.16 vs. 0.62 ± 0.03; p < 0.0001) and severe exacerbations per year (0.41 ± 0.05 vs. 0.18 ± 0.01; p < 0.0001). BODE (5.0 ± 0.3 vs. 2.6 ± 0.1; p < 0.0001), MMRC (3.31 ± 0.19 vs. 1.90 ± 0.04; p < 0.0001) and SGRQ scores (54.9 ± 2.9 vs 53.3 ± 0.6; p < 0.0001) were higher. They also had a higher percentage of emphysema (22.4 ± 1.9 vs. 14.0 ± 0.4;%, p = <0.0001) on CT scan. COPD patients that report using chronic systemic corticosteroids have more severe clinical, physiologic, and radiographic disease.
Keywords :
Introduction
Long-term therapy with systemic corticosteroids is not recommended in the treatment of chronic obstructive pulmonary disease (COPD) (Citation1). However, clinical experience demonstrates that about 5% of patients with severe or very severe COPD are treated with low dose, chronic oral steroid therapy to manage their disease. The demographic, physiologic and radiologic characteristics of COPD patients treated with chronic systemic steroid therapy are unknown.
The Genetic Epidemiology of COPD Study (COPDGene®) is a nationwide observational study that obtains demographic, radiologic and genomic characteristics in smokers ranging from normal lung function to very severe COPD. We describe the baseline characteristics, pulmonary physiology and radiographic characteristics of COPD patients in COPDGene® who report using chronic oral corticosteroids. We compare this group with those not on oral corticosteroids to determine whether COPD patients on chronic systemic corticosteroids represent a distinct clinical phenotype. Additionally, we evaluated this cohort to determine if the subset of patients on oral steroids is more likely to have phenotypic characteristics similar to asthmatics with a history of atopy and asthma (e.g., a component of reversible airflow obstruction, and a lesser degree of emphysema on high resolution chest CT imaging).
Methods
Study design
Details of the COPDGene® Study design were previously reported and are summarized here (Citation2). COPDGene® is a multicenter observational study designed to identify genetic factors associated with COPD and to characterize chest CT phenotyes in COPD subjects. The study plans to enroll 10,000 smoking subjects including all disease severities and both genders. At the time of this analysis, data for 2500 subjects were available. Subjects are being recruited at 21 clinical study centers throughout the United States. IRB approval was obtained at each of the clinical sites and all subjects provided informed consent prior to study participation.
Inclusion and exclusion criteria
Criteria for inclusion in COPDGene® include self-identified racial/ethnic category as either non-Hispanic Whites or African-Americans between ages of 45 and 80 years with a minimum of 10 pack-year smoking history (except non-smoking controls). Exclusion criteria include pregnancy, a history of other lung diseases except asthma, previous surgical excision of at least one lung lobe (or lung volume reduction procedure), active cancer under treatment, suspected lung cancer (large or highly suspicious lung mass), metal in the chest, recent exacerbation of COPD treated with antibiotics or steroids, recent eye surgery, MI, other cardiac hospitalization, recent chest or abdominal surgery, inability to use albuterol, multiple self-described racial categories, history of chest radiation therapy, and first- or second-degree relative already enrolled in the study. Subjects with a recent COPD exacerbation can be enrolled 1 month after exacerbation resolution.
