Abstract
Pharmacological lung volume reduction in COPD is an important goal in treatment with long-acting bronchodilators because in addition to airflow limitation, lung hyperinflation considerably affects COPD symptoms. Quantitative computed tomography (CT) simultaneously provides structural information about airway dimensions, emphysematous changes, and lung volumes, some of which are difficult to be evaluated by pulmonary function. Here, we evaluated changes in CT parameters and pulmonary function in 30 patients with COPD who underwent CT scans before and one year after starting tiotropium treatment and in 12 patients with COPD who were not treated with long-acting bronchodilators. Baseline pulmonary function and CT parameters did not differ between the two groups. One-year tiotropium therapy improved physiological-indices including residual volume (RV) and ratio of RV to total lung capacity (RV/TLC) (−235 mL, p = 0.005, and −2.9%, p = 0.0001, respectively), and CT-indices including wall area percent (WA%) and inner luminal area in right upper lobe apical and lower lobe basal segmental bronchi (−1.59%, p = 0.01, 2.27 mm2, p = 0.0005; and −1.33%, p = 0.0008, 3.42 mm2, p < 0.0001, respectively), low attenuation volume (LAV) and total lung volume (CT-TLV) (−92 mL, p = 0.0003, and −211 mL, p = 0.002, respectively). Changes in LAV, CT-TLV, RV, and RV/TLC were significantly greater in the tiotropium, than the non-bronchodilator group. The tiotropium-induced reduction in LAV correlated with the decrease in RV (ρ = 0.45, p = 0.01). Our findings not only indicate the value of the comprehensive CT measurements in assessing the effects of bronchodilators, including pharmacological lung volume reduction, but also further understanding of the structural changes underlying physiological improvements induced by bronchodilators.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by airflow limitation due to a mixture of airway narrowing and loss of lung elastic recoil caused by parenchymal destruction. Decreased lung elastic recoil and worsened expiratory airflow increase air trapping and lung hyperinflation (Citation1). Bronchodilators are typically used in the symptomatic management of COPD because they improve airway narrowing (Citation2), and then promote lung emptying leading to relief in lung hyperinflation, which is a major determinant of COPD symptoms (Citation3, 4).
Change in forced expiratory volume in 1 second (FEV1) is a gold standard to assess the bronchodilating effects. However, lung deflation assessed by physiological-indices such as inspiratory capacity (IC) and residual volume (RV) reflects improvement in patient-reported outcomes more sensitively than FEV1(Citation4, 5). Thus, in addition to the relief in airway narrowing, the pharmacological lung volume reduction should be an important goal in treatments with bronchodilators.
Quantitative computed tomography (CT) has been widely applied to explore the mechanism of disease progression (Citation6-8) and to clinically assess patients with COPD (Citation8–11). The dimensions of the proximal airway measured in CT images can predict the peripheral airway pathology (Citation12), and correlated with airflow limitation (Citation13) as well as clinical assessments such as respiratory symptoms (Citation14), exacerbation frequency, and a poor quality of life (Citation15). Percent low attenuation volume (LAV%), which is a standard index of emphysematous change, reflects pathological lung destruction (Citation16, 17) and lung hyperinflation (Citation13). The cumulative size distribution of low attenuation clusters follows a power law characterised by an exponent D, which is a sensitive marker of emphysema-related lung destruction and is less influenced by lung hyperinflation than LAV% (Citation6, Citation8, Citation18).
Moreover, quantitative CT provides information about lung volumes. Assessing lung volumes have been used for estimating inspiration levels during CT scans (Citation8, Citation14, Citation19), and evaluating volume reductions achieved by surgery and bronchial valve treatment (Citation20, 21). However, the pharmacological lung volume reduction induced by bronchodilators has not been evaluated by CT images. CT assessments can quantify not only the total lung volume but also volumes of lung regions damaged by emphysema-related parenchymal destruction, which cannot be evaluated by physiological assessments. Therefore, we postulated that comprehensive CT assessments including lung volumes as well as emphysematous change and airway lesions could provide additional information about the effect of bronchodilators.
