Abstract
Background: Automatic CPAP devices have demonstrated good results in obtaining optimal fixed CPAP pressure to eliminate respiratory events in patients with sleep apnea-hypopnea syndrome (SAHS). However, automatic CPAP has not been fully studied in patients with COPD plus SAHS. Objectives: To analyse the performance of an automatic CPAP in severe COPD patients compared with SAHS patients with no associated co-morbidity. Methods: We compared 10 consecutive patients with SAHS and no associated co-morbidity and 10 patients with SAHS plus severe COPD who required CPAP titration. Automatic CPAP performance was studied during full-night PSG. Inadequate pressure increase periods, absence of pressure increases in reaction to respiratory events, air leak periods, and pressure behaviour in the face of erratic breathing periods were analysed. Results: The SAHS patients without co-morbidities vs. SAHS plus COPD patients presented: mean sleep efficiency, 80.2 (11.5)% vs. 76.5 (12.1)%; residual AHI, 6.3 (5.2) vs. 5.1 (7.7); residual CT90, 1 (3)% vs. 14 (1)%. The device´s performance demonstrates a mean of 1.2 (1.5) vs. 1.3 (1.2) periods of inadequate pressure increases; absence of pressure increases in reaction to respiratory events, 4.1 (5.4) vs. 0.6 (0.7) times; periods of air leaks, 1.3 (3.8) vs. 13.9 (11.7); mean optimal pressure, 9.1 (1.4) vs. 9.0 (1.9) cm H2O. Conclusion: Titration with automatic CPAP could be as effective in patients with SAHS plus severe COPD as in patients with SAHS without COPD. However, the presence of more leakages must be taken into account.
Keywords :
Introduction
Sleep apnea-hypopnea syndrome (SAHS) is characterized by sleepiness, neuropsychiatric, cardio-respiratory, inflammatory and metabolic disorders, secondary to periods of breathing cessation (apnea) and reduced breathing (hypopnea) during sleep. In Spain and the USA, the population of 30 to 70 years old, the prevalence of sleep-disorder breathing at apnea-hypopnea index (AHI) of ≥10 is 25–32% and the SAHS (AHI ≥10 plus excessive daytime sleepiness) prevalence is estimated in 3% to 3.4% (Citation1). Overnight full polysomnography (PSG) is the gold standard for diagnosis and treatment, although simplified alternative methods are very useful in a large percentage of patients and studies (Citation2–7).
Chronic obstructive pulmonary disease (COPD) is characterized by chronic and partially reversible airflow obstruction, associated with tobacco smoke. COPD is a progressive, preventable and treatable disease with systemic impact. Diagnosis is suspected when a patient has a history of smoking, cough, sputum production and dyspnea. It is confirmed by spirometry. Its prevalence, at 6–9%, is considered to be underestimated (Citation8).
COPD and sleep apnea are known to have harmful effects on the respiratory physiology of patients. The prevalence of sleep disorder breathing among patients with COPD is similar to that of the general population (22% and 25–29%, respectively), yet the prevalence of obstructive airway disease among patients with obstructive sleep apnea is even higher (29–40%) (Citation9,10). On their own, these diseases individually compromise gas exchange, oxygenation, and overall mortality and morbidity in affected patients. The combination has been shown to have an even more profound negative effect on patient's oxygenation, gas exchange, and breathing patterns (Citation11). Therefore, early recognition and prompt treatment of coexistent SAHS in patients with COPD is essential for reducing their morbidity and mortality (Citation12).
In a large multicentre study, the efficacy of an automatic CPAP device has been demonstrated as obtaining optimal fixed CPAP pressure to eliminate all respiratory events and normalize sleep architecture (Citation13). However, in this study almost 20% of patients were excluded because of notable associated co-morbidities, such as cancer, chronic pain, renal failure, and moderate or severe chronic obstructive pulmonary disease. Therefore, adequate selection of patients is very important to use the automatic CPAP for titration effectively and avoid a second titration by full PSG that would not be cost-effective.
As COPD and SAHS are very common diseases and as severe COPD patients may have intrinsic problems with respect to automatic titration we have analysed the performance of automatic CPAP in this group of patients. Our aim in this study was to analyse the performance of an automatic CPAP in severe COPD patients compared with SAHS patients with no associated co-morbidity. Specifically, we have analysed 1) whether inadequate pressure increase periods; 2) whether absence of pressure increases in reaction to respiratory events (apnea, hypopnea, snoring, periods of airflow limitation); 3) whether leaks occur 4) the behaviour of the device during erratic breathing periods when the patient is awake or during the arousals periods and, finally 5) the optimal pressure required to abolish the greater part of respiratory events.