Patient selection
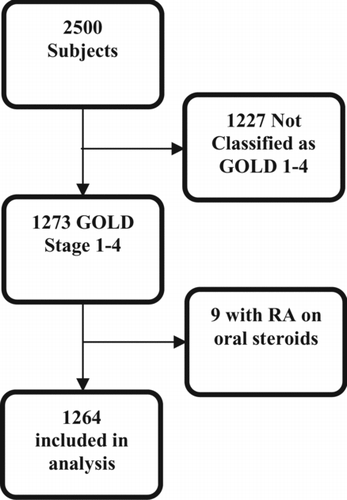
Subjects that had GOLD stage I-IV COPD as classified by spirometry were included in the analysis. Additional sub-group analyses were conducted in GOLD stage III and IV subjects. Subjects were divided into two groups and analyzed based on their response to the question regarding whether or not they take systemic corticosteroids. If a subject did not answer the question regarding whether or not they take oral corticosteroids, the answer was assumed to be ‘no’. Subjects who reported a COPD exacerbation within the past month were excluded from the study. In addition, all subjects in the study answered ‘no’ to the question ‘have you been on new or increased prednisone in the last month’. Therefore reported corticosteroid use was assumed to be part of the subjects’ chronic medication regimen rather than treatment for an acute exacerbation. Subjects with rheumatoid arthritis who reported the use of systemic corticosteroids were excluded from the analysis ().
Clinical characterization
Information collected from each subject included a modified American Thoracic Society (ATS) Respiratory Epidemiology Questionnaire and St. George's Respiratory Questionnaire (SGRQ). Height, weight, blood pressure and room air oxygen saturation were recorded for each subject. Demographic information including medical history and current medication use was collected and recorded based on patient recall. Subjects were asked to answer ‘yes’ or ‘no’ to questions regarding the presence of medical conditions such as asthma, hay fever, and pneumonia for example. Subjects were asked “At present do you use medications to treat your breathing problems? If yes, mark all that apply.” Dyspnea was assessed using the Modified Medical Research Council (MMRC) scale and health-related quality-of-life was assessed using the St. George's Respiratory Questionnaire (SGRQ).
Determination of exacerbation history
The exacerbation history of each subject was recorded. Patients were asked “Have you had a flare-up of your chest trouble in the past 12 months?” and, if yes, “how many of these episodes occurred in the last 12 months.” Additionally, subjects recorded the degree of care they received for each exacerbation (no special treatment, an increase in usual medications, an additional antibiotic or steroid which the subject kept at home, consultation with a physician who prescribed an additional antibiotic or steroid, or hospitalization). Severe exacerbations were defined as COPD exacerbations requiring hospitalization. Subjects were asked to recall the frequency of emergency room visits for COPD in the past year.
Pulmonary function testing
Each subject underwent pre- and post-bronchodilator spirometry using a standard protocol and spirometer (ndd EasyOne™ spirometer Zurich, Switzerland). Each spirometer was tested against the ATS 24 and 26 standard waveforms. Each clinical coordinator was certified by the PFT Core after spirometry training. Bronchodilation was performed with 2 puffs of albuterol using a spacer, and post-bronchodilator testing was performed 15–40 minutes after administration. Each subject's usual bronchodilator regimen was not withheld prior to testing in order to facilitate completion of all procedures in a single study visit. Predicted values were obtained using NHANES III data (Citation3). Bronchodilator reversibility was defined according to the ATS/ERS guidelines (an increase in FEV1 and/or FVC ≥12% and an increase of ≥200 mL above baseline) (Citation4). Six-minute walk test distance (Citation6 MWT) was measured in a standardized fashion (Citation6). Subjects using supplemental oxygen therapy continued their usual oxygen dose.
Thoracic Imaging. High resolution chest CT scans were acquired using multi-detector CT scanners with at least 16 detector channels. Volumetric CT acquisitions were obtained on full inspiration (200 mAs) and at the end of normal expiration (50 mAs). Image reconstruction utilized sub-millimeter slice thickness. Devices were calibrated using a standardized lung phantom. Thin-slice collimation with slice thickness of <l mm and intervals of <l mm were used to enhance spatial resolution. Quantitative image analysis to determine lung volumes, degree of hyperinflation and gas-trapping, and airway wall thickness was performed using VIDA and SLICER software. All data are stored in the COPDGene® Data Coordinating Center (DCC) at the Division of Biostatistics and Bioinformatics at National Jewish Health.