Tiotropium is a an anti-cholinergic drug that is inhaled daily and can provide sustained improvements in airflow limitation, health-related quality of life and frequency of COPD exacerbations over the long term (Citation22). Hyperinflation assessed as pulmonary function (Citation3, Citation23, Citation24) and airway dimensions assessed by CT (Citation2) are also improved over the short term. However, the long-term effect on hyperinflation and airway dimensions is unclear. In addition, the influence of long-acting bronchodilators on CT parameters of emphysematous changes or lung volume has not been reported.
The present study explores whether CT can detect pharmacological lung volume reduction and improvement in airway diseases after one year of tiotropium therapy. Furthermore, we investigated the association between improved CT parameters and improved pulmonary function to clarify the structural changes underlying physiological improvements induced by bronchodilators.
Methods
Patients. his historical cohort study comprised subjects selected from our prospective observational study investigating COPD exacerbation (Citation25–27), based on a dataset of high resolution CT and pulmonary function tests performed at one-year intervals. From May 2006 to May 2009, we recruited 121 COPD patients who agreed to prospectively record exacerbations. Among them, 92 patients were classified as having moderate to very severe COPD (confirmed by a post-bronchodilator forced expiratory volume in 1 second [FEV1] ≤ 80% of the predicted value), and their regular treatment did not include tiotropium or a salmeterol/fluticasone combination (SFC). According to the GOLD guideline (Citation1), patients with moderate to very severe COPD were recommended to inhale long-acting bronchodilators. Of the 92 recruited patients with moderate to very severe COPD, 45 started tiotropium therapy after the first evaluation upon entry to the study.
Among them, 30 underwent high resolution CT scans and pulmonary function tests before and one year of tiotropium treatment. Thus, we assigned these 30 patients to the tiotropium group. On the other hand, 12 patients declined treatment with long-acting bronchodilators despite their physicians’ recommendations because they felt that their breathlessness was minimal or modest. Alternatively, all of them were treated with inhaled corticosteroid to prevent acute exacerbation. We assigned these 12 patients to the non-bronchodilator group.
Dyspnea was assessed by the modified Medical Research Council (mMRC) Dyspnea Scale. Any regular COPD medications were not changed in the two groups from 6 months before entry to the end of the one-year follow-up, except for the initiation of tiotropium treatment in the tiotropium group. Smoking status did not change in any of the patients during the one-year follow-up. The ethics committee of Kyoto University approved the study (approval No. E182), and all patients provided written informed consent prior to their participation.
Pulmonary function tests, CT acquisition, and the calibration of CT numbers
After inhaling short-acting bronchodilators, pulmonary function tests (Chestac-65V; Chest MI Corp.; Tokyo, Japan) and CT scans (Aquilion 64; Toshiba; Tokyo, Japan,slice thickness, 0.5 mm) were performed as described (Citation8, Citation10). Spirometry was also performed 6 months after patients’ entry into the study. Lung volumes and diffusion capacity were measured by helium dilation and the single-breath method, respectively. Inter-scanner variability was avoided by using one CT scanner, as in our previous longitudinal studies (Citation8, Citation28).
In addition to routine calibration using an air and water phantom, CT numbers in all images were corrected using tracheal air densities to eliminate any influence of an aging X-ray tube according to our previous studies (Citation8, Citation28). The tracheal lumen was firstly identified using a threshold of -800 Hounsfield units, and the luminal area (Atr) was measured. The mean radius (Rtr) of the lumen was calculated, and then the mean CT number (CTair) of the region within a circle of half mean radius (Rtr/2) from the gravity center of the lumen was calculated. Finally, the original CT numbers of all pixels for each patient were corrected using the formula: corrected CT number = (-1000 × original CT number)/CTair.
