Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by inflammation of lung parenchyma and pulmonary hypoxemia with a proven systemic component. Tobacco smoke is the most important risk factor and plasma membrane plays a major role in the disease pathology and progression. The properties of biological membranes are a function of their lipid composition. Any change in its composition may lead to the pathophysiology. In COPD research, erythrocytes are emerging as a new therapeutic venture, as their shape and properties change in the disease. Therefore we studied the lipid composition of the erythrocyte membranes of COPD patients. The study included 30 patients having COPD, 10 healthy smokers and 10 non-smokers. Erythrocytes were separated from peripheral blood and their membranes prepared, followed by estimation of proteins, cholesterol and phospholipids. Individual phospholipids were identified and separated by TLC and fatty acid composition determined by gas chromatography. The data were analyzed statistically and P < 0.05 was considered significant. Our results demonstrate that in very severe COPD, proteins decrease, whereas phospholipids and cholesterol contents increase significantly, which showed a consistent negative correlation with FEV1%. The fatty acid analysis showed preponderance towards saturated fatty acids mainly arachidic and behenic acid, suggesting a decrease in membrane fluidity or a closer packing of lipid rafts. We are the first to report about preponderance of saturated fatty acids in plasma membrane of erythrocytes of COPD patients which may decrease the membrane fluidity and possibly impair the functions of the plasma membrane in the disease.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most important causes of impaired respiratory function (Citation1). It is characterized by inflammation of lung parenchyma and pulmonary hypoxemia with a proven systemic component (Citation2). Tobacco smoke is by far the most important risk factor for COPD worldwide (Citation3). COPD has recently been extensively explored from a cellular and molecular perspective, which shows that lung inflammation can produce oxidative stress, which may extend beyond the lung and contribute to several of the systemic manifestations of COPD such as systemic inflammation, cachexia, anemia, osteoporosis, skeletal muscle dysfunction and risk for cardiovascular disease (Citation4–9).
The red blood cells are emerging as a new therapeutic venture for COPD, as, changes in erythrocyte shape and rheological properties are proven to play major role in the disease pathology and progress which may affect the signal transduction pathway and eventually cause defects in signaling, and also block the lung capillaries, thus propagating the disease (Citation10). Therefore, it is very important to understand the changes in structural components of the erythrocytes in COPD.
Erythrocyte membrane, like other plasma membranes, is composed of double layer of phospholipids and cholesterol, with occasional proteins intertwined. The main phospholipids in it are phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI) and sphingomyelin (Sph) (Citation11). Cholesterol plays a vital role in determining the physicochemical properties of cell membranes. It's incorporation in the hydrocarbon core of erythrocyte membrane enhances oxygen diffusiveness (Citation12). The length and degree of unsaturation of fatty acid chains have a profound effect on membranes fluidity as unsaturated lipids create a kink, preventing the fatty acids from packing together tightly, thus decreasing the melting point (increasing the fluidity) of the membrane (Citation12).
A number of experimental studies have revealed the existence of highly ordered glycolipoprotein microdomains rich in sphingomyelin and cholesterol called lipid rafts (Citation13). They have been suggested to take part in a variety of dynamic cellular processes such as membrane trafficking and signal transduction, creating cell compartmentalization (Citation14). The difference in packing of lipid rafts is due to the saturation of the hydrocarbon chains in fatty acids of sphingolipids and phospholipids, and intermolecular hydrogen bonds (Citation15). Evidently, lipid composition determines the fluidity of biological membranes and a definite composition of lipid rafts is essential for the normal physiology of the cell (Citation12).
Any change in the composition and metabolism of the lipids may thus change the quality of the plasma membrane and their properties. These changes may in turn lead to the changes in the orientation of cell surface receptors, signal transduction mechanisms, cell functions and ultimate pathophysiology that may lead to the manifestation of the disease. Therefore we performed our studies on lipid composition of the red cell membranes of COPD patients to infer the integrity of the plasma membrane and its lipid rafts that may influence the signal transduction process. Because smoking is one of the important risk factors, the findings were compared with healthy smokers besides healthy non-smoker subjects.