Data analysis
Analysis was performed using JMP Statistical Discovery software from SAS. Values are expressed as mean ± SD. Categorical variables were compared between groups using Chi square test. Continuous variables were evaluated using one-way ANOVA or 2 tailed unpaired t-test. A p-value <0.05 was considered significant.
Results
Patient characteristics
This analysis was based on the first 2500 subjects enrolled in COPDGene® (November 2010 COPDGene® Data Set). Then, 1,273 subjects were classified as having GOLD Stages I-IV COPD. Nine of the subjects who reported using oral corticosteroids reported having an additional diagnosis of rheumatoid arthritis and were excluded from analysis. Of the 1264 subjects included in the final analysis (); 58 patients (4.5%) reported chronically using systemic corticosteroids.
presents baseline demographic information in all subjects. There were no differences in age, race, co-morbid conditions (other than asthma, hay fever, and allergic type symptoms), or body mass index (BMI) between patients with COPD who reported using chronic systemic corticosteroids and those that did not. There was a greater proportion of GOLD stage IV (41% vs. 14%; p < 0.0001) subjects in the group that reported using chronic systemic corticosteroids compared to those that did not.
Table 1. Patient demographics and past medical history
Those who reported the use of systemic corticosteroids were more likely to report a history of asthma (p = 0.0005) as well as allergic type symptoms including watery, itchy, burning eyes (p = 0.009) and hay fever (p = 0.03). The GOLD III/IV group on chronic systemic steroids also reported a frequent history of asthma (46% vs. 27%; p = 0.01) and allergic type symptoms (60% vs. 42%; p = 0.01). There was variation between centers with regards to steroid use. Ten of the 18 centers enrolled subjects that reported the use of chronic corticosteroids. Corticosteroid use varied with severity of disease; centers with a greater percentage of subjects reporting corticosteroid use had similarly high percentages subjects with GOLD stage IV disease.
Smoking pack-years were similar between the groups (52.7 ± 1 vs. 53.8 ± 3.8; p = 0.8), although those on systemic corticosteroids who reported current smoking said they smoked about half the number of cigarettes compared to those not on corticosteroids (p = 0.02). Expected side effects such as diabetes, osteoporosis and pneumonia were no more frequent in the whole cohort on systemic corticosteroids including the GOLD III/IV subgroup on systemic corticosteroids ().
Medication use
The cohort on chronic systemic corticosteroids reported using more inhaled medications including short-acting beta-agonists (p = 0.01), long-acting beta agonists (p = 0.04), combination inhaled corticosteroids and beta-agonists (p = 0.02), and tiotropium (p < 0.0001). Additionally, subjects using systemic corticosteroids were more likely to report the use of nebulized medications (p < 0.0001) as well as theophylline (p = 0.001) compared to those not on chronic systemic corticosteroids (). The GOLD III/IV group on chronic systemic steroids reported more frequent use of nebulized medications (83 vs. 50; p < 0.0001).
Table 2. Medication use
Pulmonary physiology, exercise and exacerbations
Subjects who reported using chronic systemic corticosteroids had more severe COPD indicated by a lower FEV1/FVC (41 ± 1.7 vs. 51 ± 0.4; p < 0.0001) and a lower FEV1% predicted post-bronchodilator (35 ± 3 vs. 57 ± 0.7; p < 0.0001). There were no significant differences between groups in regards to bronchodilator reversibility () including the GOLD III/IV subgroup.
Table 3. Pulmonary function testing, exercise testing, and exacerbation history
The group using chronic systemic corticosteroids had a slightly lower resting room air oxygen saturation (93 ± 0.49 vs. 95 ± 0.10;%, SaO2; p = 0.001) and used more oxygen (L/min) during 6 MWT (2.31 ± 0.21 vs. 0.59 ± 0.05; p < 0.0001). The GOLD III/IV group on chronic systemic steroids also used more oxygen (L/min) during 6 MWT (2.7 ± 0.3 vs. 1.2 ± 0.1; p < 0.0001). Subjects who reported using chronic systemic corticosteroids walked a shorter distance on 6MWT (245.4 ± 17.4 vs. 367.2 ± 3.9 meters; p < 0.0001) (). This pattern persisted when analyzing only GOLD III and IV subjects. The GOLD III/IV group on chronic systemic steroids walked less far than the GOLD III/IV group not on chronic steroids (234.3 ± 18 vs. 303.6 ± 6 meters; p = 0.0002).