Evaluation of airway dimensions, emphysematous changes, and lung volumes
Airway dimensions of the apical segmental bronchus of right upper lobe and basal segmental bronchus of right lower lobe were measured using the full width at half-maximum principle (Citation13, Citation28). Airway wall thickness and inner luminal area were measured, and wall area percent (WA%) was then calculated. According to our previous findings (Citation8), we defined the threshold between low attenuation voxel and normal density voxel as -960 Hounsfield units and performed low attenuation cluster analysis. The cumulative frequency distribution of low attenuation cluster size, Y, can be described by a power law of the cluster size X with the formula Y = K · X−D. We calculated D, LAV%, CT-derived total lung volume (CT-TLV), and low attenuation volume (LAV) using all images obtained for the entire lung.
Statistical analysis. ata were statistically analyzed using JMP 7 software (SAS Institute, Cary, NC, USA). Data are expressed as medians (25th and 75th percentiles) unless otherwise indicated. Baseline and follow-up findings were compared using the Wilcoxon signed-rank test. Relationships between changes in pulmonary function and CT parameters were assessed by Spearman's rank correlation tests. A p-value < 0.05 was considered significant.
Results
Patient characteristics
Tables and show the baseline characteristics and CT parameters of the tiotropium group (n = 30) and non-bronchodilator group (n = 12). Age, height, weight, smoking status, FEV1, residual volume (RV), total lung capacity (TLC), ratio of RV to TLC (RV/TLC), inspiratory capacity (IC), ratio of inspiratory capacity IC to TLC (IC/TLC), diffusion capacity of carbon monoxide (DLCO), all CT parameters including WA%, LAV%, D, CT-TLV, and LAV, and dyspnea assessed as mMRC dyspnea scale did not significantly differ between the two groups.
Table 1. Baseline characteristics of patients
Table 2. Baseline CT parameters of patients
In the tiotropium group, 12 patients received a treatment with inhaled corticosteroid, and 8 received a treatment with long-acting ®2-agonist. All the patients in the non-bronchodilator group received a treatment with inhaled corticosteroid. Both groups were instructed for a rescue use of short-acting β2 and/or anti-cholinergic agonists. No patient was treated with SFC.
Longitudinal changes in pulmonary functions and CT parameters of the lung
Six months after tiotropium treatment, the FEV1 was significantly increased whereas the FEV1 in the non-bronchodilator group did not change at 6 months after the entry (). One year after tiotropium treatment, IC and IC/TLC were significantly increased, RV and RV/TLC were significantly decreased, and FEV1, TLC, and DLCO did not significantly differ. In the tiotropium group, significant decreases in WA% at the right apical and basal bronchus, CT-TLV, and LAV were found ().
Table 3. One-year changes in pulmonary function
Table 4. One-year changes in CT parameters
Tiotropium significantly increased inner luminal area both in the right apical and basal segmental bronchus (median changes; 2.27 mm2, p = 0.0005, and 3.42 mm2, p < 0.0001, respectively), but not airway wall thickness (median changes; -0.00 mm, p = 0.40, and 0.03 mm, p = 0.07, respectively). In the non-bronchodilator group, there were no significant changes in pulmonary function or CT parameters. Among the significantly changed factors in the tiotropium group, the changes in FEV1 6 months after entry, as well as RV, RV/TLC, CT-TLV, and LAV, one year after entry were greater than those in the non-bronchodilator group (Tables and ). These improvements were considered to be tiotropium-related improvements.
Among the tiotropium-related improvements in CT parameters and pulmonary functions, we found that the changes in LAV and RV were significantly correlated (ρ = 0.45, p = 0.01; ), and the changes in LAV tended to be correlated with those in RV/TLC (ρ = 0.35, p = 0.05), whereas the changes in CT-TLV did not correlate with any changes in lung function ().
