Abstract
Chronic obstructive pulmonary disease (COPD) is a complex systemic disorder characterized by both local pulmonary and systemic inflammation. Many studies suggested that activation of circulating inflammatory cells and increased circulating levels of inflammatory cytokines occur in COPD. Interleukin (IL)-18 is a unique proinflammatory cytokine that mediates its effects by binding to the IL-18 receptor (IL-18R). In the present study, the expression of IL-18 in serum and IL-18R on peripheral blood T lymphocytes was analyzed. Enzyme-linked immunosorbent assay (ELISA) was used to determine the serum levels of IL-18 and interferon (IFN)-©, and high sensitivity C-reactive protein (hsCRP) were measured by chemiluminiscent immunoassay. Expression of IL-18R was examined using a three-color flow cytometry method. In total, 120 subjects were recruited including 32 nonsmokers, 30 current smokers and 58 stable COPD patients. Serum levels of IL-18 and hsCRP were significantly higher in stable COPD patients than those in nonsmokers and current smokers. A significant negative correlation existed between pulmonary function and serum level of IL-18 rather than hsCRP in stable COPD patients. The proportions of IL-18R〈-expressing T lymphocytes and CD8+ T lymphocytes were significantly higher in stable COPD patients than in nonsmokers and current smokers. The current study extended prior analyses by examining IL-18R expression in peripheral blood. The results suggested that IL-18/IL-18R system was active in peripheral blood of COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality throughout the world, which is expected to be the third leading cause of death by 2020 (Citation1). It is a condition characterized by an abnormal inflammatory response in the lung to noxious particles or gases (Citation2). Smoking is recognized as the largest risk factor for COPD, and considered to be the most pivotal factor resulted in the airway inflammation so far (Citation3). This specific inflammation pattern is involved in increased numbers of CD8+ T cells, alveolar macrophages and neutrophils in the lungs of COPD patients, and lymphocyte infiltration with enhanced accumulation of CD8+ T cells is a prominent finding (Citation4,5). These activated inflammatory cells can release various mediators, including T-help cell (Th) types 1 and 2 cytokines. The chronic inflammation of COPD is believed to result in progressive respiratory disorders and airflow limitation.
Although COPD affects the lungs, it also produces significant systemic consequences. There is increasing evidence of extrapulmonary effects in patients with COPD. Previous studies have shown that circulating markers of systemic inflammation, such as C-reactive protein (CRP), are elevated in patients with stable COPD and further increase during COPD exacerbations (Citation6), which may be a key link to most COPD-related comorbidities or systemic consequences. However, previous studies showed there was no association between pulmonary function test measurements (such as forced expiratory volume in the first second) and CRP levels in COPD patients (Citation7).
Interleukin-18 (IL-18) was described in 1995 as interferon-γ (IFN-γ) inducing factor and shown to be a member of the IL-1 cytokine superfamily (Citation8–10). It is a proinflammatory cytokine produced intracellularly from a biologically inactivated precursor, pro-IL-18, and the mature IL-18 is secreted after cleavage of pro-IL-18 by caspase-1. Pro- and mature IL-18 can be produced in a wide range of cells (Citation11–12). It is a very unique cytokine functioning at the interface of innate and acquired immunity that regulates both Th1 and Th2 immune responses (Citation13–15). IL-18 mediates its effects by binding to the receptor (IL-18R) consists of a ligand binding α subunit (IL-18Rα) and a signaling β subunit (IL-18Rβ), both of which belong to the IL-1R family (Citation16,17). IL-18Rα plays a key role in exerting responsiveness to IL-18 because IL-18Rα-deficient mice fail to respond to IL-18 (Citation16).
IL-18R signaling has also been demonstrated to play a critical role in the pathogenesis of cigarette smoke-induced inflammation and emphysema in a murine modeling system (Citation18). IL-18 proteins were significantly increasedly expressed in alveolar macrophages and CD8+ T cells in the lungs of COPD patients (Citation19). They both also reported that patients with COPD had elevated levels of IL-18 in the serum (Citation18,19). Recently, Freeman and coworkers used flow cytometry to analyze the expression of IL-18R on human lung CD8+ T lymphocytes from COPD patients and found that IL-18R expression showed a significant correlation with disease severity (Citation20). However, the expression of IL-18R on T lymphocytes in peripheral blood in COPD has not been studied yet.