Material and Methods
Patients
The study population consisted of 10 consecutive patients with SAHS without clinical evidence of pulmonary or cardiovascular co-morbidity associated and 10 patients with SAHS and severe COPD without clinical evidence of other disease who required CPAP titration, both groups paired in terms of demographic characteristics and in severity of SAHS and nocturnal desaturation.
Sleep study
Automatic CPAP performance was studied during full-night PSG in each patient. PSG was performed in the usual manner (Citation14). Briefly, the registered variables (Jaeger Sleep Lab 1000P, Jaeger, Warwick, UK) were electroencephalogram, chin electromyogram, electrooculogram and tibial electromyogram. Breathing variables were scored based on the flow tracing provided by a pneumotachograph connected to the nasal mask. Thoracic and abdominal bands measured thoracoabdominal motion. Oxygen saturation was recorded by a finger-pulse oximeter.
Sleep staging was performed manually, using the criteria of the American Academy of Sleep Medicine scoring rules (Citation14). Apnea was defined as the absence of airflow (≥90% reduction) for ≥10 s. Hypopnea was defined as a reduction in the amplitude of the airflow signal ≥50% of the basal of at least 10 s duration ending in an arousal and/or association with a ≥3% desaturation (Citation14).
Sleep architecture was considered normal when the stages N1 of non-rapid eye movement sleep (NREM 1 sleep) accounted for 5–10%, stages N2 (NREM 2 sleep) accounted for 40–50%, stages N3 (NREM 3 sleep) accounted for 20–25% and Stage R of rapid eye movement (REM) sleep accounted for 20–25%, along with more than 80% of sleep efficiency and 4 to 6 cycles (90–110 minutes each cycle) of sequence NREM and REM sleep.
Study design
Before the sleep study, patients were trained to use masks and the device during a 60-minute daytime period. The device used was Autoset-T, ResMed, Sydney, Australia. The pressure was set to start automatically after a 20-minute standby period (from 4 cm H2O up to a maximum of 16 cm H2O). The same model of nasal mask (Ultra Mirage; ResMed) was used for all the patients.
As mentioned, two groups were analysed. Group 1 comprised SAHS patients without clinical evidence of pulmonary or cardiovascular co-morbidity associated, were considered as SAHS patients with no co-morbidities and group 2, SAHS patients with severe COPD with no clinical evidence of other disease. Analyses of the results were performed in two different phases. 1) Classical PSG analysis for scoring the neurological variables and the respiratory variables followed the AASM criteria (Citation14). 2) The examination of the behaviour of the autoadjusted CPAP device was performed blind in both groups of patients, according to the following steps and criteria.
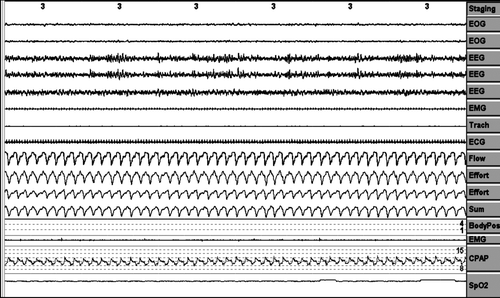
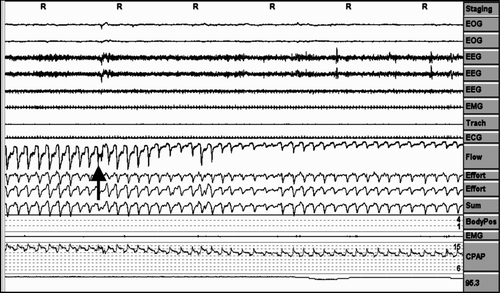
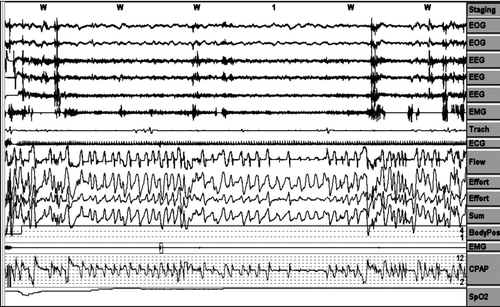
Analysis was performed on a 3-minute (6-epoch) screen for all the variables measured, as can be seen in , and every “period” mentioned below was considered as a event in a 3-minute screen over the entire night.
A period of pressure change was considered when the pressure changes ≥2 cm H2O. ().