Materials and Methods
Chemicals and biochemicals
Various chemicals viz amino-naphtho-sulfonic acid (ANSA), bovine serum albumin, petroleum ether, phospholipids viz. phosphatidyl choline (PC), phosphatidyl ethanolamine (PI), phosphatidyl inositol (PI), phosphatidyl serine (PS), sphingomyelin (Sph), and standards of fatty acid methyl ester were purchased from Sigma Chemical Co, St. Louis, Missouri, USA. Heparin (5000 I.U./ml) was purchased from Biological Evans ltd, Hyderabad, India. Other chemicals were obtained from Sisco Research Laboratories, Mumbai, India. Silica gel G was obtained from E. Merck, Mumbai, India.
Study Design
A total number of 50 subjects were enrolled in the study of which, 30 were patients having chronic obstructive pulmonary disease (COPD), 10 healthy volunteers and another 10 were healthy smokers. Informed written consent was obtained from each subject. The patients were divided into three groups viz Group I (moderate), II (severe), and group III (very severe). Peripheral blood was drawn and erythrocyte membranes prepared from them. After estimating proteins, membrane lipids were extracted from a suitable aliquot. Total membrane cholesterol and phospholipid contents were determined. Subsequently, individual phospholipids were resolved by thin layer chromatography. The phospholipids were extracted from the silica gel TLC plates and their fatty acid methyl esters prepared for analyzing fatty acids by gas chromatography. The data were analyzed statistically and P<0.05 was considered significant. The study was approved by Institutional Ethics Committee.
Subjects
Patients having COPD
The COPD patients were selected from Viswanathan Chest Hospital of Vallabhbhai Patel Chest Institute, University of Delhi, Delhi, India. Both freshly diagnosed and old follow up cases of either sex were selected for the study after ensuring proper diagnosis as per the guidelines issued jointly by American Thoracic Society and European Society of Respirology, revised in 2008 (Citation1). Based on FEV1% predicted values, the patients of COPD were classified into 4 stages viz Stage I: Mild (≥ 80% FEV1); Stage II: Moderate (50–80% FEV1); Stage III: Severe (30–50% FEV1); Stage IV: Very Severe (< 30% FEV1) (1).
The inclusion criteria included the patients of COPD of stage II (moderate), stage III (severe) and stage IV (very severe), age ranging between 18 to 65 years, not on any medications including any cholesterol lowering medicine, did not have a dietary history of past 24 hours for any fat rich food and not used any bronchodilators, during the past 24 hours before collecting the blood sample.
The exclusion criteria included the patients of COPD of Stage I (mild) (as their presentation in hospital is rare), pregnant and lactating females and patients with any other systemic disease.
Healthy subjects
Healthy volunteers (Citation20), age and sex matched, were selected as controls for the study, of which 10 were smokers and 10 non-smokers. A smoker was defined as a person who smoked tobacco in any form, equivalent to 10–40 cigarettes per day or a total of 2–15 pack years (Citation16, 17). One pack year signifies 20 g tobacco (20 cigarettes) smoked each day over a course of 1 year (Citation18). Only ‘current smokers’ were included in this group (a person who has smoked >100 cigarettes in his lifetime and is still smoking regularly). Ex-smokers (not smoking for past 1 month) and occasional smokers were excluded (Citation16). shows the demographic details of all the subjects.
Table 1. Clinicophysiological parameters of the subjects
Methodology
Preparation of RBC lysate
Blood (10 ml) was withdrawn in a pre-heparinized centrifuge tube and an aliquot taken for cell counting in the automated coulter (Sysmex PoCH-100i automated cell coulter). The remaining sample was then centrifuged at 650×g for 30 minutes at 4°C to obtain the RBC pellet followed by 3 washings. The membrane of the red blood cells was prepared by the method of Hanahan and Ekholm by giving osmotic shock after putting them in 14 volumes of lysis buffer (0.155 M NaH2PO4.H2O and 0.103 M Na2HPO4.7H2O; pH 8) and processing in high speed centrifuge (Sorvall RC-5B refrigerated high-speed centrifuge using fixed angle rotor SS 34) at 12,000×g for 40 minutes (Citation19). A suitable aliquot of the pellet was saved for protein estimation, and the rest was processed for lipid extraction. The protein contents were estimated by the method of Lowry et al. (Citation20).