In the present study, we analzsed the expression of IL-18 in the sera of COPD patients in order to evaluate its relationship with pulmonary function. We also examined the expression of IL-18R on T lymphocytes in peripheral blood of patients with COPD.
Materials and Methods
Study subjects
A total of 58 COPD patients were recruited prospectively into the study from the out-patient department of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, between 2010 and 2011. All COPD patients were diagnosed on the basis of clinical history, physical examination, chest radiograph and pulmonary function tests in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) clinical criteria for the diagnosis and severity of COPD (Citation21). Inclusion criteria were: male aged from 40 to 75 years and cigarette smoking history of at least 20 pack-years (either current smoker or former smoker status is allowable). Exclusion criteria were: chronic lung conditions, such as asthma, bronchiectasis and interstitial lung diseases; cardiac, hepatic and renal failure; active tuberculosis or malignant tumor; and current oral steroid therapy.
All patients with COPD were examined in stable condition. Lung diseases such as sarcoidosis and infectious diseases were carefully excluded in control subjects who were recruited from the health screening center of our hospital. Ex-smokers were carefully excluded from the group of nonsmokers. The study was approved by the hospital ethics committees, and all subjects gave written informed consent.
Pulmonary function tests
Forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were obtained from the flow-volume curve using an appropriately calibrated spirometer (Jaeger, Wurzburg, Germany) before and 20 minutes after β-agonist (salbutamol 200 mcg) inhalation. Three technically acceptable measurements were performed on each patient, and the highest value was selected and expressed as a percentage of reference values. The predicted FEV1 was calculated using the following prediction equations and adjusted using the race/ethnic adjustment factor of 0.94 recommended by the ATS/ERS Task Force 2005 (Citation22) (Predicted FEV1 = 4.30×height in meters-0.029×age-2.49).
Sample collection
In all subjects, peripheral venous blood samples from the antecubital vein were collected in the morning into two test tubes, each 2 mL. One was anticoagulated with Ethylenediaminetetra-aceticacid (EDTA) and used for flow cytometry analysis immediately. The other was not anticoagulated, serum was separated from blood cells by centrifugation at 4000 cycles/min for 10 minnutes and stored at -70°C until analyzed.
Biochemical analysis
Serum IL-18 and IFN-γ levels were measured with commercially available ELISA kits (supplied by Medical and Biological Laboratories Co. Nagoya, Japan for IL-18 and eBioscience Bender MedSystems GmbH. Vienna, Austria for IFN-γ). Serum high sensitivity CRP (hsCRP) levels were measured by chemiluminiscent immunoassay (Tina-Quant, Roche Diagnostics GmbH. Mannheim, Germany). The analytical sensitivity of this CRP assay is 0.1 mg/L.
Flow cytometric analysis
Expression of IL-18Rα was examined using a three-color flow cytometry method. Briefly, 1) aliquot 100 μl of whole blood to each tube. 2) Add three labeled antibodies (20 μl of phycoerythrin(PE)-labeled anti-IL-18Rα monoclonal antibody (mAb) (eBioscience, Cat. No. 12–7183), 5 μl of Peridinin Chlorophyll Protein Complex (PerCP)-labeled mAb against CD3 and 3 μl of Allophycocyanin(APC)-labeled mAb against CD8 (Becton Dickinson, Mountain View, CA)) to cells and pulse vortex gently to mix. 3) Incubate for 30 minutes in the dark. 4) Add 2 mL of room temperature 1× Red Blood Cell (RBC) Lysis Buffer (eBioscience) to each tube and gently pipet up and pulse vortex briefly. 5) Incubate in the dark at room temperature for 10 minutes. 6) Centrifuge samples at 1200 cycles/min for 5 minutes, discard supernatant. 7) Wash the cells twice with Flow Cytometry Staining Buffer (eBioscience). 8) Resuspend stained cells in Flow Cytometry Staining Buffer. 9) Acquire data on the flow cytometer. Immunofluorescence analysis was performed on a FACS-Calibur (Becton Dickinson).