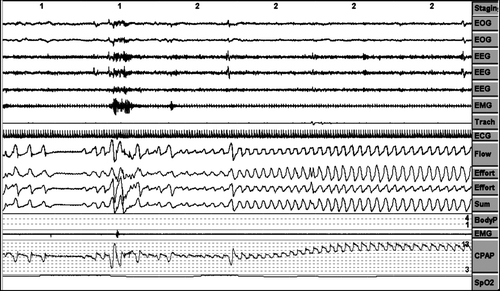
Inadequate pressure increases periods (increases in pressure without respiratory events). ().
Absence of pressure increases against respiratory events. ()
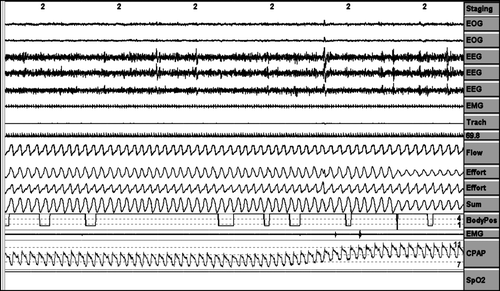
Air leaks periods were counted as episodes in which the pneumotachograph airflow signal was displaced out of the airflow channel on ≥1/3 of the 3-minute screen (at least 1 minute of continue air leakage). ()
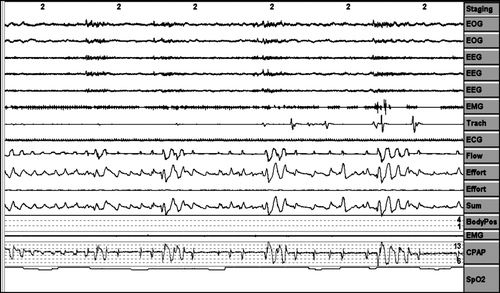
Pressure behaviour in the face of erratic breathing periods when the patient is awake. ()
The optimal pressure was determined visually on the raw data of the automatic CPAP device.(Citation13)
Data analysis
Parametric data were described as mean and standard deviation (SD) and were analysed with t-test and no parametric data were described as median and range and were analysed with the Wilcoxon signed-rank test. A p-value of <0.05 was considered to be statistically significance. All analyses were developed with the “Statistical Package for Social Sciences” (SPSS, 18. for Windows; SPSS Inc., Chicago, IL).
Results
The baseline data are shown in . Two groups were paired in terms of demographic and sleep characteristics: (Citation1) Ten patients with SAHS with no co-morbidity associated. Age, 60 (Citation12) years; male/female, 7/3; BMI 32.3 (4.3); AHI, 62.1 (34.4); CT90 (percentage of the night spent with a saturation less than 90%), 31.2 (18.6) and 2) 10 patients with SAHS and severe COPD without reported cardiovascular disease, age, 64 (Citation8) years; male/female, 10/0; BMI 32.1 (6.2); AHI 50.9 (37.5); CT90 28.5 (21.6); all with FEV1/FVC < 70% with a FEV1 38.4% (7.7).
Table 1. Baseline data of SAHS and SAHS plus COPD patients
In the group of SAHS patients with no associated co-morbidity (), the mean sleep efficiency was 80.2 (11.5) (N1, 9.2%; N2, 44.4%; N3, 18.0%; and REM, 28.1%). The arousal index was 12.4 (6.1). Residual AHI was a mean of 6.3 (5.2), and the CT90 of 1.3 (3.5)%. The performance of the device showed: 1) A mean of 1.2 (1.5) periods of inadequate pressure increases; 2) Absence of pressure increases in reaction to respiratory events was observed in a mean of 4.1 (5.4) times. 3) A mean of 1.3 (3.8) periods of air leaks. In this group of patients, 8 did not have any leak periods, 1 had 30 minutes of leaks and another 3 minutes. The device did not show any changes in pressure in the face of irregular breathing during periods of wakefulness. The mean optimal pressure required to abolish the greater part of a respiratory event was 9.1 (1.4) cm H2O.