Extraction of total lipids, quantification of cholesterol and total membrane phospholipids
For extracting lipids, the membrane preparation was mixed with equal volume of acidified chloroform: methanol: HCl (200:100:1, v/v/v) and centrifuged at 200g, followed by determination of cholesterol by the method of Zak et al. (Citation21) and phospholipid contents by estimating inorganic phosphorus by the method of Fiske and Subbarow (Citation22).
Determination of constituent phospholipids
Individual phospholipids were resolved by thin layer chromatography on silica gel G using TLC spreader designed by Shandon Scientific Co. Ltd, London, in a solvent system of chloroform: methanol: 7N ammonia:: 230: 90: 15 (v/v/v) (Citation23). Standard phospholipids were co-chromatographed. The lipid classes separate out as bands, were visualized by exposure to iodine vapors for 2–3 minutes. The bands were scraped and inorganic phosphorus estimated using direct gel digestion method (Citation24). Iodine was not used for the bands scraped for extraction of fatty acid.
Determination of fatty acids of individual phospholipids
The phospholipid bands (unexposed to iodine) were scraped from the TLC plates and their lipids extracted using 1ml of chloroform: methanol (2:1). For determination of fatty acids of individual phospholipids, their methyl esters were prepared. The lipid extract (1 ml) was taken in screw capped test tube and 0.2 ml of 2N KOH in methanol was added. The tube was incubated in a water bath at 50 °C for 10 minutes with occasional shaking and then cooled at 4 °C for 10 minutes. Then, 1 ml of 5% HCl in methanol was added, the contents incubated in a water bath at 70 °C for 10 minutes with occasional shaking and cooled at 4 °C for 10 min.
Then, 2 ml petroleum ether was added to the tube, centrifuged at 200×g to extract the fatty acid methyl ester (upper ether layer) and the extract dried under the stream of nitrogen gas. The dried sample was reconstituted in petroleum ether (200 μl) before injecting in GC (Aimil-Nucon Series 5700 Gas Chromatograph). The fatty acids were resolved using a C18 (SS, 30m) column and data analyzed by the WinAcds software provided by the manufacturers. Standard fatty acid methyl esters (a combination of 4 to 5 fatty acids in one standard vial having a fixed pattern viz. palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2) and linolenic acid (C18:3) (Sigma catalogue no. 1891) and stearic acid (C 18:00), nonadecanoic acid (C19:00), arachidic acid (C20:00), heneicasanoic acid (C21:00), behenic acid (C22:00) (Sigma catalogue no. 1899) were run on gas chromatograph in varying known concentrations to draw a standard curve. This also gave the retention times of the standard fatty acids. On the basis of the standard graph (slope) and the retention times of known fatty acids, the samples were analyzed to determine the fatty acid composition of the phospholipids as well quantify them.
Statistical analysis
The data were subjected to statistical analysis using computer-based software ‘Prism’. The tests applied were column statistics, one way ANOVA, Bon Ferroni Multiple Comparison Test and correlation coefficient by Pearson. P<0.05 was considered significant.
Results
In the present study a total number of 50 cases were included, who were divided into 5 groups viz. healthy non-smokers, healthy smokers and 3 groups of patients diagnosed with COPD, classified as group I (moderate), group II (severe) and group III (very severe) as described in the materials and methods section. Their demographic, smoking and pulmonary function details are given in . One patient of group I, 2 patients of group II and 1 patient of group III were found to be still smoking actively. The rest of the patients had stopped smoking for an average of (4.55 ± 0.87) year. (Range = 0–17years).