Figure 1. Relationship between changes in residual volume (RV) and low attenuation volume (LAV) in the tiotropium-treated patients. Changes in RV and LAV (ρ = 0.45, p = 0.01) were significantly correlated.
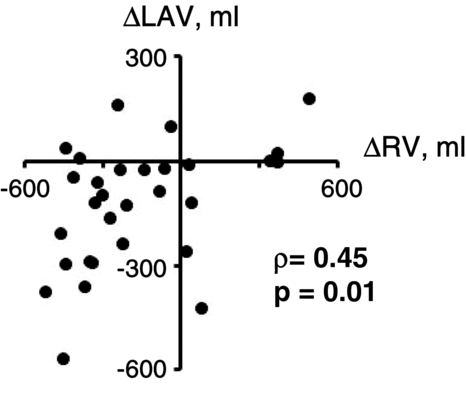
Table 5. Spearman's rank correlation coefficients between changes in pulmonary function and CT parameters 1 year after starting tiotropium treatment (n = 30)
Discussion
Measuring lung volume by CT, we found pharmacological lung volume reduction 1 year after the initiation of tiotropium treatment. Moreover, we showed a significant correlation between the reduction of LAV and RV. Although previous studies have shown that tiotropium improves hyperinflation assessed by IC, RV, slow vital capacity, functional residual capacity, RV and RV/TLC at rest and during exercise (Citation3, Citation23), to our knowledge, this is the first study to determine long-term improvements in lung hyperinflation using both physiological and structural means to evaluate volumes of both the whole lung and emphysematous lesions using CT indices.
Lung hyperinflation responds to bronchodilators more sensitively than FEV1(Citation4, 5), which the present results support. We detected improvements in hyperinflation assessed by RV and lung volume reduction measured by CT at one year after starting tiotropium therapy, even though FEV1 returned to the baseline value after temporary improvement at 6 months.
Quantitative CT is important for understanding the mechanism of disease progression (Citation6–8) and assessing treatment responses (Citation29, 30) because it can provide information about airway dimensions, emphysematous changes, and lung volumes (Citation8, Citation13, Citation16, 17). The D value (index of the low attenuation cluster analysis) can provide additional information, although LAV% is a standard index of emphysema (Citation6, Citation8, Citation18). As emphysema progresses, neighboring low attenuation clusters merge and become larger (Citation6, Citation31). The coalescence of clusters reflecting parenchymal destruction is required for a decrease in D.
We also previously demonstrated that COPD exacerbation is associated with progressive emphysema because changes in LAV% and D were greater in patients with exacerbations than those without exacerbations (Citation8). Our model simulation confirmed that when LAV% increases, D cannot be decreased without coalescence of the clusters. Moreover, the changes in CT-TLV independently correlated with those in LAV%, but not in D. Collectively, these findings indicate that compared with LAV%, a change in D is more specific for emphysema-related parenchymal destruction, and is less influenced by changes in lung volume, which can reflect inspiration levels during CT scanning as well as hyperinflation.
Other studies showing that the low attenuation cluster analysis can help to discriminate emphysematous change and air trapping in patients with COPD support this notion (Citation32), as well as in asthmatic smokers and non-smokers (Citation33). The D value in the present study did not significantly differ over our observational period. We speculated that hyperinflation, rather than parenchymal destruction, contributes to the changes in LAV and LAV%. Thus, the effects of inhaled bronchodilators on hyperinflation should be considered when interpreting CT values.
Although LAV% did not change during the observational period, LAV and CT-TLV were significantly decreased at 1 year after tiotropium treatment, and these changes significantly differed from those in the non-bronchodilator group in whom LAV and CT-TLV were not reduced. The reduction in LAV was significantly correlated with improvements in hyperinflation assessed by RV and tended to correlate with the decrease in RV/TLC.