Table 2. CPAP performance in patients with SAHS and no co-morbidity and in patients with SAHS and COPD
In the group of patients with SAHS plus COPD, the mean sleep efficiency was 76.5 (12.1)% (N1, 12.2%; N2, 47.3%; N3, 13.8%; and REM, 26.7%). The arousal index was 12.8 (5.9). Residual AHI presented a mean of 5.1 (7.7), and CT90 of 13.9 (1)%. The device's performance was: 1) Periods of inadequate increases in pressure, 1.3 (1.2) times in the overall study. 2) Periods of absence of pressure increases in reaction to respiratory events, 0.6 (0.7) times. 3) Air leaks, 13.9 (11.7) periods. In this group of patients, 2 did not have any leak period, one had three 3 of leaks, another had 18 minutes of leaks, another had 30 minutes of leaks and, finally, 5 had 73 minutes of leaks. The pressure in the face of irregular breathing during wakefulness periods was maintained without any changes. The mean optimal pressure required to abolish the greater part of the respiratory events was 9.0 (1.9) cm H2O.
When the CPAP performance was compared between groups, the device showed more residual CT90 and periods of air leaks in the group of SAHS plus COPD than in the group of SAHS with no co-morbidity [CT90 of 13.9 (31.6)% vs. CT90 of 1.3 (3.5)% (p < 0.05) and air leaks, 13.9 (11.7) periods vs. air leaks of 1.3 (3.8) periods (P = 0.005)]. The SAHS plus COPD group showed fewer periods of absence of pressure increases in reaction to respiratory events than the SAHS with no co-morbidity group [mean 4.1 (5.4) times vs. 0.6 (0.7) times, (p = 0.075)] however was not statistically significant. Sleep efficiency, arousal index, residual AHI, periods of inadequate pressure increases and final mean optimal pressure did not show any significant differences.
Discussion
In the latest update, the American Academy of Sleep Medicine (AASM) established that patients with significant lung disease such as chronic obstructive pulmonary disease are currently not considered candidates for automatic CPAP titration. This recommendation is based on the fact that most automatic CPAP studies excluded these patients because the sensors and algorithms identifying respiratory events may not be sensitive or specific in these cases. Areas for future research are recommended by the AASM at the end of this document, suggesting a need to clarify which patients would be suitable for automatic CPAP devices, paying particular attention to subjects with mild SAHS or co-morbidities (Citation15).
Consequently, the aim of this study was thus to analyse the performance of an automatic CPAP device in severe COPD patients, compared with SAHS patients with no cardiovascular or pulmonary co-morbidity associated, to evaluate whether the presence of severe co-morbidities such as COPD could result in a suboptimal titration with an automatic CPAP.
In this study, we observed no statistically significant differences in either the sleep efficiency or the arousal index of patients with SAHS, compared to SAHS plus COPD after titration with automatic CPAP. There were no differences between the two groups in the residual AHI, in the periods of inadequate pressure increases and in the optimal pressure. These results confirm the efficacy of an automatic CPAP device in the titration of SAHS with COPD.
However, we also found more air leak periods in the SAHS plus COPD group. This is a fact to keep in mind because no known studies in the literature that shows acute air leaks in the first night of titration with CPAP in patients with respiratory diseases. The presence of significant leakages in our severe COPD patients with SAHS could be related to the pathophysiology of COPD. The COPD is characterized by a non-reversible airway obstruction and hyperinflation and accordingly auto-positive end-expiratory pressure (Citation16). Thus, CPAP treatment could have an acute effect, increasing the auto-positive end-expiratory pressure (PEEP) and causing more leakage through the mouth in titration with Automatic CPAP.
Also, the abnormal pattern of breathing in these patients may contribute to the increase in the leak periods (Citation17). This effect has been described in noninvasive ventilation (Citation18). There is a reduction in the effective ventilation when a leak occurs that is compensated and minimised with most of the pressure preset devices. However, the subsequent prolongation of the inspiratory time in the presence of air leakage may lead to insufficient time for expiration, hyperinflation and trigger failure. These consequences can be especially harmful for COPD patients.
It has been well described the factors that can contribute to the development of leakages during CPAP treatment, and the permeability of the upper airway is one of the most important factors. Thus, it has been described as a cause of leaks in noninvasive ventilation, the presence of patient-ventilator asynchrony because of glotic closure and increase in the airway resistance (Citation19). This same mechanism of leaks could be involved in COPD due to the non-reversible obstruction of the airway that characterizes the disease.
In this study, 50% of patients with SAHS and COPD had more than 70 minutes of leaks during the sleep period, compared to the other group, in which only one patient showed 30 minutes of leaks. We can divide patients with SAHS and COPD into two groups: patients with severe leaks, defined as more than 30 minutes of leaks, and those with mild leaks (less than 30 minutes). In our study, COPD patients with severe leaks had a significantly increased residual CT90 in comparison with those who had mild leaks or no leaks (p = 0.02) that was not attributed to the severity of COPD (); furthermore, the residual AHI was also higher in COPD patients with severe leaks, compared to those with mild leaks, (p = 0.056), however, is not statistically significant.