Hemoglobin of COPD patients
The hemoglobin of COPD patients was determined by an automated coulter. In group I it was found to be 12.76 ± 0.86g/dl (mean ± S.D.), in group II to be 12.45 ± 0.85 g/dl and in group III to be 11.92 ± 1.13g/dl. The mean hemoglobin content in the healthy non-smoker group was found to be 13.5 ± 1.74 (g/dl) (mean ± S.D.) and of healthy smokers to be 14.47 ± 0.45g/dl. Two patients from group I, 3 patients from group II and 5 patients from group III and one subject each from the healthy non-smoker and healthy smoker groups were found to be anemic. (Criteria: Hb ≤ 12g/dl) (Citation25).
Protein, cholesterol and total phospholipid content of erythrocyte membranes
The erythrocyte membranes were prepared and the total membrane proteins, cholesterol and phospholipids determined. In COPD groups, protein values decreased gradually, with significant decrease (P = 0.0425) in group III i.e., stage IV COPD. The cholesterol value increased significantly (P = 0.021) in healthy smokers. The increase was found to be gradual in COPD groups with significant increase in group III (P = 0.015) ().
Figure 1. Cholesterol (Chol) and Phospholipid (PL) contents of erythrocyte membrane (expressed as μg/mg proteins) H = Healthy non-smokers, S = Healthy smokers, Group I = patients of COPD stage II, Group II = Patients of COPD stage III, Group III = Patients of COPD stage IV. N = 10 in each group. Data represented as mean ± S.E.M.
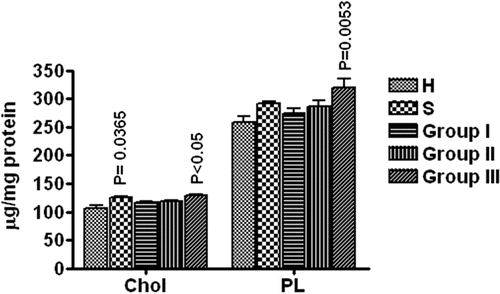
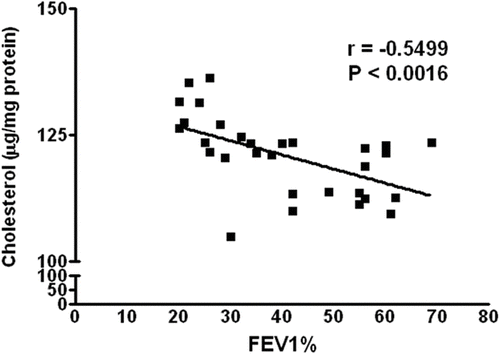
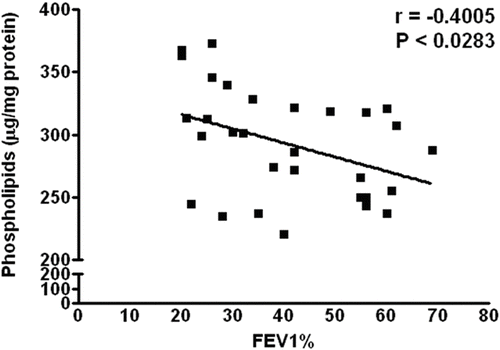
Similarly, the values of total phospholipids of the erythrocyte membrane increased in all groups as compared to healthy non-smokers; however, the significant rise was seen only in group III (P = 0.0053) (). The correlational coefficient (r) analysis revealed that FEV1% (% predicted) has a negative correlation with total membrane cholesterol contents (r = -0.5499), which is statistically significant (P = 0.0016) (). Similarly, the correlation between FEV1% (% predicted) with total phospholipids shows a negative correlation (r = -0.4005, P = 0.0283) (), suggesting that increase in membrane cholesterol and phospholipids is associated with the severity of COPD, or vice versa.