The group using chronic systemic corticosteroids reported more exacerbations per year than those not using chronic systemic corticosteroids (1.7 ± 0.16 vs. 0.62 ± 0.03; p < 0.0001) and more severe exacerbations requiring hospitalization (0.41 ± 0.05 vs. 0.18 ± 0.01; p < 0.0001). The subgroup analysis of the GOLD III/IV group on chronic systemic steroids demonstrated more exacerbations per year than those GOLD III/IV subjects not using chronic systemic steroids (1.6 ± 0.2 vs. 1.0 ± 0.1; p = 0.003).
BODE scores and health-related Quality-of-Life
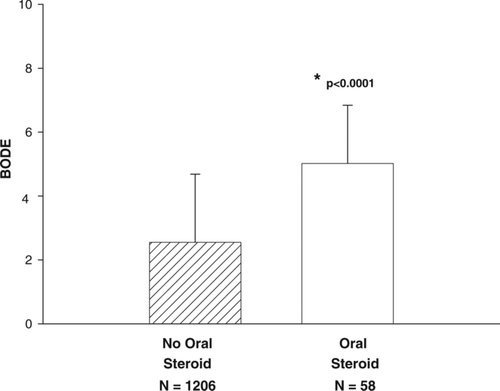
The group using chronic systemic corticosteroids had higher BODE scores (5.0 ± 0.3 vs. 2.6 ± 0.1; p < 0.0001) (). In the GOLD III/IV analysis, there were higher BODE scores in the group using chronic systemic steroids (5.5 ± 0.2 vs. 4.5 ± 0.1; p < 0.0001). Additionally, the MMRC dyspnea scores were higher for the chronic corticosteroid using group (3.3 ± 0.2 vs. 1.9 ± 0.04; p < 0.0001). This difference persisted in the GOLD III/IV subgroup analysis (3.5 ± 0.2 vs. 2.7 ± 0.1; p < 0.0001). Those on chronic systemic corticosteroids had significantly higher total SGRQ scores (54.9 ± 2.9 vs 34.2 ± 0.2; p < 0.0001) indicating worse health-related quality of life compared to those not on chronic systemic steroids. This difference persisted in the GOLD III/IV subgroup analysis (56 ± 3 vs. 47 ± 1; p = 0.007).
Radiographic characteristics
The cohort on chronic systemic corticosteroids had a higher percentage of emphysema on CT scan (22.4 ± 1.9 vs. 14.0 ± 0.4;%, p < 0.0001) and a higher percentage of gas trapping (53.0 ± 3.1 vs. 49.0 ± 0.7;%, p < 0.0001) in comparison to those not on chronic steroids. These differences did not persist in the GOLD III/IV subgroup analysis. There was a small but statistically significant increase in subsegmental wall area percent in the cohort on chronic systemic corticosteroids (66.3 ± 0.38 vs. 65.30 ± 0.08, p = 0.009).
Discussion
In the cohort of subjects enrolled in COPDGene® that met diagnostic criteria for COPD, 4.5% reported the use of chronic systemic corticosteroids despite disease management guidelines recommending against their use in the chronic management of stable, severe COPD (Citation1). Patients with COPD who reported using chronic systemic steroids were a sicker cohort as measured by multiple clinical, physiologic and radiological parameters. Those using chronic systemic steroids overall used more respiratory medications, had more severe airflow obstruction, demonstrated a greater need to use supplemental oxygen, walked less on 6 MWT, and reported having more frequent and severe exacerbations. Additionally, patients using chronic systemic steroids reported worse quality of life, more dyspnea, and higher BODE scores compared to those not using chronic systemic steroids. The cohort using chronic systemic steroids also had a greater degree of both emphysema and gas trapping on HRCT imaging.