Our findings are quite important because we discovered that a pharmacological reduction in lung volume can be determined using both physiological indices and CT measures. In previous studies, CT assessment of volume reduction has been performed only for surgical lung resection (Citation20) or bronchial valve treatment (Citation21). In addition, although LAV% is considered to be associated with hyperinflation (Citation34), we found that LAV is more sensitive to changes in hyperinflation than LAV%.
The WA% at the apical segmental bronchus of the right upper lobe and basal segmental bronchus of the right lower lobe significantly decreased in the tiotropium group. Moreover, the luminal areas increased and wall thickness did not change in both bronchi, indicating that the improvement in WA% reflects airway dilation, but not a decrease in wall thickness. These changes were detected together with lung deflation assessed as a decrease in CT-TLV, although previous studies using paired CT images at functional respiratory capacity and TLC showed that airway calibre can be increased with lung inflation in healthy individuals and in many patients with COPD (Citation35–37).
Several explanations could account for this finding. Firstly, the bronchodilatory effect of tiotropium could simply overcome the effect of the decreased lung volume. Second, since hyperinflation reduces airway calibre (Citation35, Citation37), the improved hyperinflation further promoted the increase in luminal areas in addition to the direct airway dilation. Indeed, the change in WA% at the basal segmental bronchus of the right lower lobe correlated with the change in RV/TLC (〉 = 0.40, p = 0.03), while that at the right apical segmental bronchus did not (ρ = 0.31, p = 0.09). Third, the increase in luminal areas with lung inflation can be diminished along with emphysema severity due to impaired airway-parenchymal interdependence, and lung inflation paradoxically reduces luminal areas in some patients with COPD (Citation35, Citation37). The tiotropium group in the present study might have included such patients.
We found that the changes in WA% were relatively small, and not significantly different from those in the non-bronchodilator group. This might have reflected the finding that the improvement in FEV1 at 6 months was lost one year after starting tiotropium. We speculated that tiotropium-induced improvements in airway dimensions promote lung emptying leading to pharmacological lung volume reduction, but the improvements in airway dimensions were diminished earlier than the decreases in lung volume.
We assessed total lung volume using TLC determined by helium dilution and CT-TLV. The CT-TLV decreased and TLC did not change in the tiotropium group, whereas neither of these indices changed in the non-bronchodilator group. Studies have shown that CT-TLV is similar to TLC derived from helium dilution, but smaller than that derived from plethysmography (Citation38, 39).
The discrepancy between changes in CT-TLV and helium dilution-derived TLC might have been simply due to differences in the sensitivity and accuracy of the two methods. However, considering that unlike CT-TLV, helium dilution-derived TLC might not include poorly ventilated regions (Citation40), the decrease in CT-TLV and the lack of change in the helium dilution-derived TLC suggests that tiotropium reduces pharmacological volume mainly in poorly ventilated areas. This should be investigated in future studies.
Smoking cessation can affect lung density (Citation41). However, no patient in the present study stopped or resumed smoking during follow-up. Our findings were not confounded by smoking status.
Several limitations are associated with this study. First, because the present study was not a clinical trial, a selection bias might have been introduced. To minimise the selection bias, we recruited subjects in this cohort from our previous prospective observational studies (Citation25–27) based on strict criteria. As the control, we selected patients who were not treated with any long-acting bronchodilators. This strategy enhances the validity to investigate the effect of tiotropium as a bronchodilator on CT parameters. Second, although patients were asked to fully inspire during CT scanning, inappropriate inspiration might have influenced our findings.
However, because all the subjects were instructed to hold their breath in a same way during the CT scanning, it is unlikely that only the patients treated with tiotropium held their breath inappropriately, leading to the CT-TLV reduction. Third, we assessed only proximal airways, although lesions of small airways comprise a major feature in COPD (Citation42). However, we postulated that the peripheral airway dimensions were changed in proportion to proximal airway dimensions because larger airway dimensions reflect peripheral airway pathology (Citation12) and short-term tiotropium treatment improved peripheral airway dimensions as well as proximal airway dimensions (Citation2). We believe that the influence of the location of the airways in which we measured WA% was quite small.