Constituent phospholipids of erythrocyte membrane
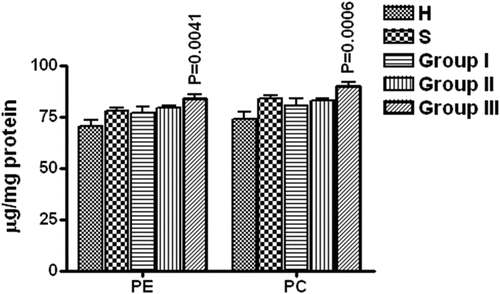
The total phospholipids were resolved by thin layer chromatography to obtain bands of individual phospholipids which were then scraped and digested with 60% PCA followed by inorganic phosphorus (Pi) estimation. The phosphoinositide (PI), sphingomyelin (Sph), phosphatidylserine (PS), phosphatidylethanolamine (PE) and phosphatidylcholine (PC) contents of each subject were estimated. There was a significant increase in PE (P = 0.0041) and PC (P = 0.0006) values in erythrocyte membrane of group III COPD patients (i.e., stage IV of COPD) as compared to healthy non-smokers ().
Figure 4. Phosphatidylethanolamine (PE) and phosphatidylcholine (PC) contents of the erythrocyte membrane (expressed as μg/mg proteins). H = Healthy non-smokers, S = Healthy smokers, Group I = Patients of COPD stage II, Group II = Patients of COPD stage III, Group III = Patients of COPD stage IV. N = 10 in each group. Data represented as mean ± S.E.M.
Fatty acid composition of individual phospholipids
The fatty acids of all the individual phospholipids were estimated using gas chromatography. The retention times of the standard fatty acids were used to identify the fatty acid and the slope of area was used to calculate the concentration of fatty acid in the sample. A representative picture of fatty acid analysis of phospholipids in all 5 groups is given in and the data are given in Tables , and . The values are expressed in μg fatty acid / μg phospholipid.
Figure 5. Chromatogram of fatty acid analysis of phosphatidylcholine (PC) as representative of the phospholipids of erythrocyte membrane in various groups of patients having COPD (Group I-III), healthy smokers (S) and healthy non-smokers (H). H = Healthy non-smokers, S = Healthy smokers, Group I = Patients of COPD stage II, Group II = Patients of COPD stage III, Group III = Patients of COPD stage IV.
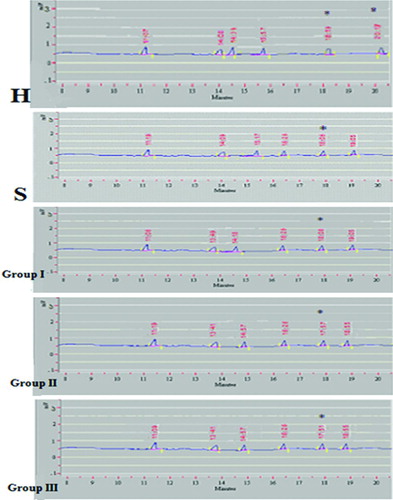
Table 2. Fatty acid composition of erythrocyte membrane of acidic phospholipids (Phoshatidylinositide, Phosphatidylserine)
Table 3. Fatty acid composition of erythrocyte membrane neutral phospholipids (Phoshatidylcholine, Phoshatidylethanolamine)
Table 4. Fatty acid composition of erythrocyte membrane sphingomyelin (Sph)
The fatty acids identified in healthy non-smoker subjects were palmitic acid (C16:00), stearic acid (C18:00), oleic acid (C18:01) and linoleic acid (C18:02). In healthy smokers and COPD patient groups, linoleic acid (C 18:02) was not found to be substantially present. Interestingly, 2 new saturated fatty acids were observed in all these 4 groups, which were identified as arachidic acid (C20:00) and behenic acid (C22:00). This pattern was found to be uniform in every constituent phospholipids. Palmitic acid is seen to be uniformly increasing in COPD patients of all groups in the phospholipids. Thus, saturation of fatty acids is apparently increasing in the phospholipids, suggesting a shift towards lesser fluidity of membrane owing to tighter and more ordered packing of phospholipids. The phospholipid/protein (P = 0.018) and sphingomyelin/phosphatidylcholine (P = 0.045) ratios were seen to increase and decrease respectively in group III COPD patients. There was no significant change in cholesterol/phospholipid, phosphatidylcholine/cholesterol, sphingomyelin/cholesterol or phosphatidylcholine/phosphatidylethanolamine ratios ().