Increased mortality in COPD has been linked to several factors that contribute to the BODE score (Citation5) including lower FEV1 (Citation7,8,Citation9), increased dyspnea (Citation10,11) and decreased exercise capacity (Citation12,13).
In addition, other factors such as oxygen use and health related quality of life have also been linked to mortality in COPD (Citation11,Citation14). The use of chronic systemic steroids may be another marker that identifies patients with COPD at greater risk of death. The cohort of patients on chronic systemic steroids had significantly lower FEV1 values, increased dyspnea, decreased exercise capacity, increased oxygen requirements, and more frequent and severe acute exacerbations of COPD (AECOPD). These differences persisted in the subgroup analysis of only the GOLD III and IV subjects, when the degree of obstruction was similarly severe between the groups.
AECOPD has been associated with increased BODE scores in the acute setting that become lower following resolution of the exacerbation (Citation15). We found a 2.5-point increase in BODE scores as well as an increased frequency and severity of AECOPD for patients using chronic systemic steroids. Although lung function has been shown to return to baseline in the majority of patients after a COPD exacerbation, there are a substantial proportion of patients for whom lung function remains decreased after an exacerbation (Citation16,17). The permanent loss of lung function after AECOPD is associated with more severe exacerbations and an accelerated decline in lung function (Citation16, Citation18). This article cannot address the decline in lung function in patients with COPD on chronic systemic steroids. However, the possibility that this patient population experiences a more rapid decline in lung function than those not on chronic systemic steroids given the increased frequency and severity of acute exacerbations should be evaluated in the future.
The significantly increased SGRQ scores reported in the group on chronic systemic steroids are consistent with prior literature correlating AECOPD and lower FEV1 with worse health related quality of life (Citation19, 20, Citation21). As discussed here, increased SGRQ scores are linked to increased mortality in COPD.
Although COPD is primarily a disease of the lungs, the presence of systemic inflammation in COPD has been well described (Citation22). Additionally, elevated levels of IL-6 and fibrinogen have been associated with a more rapid lung function decline in COPD (Citation23). Drugs with anti-inflammatory properties such as the macrolides and statins have shown promise in improving clinical parameters in COPD (Citation24, 25). Although the benefit of systemic corticoteroids in persistent asthma has been well documented (Citation26), this benefit is less clear in COPD (Citation27). Oral corticosteroids have been shown to modify eosinophilic inflammation in asthma (Citation28) and to decrease sputum eosinophil count in COPD suggesting that patients with COPD who respond to oral corticosteroids may represent a subgroup with eosinophilic airway inflammation (Citation26).
This study raises the question as to why patients with COPD are placed on chronic systemic corticosteroids despite the lack of evidence supporting their use. Our study demonstrates that patients placed on chronic oral corticosteroids have usually been otherwise maximally medically treated and remain highly symptomatic by all measurable subjective and objective clinical parameters of disease activity. This patient population may be prescribed corticosteroids as a last resort to control persistent and severe symptoms of breathlessness despite optimized medical therapy in severe and very severe COPD.
We found no increased incidence of pneumonia, diabetes, or osteoporosis in the cohort of patients that reported oral corticosteroid use. However, whether using chronic systemic steroids improves the clinical course of patients with severe but stable COPD remains unknown. Future prospective studies that evaluate the effect of systemic corticosteroids as well as other anti-inflammatory medications such as statins on markers of airway and systemic inflammation in severe stable COPD are necessary to determine if there is a sound rationale for ever prescribing oral corticosteroids in severe but stable COPD.