Statistical analysis
Results were expressed as mean±SEM. Data that were normally distributed were assessed for significance by Student's t-test or ANOVA as appropriate. Data that were not normally distributed were assessed for significance using the Mann-Whitney U-test or the Kruskal-Wallis test with Dunn's posttest for multiple comparisons as appropriate. Correlations were analyzed by simple regression. Statistical analysis was performed using Prism version 5 (GraphPad). A two-sided p-value < 0.05 was considered to be statistically significant. In addition, PROC GLM with Tukey's method for multiple comparisons was also employed to contrast the groups using SAS 9.2. Regression analysis was used to examine the relationship of age and inhaled corticosteroids (ICS) use to the relationship between FEV1%predicted and expression of various markers.
Results
Subjects characteristics
The characteristics of subjects are presented in . In total, 120 subjects were recruited to the study including 32 nonsmokers and 30 current smokers. Fifty-eight patients with COPD were clinically stable, of which 14 patients were classified as stage I, 15 patients were stage II, 16 patients were stage III and 13 patients were stage IV COPD. Twenty-one patients had stopped smoking 1–20 yrs previously (mean 7.3±1.2 yrs). Only 16 patients had received bronchodilators and/or ICS.
Table 1. Clinical characteristics of subjects in this study
Serum levels of IL-18, IFN-ã and hsCRP
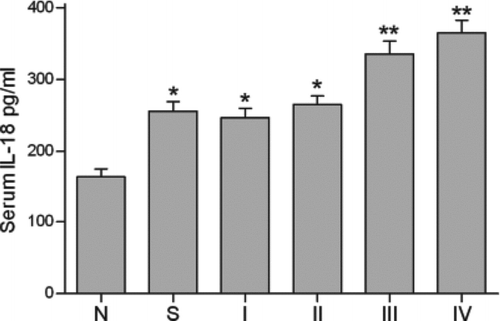
The serum levels of IL-18, IFN-γ and hsCRP are presented in . Serum IL-18 levels in stable COPD patients were significantly higher than those in current smokers (p<0.05). They were significantly higher in current smokers compared with nonsmokers (p<0.05). Next, the 58 stable COPD patients were categorised according to the GOLD classification of severity of COPD. Serum IL-18 levels in GOLD stage I (n = 14), II (n = 15), III(n = 16) and IV(n = 13) stable COPD patients were 246.7 ± 13.1, 264.6 ± 12.2, 335.5 ± 17.7, and 364.6 ± 18.1 pg/mL, respectively.
Table 2. Serum levels of interleukin (IL)-18, interferon (IFN)-γ and high sensitivity C-reactive protein (hsCRP) in subjects
Serum levels of IL-18 in GOLD stage III and IV were significantly higher than those in stage II, stage I, smokers and nonsmokers (). Serum levels of hsCRP were significantly higher in stable COPD patients than those in current smokers and nonsmokers (p<0.05). There were no significant differences in serum hsCRP levels between current smokers and nonsmokers. Serum levels of IFN-γ were not significantly increased in stable COPD patients or in current smokers. Statistically similar results were obtained after adjustment for age.
Figure 1. Serum IL-18 levels in nonsmokers (N) (n = 32), current smokers (S) (n = 30) and stable chronic obstructive pulmonary disease (COPD) patients classified according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage I (n = 14), II (n = 15), III (n = 16) and IV (n = 13). *: p<0.05 versus nonsmokers; **: p<0.05 versus current smokers, stable patients with GOLD stage I and II COPD.
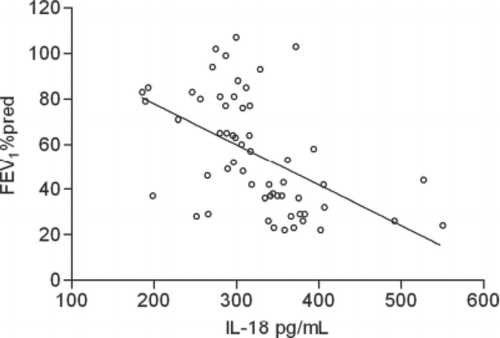
Relationship between serum levels of IL-18 and FEV1% pred
The correlation between serum IL-18 levels and pulmonary function was analyzed in stable COPD patients. There was a significant negative correlation between serum IL-18 levels and FEV1% pred (r = 0.5108; ). The strength of the correlation did not vary when adjusted for age and ICS use. In addition, we also analyzed the correlation between serum hsCRP levels and pulmonary function in stable COPD patients. There was no significant correlation between serum hsCRP levels and FEV1% pred. Statistically similar results were obtained when adjusted for age and ICS use.