Table 5. Lipid ratios in erythrocyte membranes
Discussion
COPD is primarily a disease of the adults. In the present study, the patients’ age ranged between 42 to 65 years. Of the several risk factors of COPD, tobacco smoking is the cause in 80–95% of COPD patients (Citation26). However, only 15–25% smokers develop COPD (Citation27). Thus, smoking is considered to be one of the main etiological factors for the development of COPD. Out of 30 patients, 27 were smokers and 3 non-smokers. From the data shown in , it can be seen that the duration and extent of smoking does not correlate with the severity of COPD. Thus, only certain people develop the disease, the cause of which may be related to genetic susceptibility to smoking induced damage leading to the development of COPD in certain individuals (Citation3). Anemia is a common finding in COPD which is either due to a commonly observed nutritional deficiency or due to anemia of chronic disease (Citation10). We observed 33% COPD patients to be anemic which further confirms this contention.
In COPD, the red cells have been seen to have structural deformities and its membrane proteins have been shown to have qualitative changes on specific analysis (Citation10, Citation28). In our study, the protein content of red cell membranes decreased in group III, i.e., Stage IV (very severe) COPD (P = 0.0425). The protein content of rest all groups were found to be normal. Any changes whatsoever in these stages may also be qualitative as shown by other workers (Citation10). In the pathological process, the erythrocytes are involved especially in the systemic component of the disease, where they may act as signal transducing cells (Citation29). In COPD, the membranes of erythrocytes have structural abnormalities (Citation10).
The cholesterol-sphingomyelin liquid-ordered moieties known as lipid rafts may be involved in the pathology by altering the overall fluidity of the membrane. The analysis of membrane fluidity and any possible abnormalities of membrane lipid composition may influence the signal transduction. The decrease in protein contents may be due to its decreased biosynthesis and/or increased catabolism, which may lead to quantitative and qualitative changes in the membrane proteins, which may influence the signal transduction mechanism and possibly impair the cellular functions.
Smokers are known to develop hyperlipidemia and hypercholesterolemia (Citation30). The cholesterol and lipids in their blood may be in dynamic equilibrium with the plasma membranes. In our study, we observed a significant increase (P = 0.0365) in cholesterol in smokers as well as group III COPD i.e., Stage IV, which may be due to its increased uptake in the membranes or decreased turnover. In group I and II, the values of cholesterol do not show any significant change, possibly due to body response to maintain itself in response to smoking to prevent the development of the disease.
Similarly, there was a significant increase in total phospholipid of erythrocyte membranes in group III COPD patients (P = 0.0053). This suggests that there may be a fall in phospholipase activity or an increase in the enzymes involved in the biosynthesis of phospholipids in the later stages of COPD. In the present study, we observed a significant increase in total cholesterol and phospholipid contents in group III of the patients and there was a significant correlation between FEV1% and cholesterol (P = 0.0016) and between FEV1% and total membrane phospholipid or vice versa (P = 0.0283).
This suggests an increase in lipid contents with increase in airway obstruction in COPD or vice versa and thus, we propose that the airway obstruction may be associated with decrease in some phospholipases activity. The analysis of the individual phospholipids showed that the phosphatidyl inositides, sphingomyelin and phosphatidylserine contents were comparable to that of the healthy group. However, phosphatidylcholine (P = 0.006) and phosphatidylethanolamine (P = 0.0041) increased significantly in group III COPD patients. This indicates that either the catabolism of the phospholipids is decreased or their anabolism is increased in the last stage of the disease, which may depend upon the activity of the enzymes of their respective pathways.
Further, the fatty acid analysis of the individual phospholipids showed preponderance towards saturated fatty acids in COPD patients and smokers. The levels of palmitic acid (C 16:00) increased in all the stages of COPD in various phospholipid moieties. Oleic acid (C18:01) and linoleic acid (C18:02) were decreased in all patient groups. Moreover, there was appearance of higher carbon saturated fatty acids in healthy smokers and COPD patients, which may cause the tight packing of membrane molecules, providing it possibly a more rigid structure causing dysfunction of the membranes. In healthy non-smoker subjects, we observed the presence of mainly four fatty acids in the phospholipids of the erythrocyte membranes, viz palmitic acid (C16:00), stearic acid (C18:00), oleic acid (C18:01) and linoleic acid (C18:02).