Conclusion
In conclusion, by using CT images, we could detect long-term pharmacological lung volume reduction induced by a bronchodilator even after the temporary improvement in FEV1 was lost. Our finding that tiotropium-induced improvement in hyperinflation is closely associated with the reduction of the lung volume of emphysema-related regions can deepen understanding of the structural changes underlying physiological improvements induced by bronchodilators. Quantitative CT can be a useful tool for assessing the effects of bronchodilators because it simultaneously provides structural changes in airway dimensions, emphysema, and lung volumes covering not only whole-lung but also emphysema-related region, some of which are difficult be determined by conventional physiological tests.
Declaration of Interest
Dr. Shigeo Muro and Dr. Michiaki Mishima received lecture fees and grant support from Boehringer Ingelheim. The other authors have no conflicts of interest to disclose. The authors are responsible for the content and the writing of this paper. This study was supported by Grants-in-Aid for scientific research (B) (No. 16390234) and (C) (No. 21590964) and a grant to the Respiratory Failure Research Group from the Ministry of Health, Labour and Welfare, Japan. We thank Koji Koizumi and Ryuzo Tanaka for excellent technical assistance.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of COPD. Revised 2011. htt p://www.goldcopd.com (accessed 6 February 2012).
- Hasegawa M, Makita H, Nasuhara Y, Odajima N, Nagai K, Ito Y, Relationship between improved airflow limitation and changes in airway calibre induced by inhaled anticholinergic agents in COPD. Thorax 2009 Apr;64(4):332–8.
- Celli B, ZuWallack R, Wang S, Kesten S. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest 2003 Nov;124(5):1743–8.
- Newton MF, O'Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest 2002 Apr;121(4):1042–50.
- O'Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999 Aug;160(2):542–9.
- Mishima M, Hirai T, Itoh H, Nakano Y, Sakai H, Muro S, Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 1999 Aug 3;96(16):8829–34.
- Bakker ME, Putter H, Stolk J, Shaker SB, Piitulainen E, Russi EW, Assessment of regional progression of pulmonary emphysema with CT densitometry. Chest 2008 Nov;134(5):931–7.
- Tanabe N, Muro S, Hirai T, Oguma T, Terada K, Marumo S, Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011 Jun 15;183(12):1653–9.
- Ogawa E, Nakano Y, Ohara T, Muro S, Hirai T, Sato S, Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax 2009 Jan;64(1):20–5.
- Ohara T, Hirai T, Muro S, Haruna A, Terada K, Kinose D, Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest 2008 Dec;134(6):1244–9.
- Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, CT scan findings of emphysema predict mortality in COPD. Chest 2010 Sep;138(3):635–40.
- Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005 Jan 15;171(2):142–6.
- Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000 Sep;162(3 Pt 1):1102–8.
- Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med 2010 Feb 15;181(4):353–9.
- Mair G, Maclay J, Miller JJ, McAllister D, Connell M, Murchison JT, Airway dimensions in COPD: relationships with clinical variables. Respir Med 2010 Nov;104(11):1683–90.
- Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988 Oct;94(4):782–7.
- Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995 Aug;152(2):653–7.
- Yuan R, Nagao T, Pare PD, Hogg JC, Sin DD, Elliott MW, Quantification of lung surface area using computed tomography. Respir Res 2010;11:153.
- Stoel BC, Putter H, Bakker ME, Dirksen A, Stockley RA, Piitulainen E, Volume correction in computed tomography densitometry for follow-up studies on pulmonary emphysema. Proc Am Thorac Soc 2008 Dec 15;5(9):919–24.
- Rogers RM, Coxson HO, Sciurba FC, Keenan RJ, Whittall KP, Hogg JC. Preoperative severity of emphysema predictive of improvement after lung volume reduction surgery: use of CT morphometry. Chest 2000 Nov;118(5):1240–7.