Expression of IL-18R on T lymphocytes in peripheral blood
The proportions of IL-18R-expressing T lymphocytes and CD8+ T lymphocytes in peripheral blood were measured by flow cytometry. show the flow cytometric analysis of IL-18Rα-expressing on T lymphocytes and CD8+ T lymphocytes in a nonsmoker, current smoker and stable COPD patients. In a nonsmoker, 49.9% of T lymphocytes and 50.9% of CD8+ T lymphocytes were positive for IL-18α expression.
Figure 3. A representative three-color flow cytometric analysis of IL-18Rα expression on CD3+ cells (T lymphocytes) and CD3+CD8+ cells (CD8+ T lymphocytes) in a nonsmoker. The number in each panel indicates a percentage of IL-18Rα-positive cells gated in CD3+ cells or CD3+CD8+ cells.
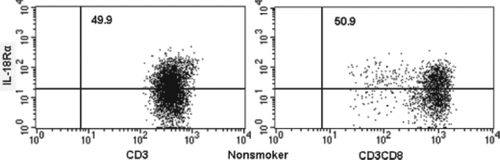
Figure 4. A representative three-color flow cytometric analysis of IL-18Rα expression on CD3+ cells (T lymphocytes) and CD3+CD8+ cells (CD8+ T lymphocytes) in a current smoker. The number in each panel indicates a percentage of IL-18Rα-positive cells gated in CD3+ cells or CD3+CD8+ cells.
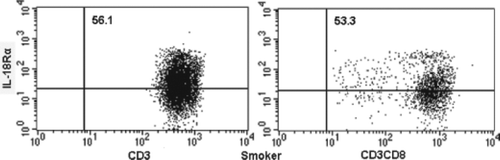
Figure 5. A representative three-color flow cytometric analysis of IL-18Rα expression on CD3+ cells (T lymphocytes) and CD3+CD8+ cells (CD8+ T lymphocytes) in a stable chronic obstructive pulmonary disease patient (COPD). The number in each panel indicates a percentage of IL-18Rα-positive cells gated in CD3+ cells or CD3+CD8+ cells.
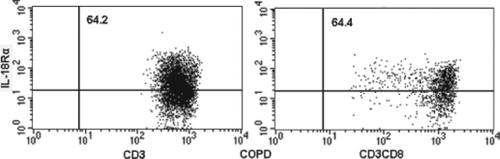
In a current smoker, 56.1% of T lymphocytes and 53.3% of CD8+ T lymphocytes were positive. In a stable COPD patient, 64.2% of T lymphocytes and 64.4% of CD8+ T lymphocytes were positive. The mean expression of IL-18Rα on T lymphocytes and CD8+ T lymphocytes for these 4 groups is shown in and . IL-18Rα on T lymphocytes and CD8+ T lymphocytes was 49.6%±1.6% and 51.4%±2.1%, respectively, in nonsmokers, 54.3%±1.9% and 51.6%±2.3%, respectively, in current smokers and 60.5%±1.6% and 59.3%±1.8%, respectively, in stable COPD patients.
Figure 6. Expression of IL-18Rα on peripheral CD3+ lymphocytes (T lymphocytes). Percentages of IL-18Rα-positive T lymphocytes in flow cytometric analysis are indicated in nonsmokers (N), current smokers (S) and stable chronic obstructive pulmonary disease patients (C). *: p<0.05 versus nonsmokers and current smokers.
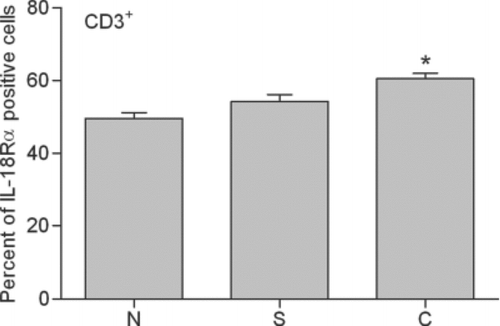
Figure 7. Expression of IL-18Rα on peripheral CD3+CD8+ lymphocytes (CD8+ T lymphocytes). Percentages of IL-18Rα-positive CD8+ T lymphocytes in flow cytometric analysis are indicated in nonsmokers (N), current smokers (S) and stable chronic obstructive pulmonary disease patients (C). *: p<0.05 versus nonsmokers and current smokers.