Linoleic acid was totally absent in all the types of phospholipids in the healthy smokers and COPD patients groups. However, two new saturated fatty acids appeared in these groups viz. arachidic acid (C20:00) and behenic acid (C22:00). This pattern was found to be uniformly present in all other phospholipids also. The arachidic and behenic acids of PC, which appeared in the healthy smokers and COPD groups, were significantly increased in all groups of COPD as compared to healthy smokers (P<0.01).
Similarly, palmitic acid uniformly increased in all the COPD patients in all the types of phospholipids. This increase in the saturated fatty acids suggests a shift towards lesser fluidity of membrane resulting into tighter and more ordered packing of phospholipids. The membrane fluidity in COPD thus appears to decrease, which may result in closer packing of lipids in the lipid rafts, suggesting the rafts to become more tightly packed and less mobile in the plane of membranes leading to their dysfunction in the presence of external stimuli and finally affecting on the signal transduction mechanism and cell response.
The ratio of various constituent lipids and other molecules in the membrane is very important in deciding the properties of the membrane. The changes in lipid composition are expected to promote greater freedom of molecular motion in membranes (Citation31). These changes include decreased chain length of fatty acids, increased degree of unsaturation of fatty acids, decreased amount of cholesterol relative to phospholipid (Chol/PL), and increased amount of phospholipid relative to protein (PL/prot), decreased amount of sphingomyelin (Sph) relative to PC, and increased amount of PC relative to PE (Citation31–33).
In our study, in group III COPD patients, there was significant increase in phospholipids as compared to proteins (P<0.001) and decrease in sphingomyelin as compared to PC (P = 0.035). Both these parameters indicate increased degree of freedom of molecules in group III patients. This possibly creates a different rearrangement of lipid rafts in later stages of COPD. Although the increased phospholipids point towards increased degree of freedom, their fatty acids are increasingly saturated suggesting tighter membranes relatively. This is likely to create different arrangement and mobility of lipid rafts and thus affect their function.
Conclusions
We may conclude that in COPD, phospholipid and cholesterol contents increase, and proteins decrease, particularly in very severe stage, suggesting possible alterations in the activity of the enzymes of their respective pathways. We propose that the decrease in protein contents may be quantitative and qualitative, possibly due to their altered metabolism. A decrease in proteins and an increase in phospholipids and cholesterol together may be due to some common mechanism relating to catabolism of proteins and anabolism of phospholipids possibly due to some changes in enzyme composition of their respective pathways. The fatty acid analysis of the individual phospholipids showed preponderance towards saturated fatty acids in COPD patients and smokers. The membrane fluidity in COPD thus appears to decrease, suggesting closer packing of lipids in the lipid rafts, which is likely to impair the functions of the plasma membranes in the disease.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- American Thoracic Society. European Respiratory Society. Standards for the Diagnosis and Management of Patients with COPD. 2004 (Updated Sept 2008) (cited 2009 Mar 4). Available from: http://www.thoracic.org/sections/copd/.
- Wouters EFM, Groenewegen KH, Dentener MA, Systemic inflammation in COPD. Proc Amer Thor Soc 2007; 4:626–34.
- Eisner MD, Balmes J, Katz BP, Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health Perspect 2005 Apr; 113(4):7–15.
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003 Oct; 22(4):672–88.
- Agusti AG, Noguera A, Sauleda J, Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003 Feb; 21(2):347–60.
- MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol 2001 Oct; 429(1–3):195–207.
- Gan WQ, Man SF, Senthilselvan A, Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004 July; 59(7):574–80.
- Agusti AG. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005 Nov; 2(4):367–70.
- MacNee W, Rahman I. Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol Med 2000 Feb; 6(2):43–62.