The main limitation to our study is the recall nature of the data collection. Medication lists, oxygen use, exacerbation and hospitalization frequency, and co-morbidities were provided by the patients themselves rather than their primary medical doctor or pulmonologist. There were only a small number of subjects on chronic oral steroids, which may have additionally affected the data analysis.
Conclusions
In conclusion, this paper offers a detailed description of the phenotypic characteristics of COPD patients that receive chronic systemic steroids for disease control. These findings demonstrate that the use of chronic oral corticosteroid therapy can be used as a marker of a sicker, more complex COPD patient population. Further research is needed in anti-inflammatory therapies to target both airway and systemic inflammation in COPD.
Declaration of Interest
This study was supported by the NHLBI U01 HL089856 and U01 HL089897. The authors report no conflicts of interest. The authors are responsible for the content and the writing of this paper.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Bethesda, MD: National Heart, Lung, and Blood Institute, April 2001 (revised 2009). (Available at http://www.goldcopd.org).
- Regan E, Hokanson J, Murphy J, Make B, Lynch D, Beaty T, Genetic epidemiology of COPD (COPDGene®) study design. COPD 2010; 7:32–43.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179–187.
- Pellefrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Series “ATS/ERS Task Force: Standardisation of lung function testing”. Eur Respir J 2005; 26:948–968.
- Celli BR, Cote CG, Marin JM, Casanova C, de Oca MM, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. NEJM 2004; 350:1005–1012.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117.
- Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J 1995; 8:1398–1420.
- Anthonisen NR, Wright EC, Hodgkin JE, and the IPPB Trial Group. Prognosis in chronic obstructive pulmonary disease. Am Rev Resp Dis 1986; 133:14–20.
- Traver GA, Cline MG, Burrows B. Predictors of mortality in chronic obstructive pulmonary disease: a 15-year follow-up study. Am Rev Respir Dis 1979; 119:895–902.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 1221:1434–1440.
- Wegner RE, Jorres RA, Kirsten DK, Magnussen H. Factor analysis of exercise capacity, dyspnoea ratings and lung function in patients with severe COPD. Eur Respir J 1994; 7:725–729.
- Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006; 173:1326–1334.
- Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 2004; 23:28–33.
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 167:544–49.
- Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centered outcomes. Chest 2007; 131:696–704.
- Seemungal TAR, Donaldson GC, Bhowmik A, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161:1608–1618.
- Sachs APE, Koeter GH, Groenier KH, van der Waaij D, Schiphuis J, Jong BM. Changes in symptoms, peak expiratory flow and sputum glora during treatment with antibiotics of exacerbations in patients with chronic obstructive pulmonary disease in general practice. Thorax 50:758–763.
- Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Connors AF Jr, Dawson NV, Thomas C, Harrell FE Jr., Desbiens N, Fulkerson WJ, Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med 1996; 154:959–967.
- Ferrer M, Alonso J, Morera J, Marrades RM, Khalaf A, Aguar MC, Chronic obstructive pulmonary disease stage and health-related quality of life: the quality of life of chronic obstructive pulmonary disease study group. Ann Int Med 1997; 127:1072–1079.
- Seemungal TAR, Donaldson GC, Paul EA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1418–1422.
- Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59:574–580.
- Donaldson GC, Seemungal TAR, Patel IS, Bhowmik A, Wilkinson TMA, Hurst JR, Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 2005; 128:1995–2004.
- Seemuungal TA, Wilkinson TAM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med 2008; 178:1139–1147.
- van Gestel YR, Hoeks SE, Sin DD, Simsek C, Weltzen G, Schouten O, Stam H, Effect of statin therapy on mortality in patients with peripheral arterial disease and comparison of those with versus without associated chronic obstructive pulmonary disease. Am J Cardiol 2008; 102:192–196.
- Brightling CE, Monteiro W, Ward R, Parker D, Morgan MDL, Wardlaw AJ, Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 2000; 356:1480–1485.
- Walters JAE, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005; 3. Art No: CD005374.
- Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med 1997; 155:542–548.