- Coxson HO, Nasute Fauerbach PV, Storness-Bliss C, Muller NL, Cogswell S, Dillard DH, Computed tomography assessment of lung volume changes after bronchial valve treatment. Eur Respir J 2008 Dec;32(6):1443–50.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008 Oct 9;359(15):1543–54.
- O'Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004 Jun;23(6):832–40.
- Verkindre C, Bart F, Aguilaniu B, Fortin F, Guerin JC, Le Merre C, The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration 2006;73(4):420–7.
- Terada K, Muro S, Ohara T, Kudo M, Ogawa E, Hoshino Y, Abnormal swallowing reflex and COPD exacerbations. Chest 2010 Feb;137(2):326–32.
- Terada K, Muro S, Ohara T, Haruna A, Marumo S, Kudo M, Cough-reflex sensitivity to inhaled capsaicin in COPD associated with increased exacerbation frequency. Respirology 2009 Nov;14(8):1151–5.
- Terada K, Muro S, Sato S, Ohara T, Haruna A, Marumo S, Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax 2008 Nov;63(11):951–5.
- Ohara T, Hirai T, Sato S, Terada K, Kinose D, Haruna A, Longitudinal study of airway dimensions in chronic obstructive pulmonary disease using computed tomography. Respirology 2008 May;13(3):372–8.
- Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006 Jun 15;173(12):1309–15.
- Dirksen A, Piitulainen E, Parr DG, Deng C, Wencker M, Shaker SB, Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J 2009 Jun;33(6):1345–53.
- Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med 2003 Sep 1;168(5):516–21.
- Gietema HA, Muller NL, Fauerbach PV, Sharma S, Edwards LD, Camp PG, Quantifying the extent of emphysema: factors associated with radiologists’ estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol 2011 Jun;18(6):661–71.
- Mitsunobu F, Ashida K, Hosaki Y, Tsugeno H, Okamoto M, Nishida K, Complexity of terminal airspace geometry assessed by computed tomography in asthma. Am J Respir Crit Care Med 2003 Feb 1;167(3):411–7.
- Nakano Y, Sakai H, Muro S, Hirai T, Oku Y, Nishimura K, Comparison of low attenuation areas on computed tomographic scans between inner and outer segments of the lung in patients with chronic obstructive pulmonary disease: incidence and contribution to lung function. Thorax 1999 May;54(5):384–9.
- Scichilone N, La Sala A, Bellia M, Fallano K, Togias A, Brown RH, The airway response to deep inspirations decreases with COPD severity and is associated with airway distensibility assessed by computed tomography. J Appl Physiol 2008 Sep;105(3):832–8.
- Bakker ME, Stolk J, Reiber JH, Stoel BC. Influence of inspiration level on bronchial lumen measurements with computed tomography. Respir Med 2012 May;106(5):677–86.
- Diaz AA, Come CE, Ross JC, San Jose Estepar R, Han MK, Loring SH, Association between airway caliber changes with lung inflation and emphysema assessed by volumetric CT In subjects with COPD. Chest 2012 Mar;141(3):736–744.
- O'Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD? Chest 2010 May;137(5):1108–15.
- Parr DG, Sevenoaks M, Deng C, Stoel BC, Stockley RA. Detection of emphysema progression in alpha 1-antitrypsin deficiency using CT densitometry; methodological advances. Respir Res 2008;9:21.
- Rodenstein DO, Stanescu DC. Reassessment of lung volume measurement by helium dilution and by body plethysmography in chronic air-flow obstruction. Am Rev Respir Dis 1982 Dec;126(6):1040–4.
- Ashraf H, Lo P, Shaker SB, de Bruijne M, Dirksen A, Tonnesen P, Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax 2011 Jan;66(1):55–60.
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004 Jun 24;350(26):2645–53.