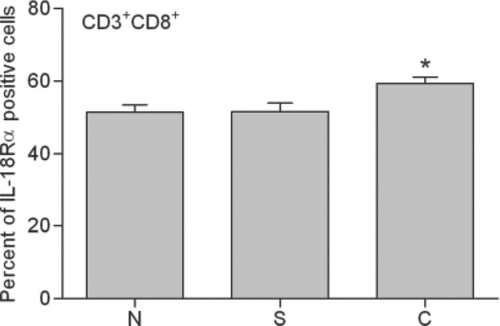
Table 3. The proportion of interleukin (IL)-18R-expressing T lymphocytes and CD8+ T lymphocytes in subjects
Stable COPD patients contained significantly higher proportions of IL-18Rα-expressing T lymphocytes and CD8+ T lymphocytes compared with current smokers and nonsmokers (p<0.05). There were no significant differences between current smokers and nonsmokers. No significant correlation was found between the proportion of IL-18Rα-expressing T lymphocytes or CD8+ T lymphocytes and pulmonary function in stable COPD patients. Statistically similar results were obtained after adjustment for age and ICS use.
Discussion
COPD is an insidious, highly heterogeneous condition that primarily affects the lungs, and also is associated with significant systemic inflammation (Citation23). Many studies investigating systemic manifestation of COPD suggested that activation of circulating inflammatory cells and increased circulating levels of inflammatory cytokines and acute phase proteins occurred in stable disease (Citation24–26).
A previous study reported that cigarette smoke induced IL-18 production in the lungs of mice and that serum levels of IL-18 were increased in COPD patients (Citation18). In the present study, we found that the levels of IL-18 were significantly greater in the sera of patients with GOLD stage III and IV COPD than in current smokers or nonsmokers. A significant correlation was also found between circulating level of IL-18 and pulmonary function in stable COPD patients. This finding was in line with that reported by Imaoka (Citation19).
However, Freeman and coworkers found no significant relationship between IL-18 protein expression in the lung and lung function (Citation20). This likely reflects differences between lung and peripheral IL-18 expression. Previous studies suggested that serum CRP levels were elevated in patients with stable COPD (Citation6). The present study showed that serum levels of hsCRP in stable COPD patients were significantly higher than those in current smokers and nonsmokers. However, in our study, we found that there was no significant correlation between serum hsCRP levels and pulmonary function in stable patients. This was inconsistent with a previous report that post-bronchodilator FEV1 was inversely related to serum concentrations of CRP (Citation27). A meta-analysis suggested that there were no statistically significant differences in serum CRP concentrations between healthy subject groups and any of the COPD stages (Citation28). In this respect, IL-18 in the sera is more relevant than hsCRP to disease severity in COPD.
Several studies of COPD have reported changes in various inflammatory cells including lymphocytes and neutrophils in peripheral blood. de Jong and coworkers reported that the percentage of CD8+ cells in peripheral blood was significantly higher in subjects with COPD compared with control subjects (Citation29). It has been recently reported that the expression of IL-18R on human lung CD8+ T lymphocytes from COPD patients was correlated with disease severity (Citation20).
However the expression of IL-18R on T lymphocytes in peripheral blood has not been studied yet. In the present study, we found that stable COPD patients contained significantly higher proportions of IL-18Rα-expressing T lymphocytes and CD8+ T lymphocytes compared with current smokers and nonsmokers. Unfortunately, the results showed no significant correlation between the proportion of IL-18Rα-expressing T lymphocytes or CD8+ T lymphocytes and pulmonary function in stable COPD patients, even when adjusted for age and ICS use.
Previous studies suggested that cigarette smoke was a potent stimulator of IL-18 in the murine lung and this stimulation was associated with IL-18 activation. The inflammation and emphysema were mediated via mechanisms that involved IL-18Rα, and cigarette smoke induction of apoptosis and stimulation of caspases, proteases and chemokines were mediated by IL-18Rα-dependent pathways (Citation18). In the present study, we found that both serum levels of IL-18 and the expression of IL-18Rα on peripheral blood T lymphocytes and CD8+ T lymphocytes were elevated in stable COPD patients. These results suggested that IL-18/IL-18R system in peripheral blood was active in COPD. However, further investigation is needed to verify the hypothesis that IL-18/IL-18R system in peripheral blood is involved in the pathogenesis of systemic manifestation of COPD.