- Santini MT, Straface E, Cipri A, Structural alterations in erythrocytes from patients with chronic obstructive pulmonary disease. Haemostasis 1997; 27(4):201–10.
- Dodge JT, Philips GB. Composition of phospholipids and of phospholipids fatty acids and aldehydes in human red cells. J Lipid Res 1967 Nov; 8(6):667–75.
- Dumas D, Muller S, Gouin F, Membrane fluidity and oxygen diffusion in cholesterol enriched erythrocyte membrane. Arch Biochem Biophys 1997 May; 341(1):34–49.
- Simons K, Ikonen K. Functional rafts in cell membranes. Nature 1997 June; 387:569–72.
- Samsonov A. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys J 2001 September; 81(3):1486–500.
- Devaux PF, Morris R. Transmembrane asymmetry and lateral domains in biological membranes. Traffic 2004 April; 5(4):241–6.
- Lazarus SC, Chinchilli VM, Rollings NJ, Smoking affects response to inhaled corticosteroid or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med 2007 April;175(8):783–90.
- Kacmaz M, Serdarozturk H, Cete S, The effects of smoking on antioxidant defense system and membrane free fatty acid contents of erythrocytes and plasma lipid parameter: protective role of antioxidant vitamins. Nutr Res 1997 June; 17(6):931–40.
- National Cancer Institute. Definition of Pack Year. (Cited 2009 Feb 8). Available from: http://www.cancer.gov/Templates/db_alpha.aspx?CdrID = 306510.
- Hanahan DJ, Ekholm JE. The preparation of red cell of ghosts (membranes). Colowick SP, Kaplan NO, editors. Methods in Enzymology. New York: Academic Press. 1974, Vol 31:168–72.
- Lowry OH, Rosenbrough NJ, Farr AL, Protein measurement with the Folin Phenol reagent. J Biol Chem 1951 November; 193(1):265–75.
- Zak B, Dickenman RC, White EG, Rapid estimation of free and total cholesterol. Am J Clin Pathol 1954; 24:1307–15.
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem 1925 December; 66(2):375–400.
- Vladimir PS, Marion B. Thin layer chromatography of lipids. Colowick SP, Kaplan NO, editors. Methods in Enzymology. New York: Academic Press. 1969. p 530–602.
- Misra UK. Thin layer chromatographic estimation of rat plasma and tissue lipids. Biochem Biol Sper 1968; 7:57–9.
- Kumar V, Abbas AK, Fausto N, Robbins Basic Pathology, 8th ed. Philadelphia: Saunders, an imprint of Elsevier. 2007; 432.
- Silva GE, Sherill DL, Guerra S, Asthma as a risk factor for COPD in a longitudinal study. Chest 2004 July; 126(1):59–65.
- Rennard S, Vestbo J. COPD: The dangerous underestimate of 15%. Lancet 2006 April; 367(9518):1216–9.
- Stepovaia EA, Novitskii VV, Riazantseva NV, Chronic bronchitis: participation of red cells in pathological process. Klin Med (Mosk) 2004; 82:53–6.
- Lucantoni G, Pietraforte D, Mataresse P, The red blood cell as a biosensor for monitoring oxidative imbalance in COPD: An ex vivo and in vitro study. Antioxid Redox Signal 2006; 8:1171–82.
- Penn A, Keller K, Snyder C, The tar fraction of cigarette smoke does not promote arteriosclerotic plaque development. Environ Health Perspect 1996 Oct;104(10):1108–13.
- Ingraham LLM, Burns CP, Boxer LA, Fluidity properties and composition of erythrocyte membranes in Chediak-Higashi syndrome. J Cell Biol 1981 June; 89(3):510–6.
- Force A, Grizard G, Giraud MN, Membrane fluidity and lipid content of human spermatozoa selected by swim-up method. Intl J Androl 2001; 24:327–34.
- Stibler H, Beauge F, Leguicher A, Biophysical and biochemical alterations in erythrocyte membranes from chronic alcoholics. Scand J Clin Lab Invest 1991; 51:309–19.