Table 3. Mild in severe leaks in patients with SAHS without co-morbidity and in patients with SAHS and COPD
Six months after CPAP titration, two patients in the SAHS and COPD group required change of nasal interface to oronasal interface and of these, one patient did not use the CPAP for lack of compliance. One patient in the SAHS with no co-morbidity group required change of nasal interface to oronasal and did not use the CPAP for lack of compliance. The rest of patients in both groups showed good compliance and no required another titration neither changes on pressure. For this reason, and in the light of our results, we believe that the titration with automatic CPAP can be performed in SAHS and COPD patients. However, it is necessary to pay special attention to the possibility of leaks that can require changing the interface.
Our study has some limitations: first, the small number of patients analysed and, second, the absence of long-term monitoring to confirm the effective pressure of automatic CPAP, despite the presence of air leaks in the group of SAHS plus COPD.
In conclusion, titration with an automatic CPAP in patients with SAHS and severe COPD could be as effective as in patients without COPD. However, the presence of more leakages must be taken into account with respect to titration with automatic CPAP, in order to optimize results and improve compliance. Closer monitoring should probably be recommended in COPD patients, especially at the beginning of the CPAP, on the first night of CPAP titration.
Declaration of Interests
No financial or other potential conflicts exist in any of the authors with respect to the subject of this manuscript. The authors are responsible for the content and the writing of this paper.
References
- Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001 Mar; 163(3 Pt 1):685–9.
- Masa JF, Montserrat JM, Duran J. Diagnostic access for sleep apnea in Spain. Am J Respir Crit Care Med 2004 Jul 15; 170(2):195–6.
- Masa JF, Corral J, Pereira R, Duran-Cantolla J, Cabello M, Hernandez-Blasco L, Effectiveness of home respiratory polygraphy for the diagnosis of sleep apnoea and hypopnoea syndrome. Thorax 2011 Jul;66(7):567–73.
- Masa JF, Corral J, Pereira R, Duran-Cantolla J, Cabello M, Hernandez-Blasco L, Therapeutic decision-making for sleep apnea and hypopnea syndrome using home respiratory polygraphy: A large multicentric study. Am J Respir Crit Care Med Oct 15, 184(8):964–71.
- Guerrero A, Embid C, Farre R, Navajas D, Masa JF, Duran J, Sleep breathing flow characteristics as a sign for the detection of wakefulness in patients with sleep apnea. Respiration 2010; 80(6):495–9.
- Hernandez L, Torrella M, Roger N, Llunell A, Ballester E, Quinto L, Management of sleep apnea: concordance between nonreference and reference centers. Chest 2007 Dec; 132(6):1853–7.
- Flemons WW, Littner MR, Rowley JA, Gay P, Anderson WM, Hudgel DW, Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest 2003 Oct; 124(4):1543–79.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001 Apr; 163(5):1256–76.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993 Apr 29; 328(17):1230–5.
- Sanders MH, Newman AB, Haggerty CL, Redline S, Lebowitz M, Samet J, Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med 2003 Jan 1; 167(1):7–14.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008 Feb 15; 5(2):237–41.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010 Aug 1; 182(3):325–31.
- Masa JF, Jimenez A, Duran J, Capote F, Monasterio C, Mayos M, Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med 2004 Dec 1; 170(11):1218–24.
- Iber C, Ancoli-Israel S, Cheeson A, Quan SF. The AASM manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. In: American Academy of Sleep Medicine, editor. 1st ed. Westchester, Illinois: 2007.
- Morgenthaler TI, Aurora RN, Brown T, Zak R, Alessi C, Boehlecke B, Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine Report. Sleep 2008 Jan 1; 31(1):141–7.
- Purro A, Appendini L, Patessio A, Zanaboni S, Gudjonsdottir M, Rossi A, Static intrinsic PEEP in COPD patients during spontaneous breathing. Am J Respir Crit Care Med 1998 Apr; 157(4 Pt 1):1044–50.
- Dellaca RL, Rotger M, Aliverti A, Navajas D, Pedotti A, Farre R. Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur Respir J 2006 May; 27(5):983–91.
- Highcock MP, Shneerson JM, Smith IE. Functional differences in bi-level pressure preset ventilators. Eur Respir J 2001 Feb; 17(2):268–73.
- Rabec CA, Reybet-Degat O, Bonniaud P, Fanton A, Camus P. [Leak monitoring in noninvasive ventilation]. Arch Bronconeumol 2004 Nov; 40(11):508–17.