In conclusion, serum IL-18 level was inversely related to pulmonary function in stable COPD patients. IL-18R-expressing T lymphoctyes accumulated in peripheral blood of COPD patients. IL-18/IL-18R system was active in peripheral blood of patients. However, whether IL-18/IL-18R system in peripheral blood is involved in the pathogenesis of systemic manifestation of COPD remains uncertain. Further studies are needed to verify this hypothesis.
Declaration of interests:
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
This study was supported in part by research grant No. 2007353 from the Clinical Speciality Key Program for Hospitals Affiliated to the Ministry of Health of China, No. 201002008 from the Health Public Service Sectors Research Special, and No. 30900648 from the National Natural Science Foundation of China.
References
- Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997; 349:1498–504.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–55.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–20.
- Saetta M, Di Stefano A, Maestrelli P, Activated T lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitis. Am Rev Respir Dis 1993; 147:301–6.
- Saetta M, Baraldo S, Corbino L, CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160:711–17.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59:574–80.
- Pinto-Plata VM, Müllerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, Celli BR. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006; 61:23–8.
- Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol 2003; 73:213–24.
- McInnes IB, Liew FY, Gracie JA. Interleukin-18: a therapeutic target in rheumatoid arthritis? Arthritis Res Ther 2005; 7:38–41.
- Reddy P. Interleukin-18: recent advances. Curr Opin Hematol 2004; 11:405–10.
- Dinarello CA. IL-18: a Th1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol 1999; 103:11–24.
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 2001; 19:423–74.
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995; 378:88–91.
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 2001; 19:423–74.
- Dinarello CA. Interleukin-18, a proinflammatory cytokine. Eur Cytokine Netw 2000; 11:483–6.
- Hoshino K, Tsutsui H, Kawai T, Takeda K, Nakanishi K, Takeda Y, Akira S. Cutting edge: generation of IL-18 receptor-deficient mice: evidence for IL-1 receptor-related protein as an essential IL-18 binding receptor. J Immunol 1999; 162:5041–4.
- Debets R, Timans JC, Churakowa T, Zurawski S, de Waal Malefyt R, Moore KW, IL-18 receptors, their role in ligand binding and function: anti-IL-1RAcPL antibody, a potent antagonist of IL-18. J Immunol 2000; 165:4950–6.
- Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol 2007; 178:1948–59.
- Imaoka H, Hoshino T, Takei S, Kinoshita T, Okamoto M, Kawayama T, Interleukin-18 production and pulmonary function in COPD. Eur Respir J 2008; 31:287–97.
- Freeman CM, Han MK, Martinez FJ, Murray S, Liu LX, Chensue SW, Cytotoxic potential of lung CD8+ T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J Immunol 2010; 184:6504–13.
- GOLD. Global initiative for chronic obstructive lung disease (GOLD): report of the GOLD workshop, global strategy for diagnosis, management and prevention of COPD. Updated 2009. http: //www.goldcopd.org Accessed: 15 March 2010.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–68.
- Wouters EF. Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2:26–33.
- Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: Data from the third national health and nutrition examination. Am J Med 2003; 114:758–62.
- Dahl M, Tybjaerg-Hansen A, Vestbo J, Lange P, Nordestgaard BG. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164:1008–11.
- Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996; 154:1055–60.
- Garcia-Rio F, Miravitlles M, Soriano JB, Systemic inflammation in chronic obstructive pulmonary disease: a population-based study. Respir Res 2010; 11:63.
- Franciosi LG, Page CP, Celli BR, Markers of disease severity in chronic obstructive pulmonary disease. Pulm Pharmacol Therap 2006; 19:189–99.
- de Jong JW, van der Belt-Gritter B, Koeter GH, Postma DS. Peripheral blood lymphocyte cell subsets in subjects with chronic obstructive pulmonary disease: association with smoking, IgE and lung function. Respir Med 1997; 232;91:67–76.