Abstract
Background and Aim: Under-diagnosis of COPD is a widespread problem. This study aimed to identify previously undiagnosed cases of COPD in a high-risk population identified through general practice. Methods: Participating GPs (n = 241) recruited subjects with no previous diagnosis of lung disease, >35 yrs, and at least one respiratory symptom. Age, smoking status, pack-years, BMI, dyspnoea score (MRC), and pre-bronchodilator spirometry data was obtained. Subjects with airway obstruction (FEV1/FVC ≤ 0.7) at initial spirometry were tested for reversibility, according to Danish COPD guidelines, with bronchodilator and, if necessary, corticosteroids in order to confirm a diagnosis of COPD. Results: A total of 4.049 (49% females) subjects were included; mean age 58 yrs, BMI 27, and 32 pack-years. The COPD prevalence was 21.7%; 8.3% in subjects younger than 48 years. Most patients were classified in GOLD stages I and II (36% and 50%, respectively). The number needed to screen (NNS) for a new diagnosis of COPD was 4.6. COPD diagnosis was related to gender, age, BMI (p < 0.001), pack-years, and cough (p < 0.001), wheezing (p < 0.001) and sputum production (p = 0.002). A threshold of 10% pre-test risk of COPD would have reduced the number of spirometry tests by 35% although 90% of the patients with COPD would still have been identified (NNS 3.9). Conclusions: Of the at-risk subjects studied, 22% were diagnosed with COPD. A case-finding strategy providing questionnaire assessment and diagnostic spirometry to high-risk subjects in primary care, and therefore, identifies a large proportion of undiagnosed COPD patients, especially in the early stages of the disease.
Introduction
Chronic obstructive pulmonary disease (COPD) represents a significant burden not only for the health care systems worldwide but also for the individual patient and their relatives (Citation1,2). COPD is characterised by airflow limitation that is not fully reversible (Citation1,2). The development of COPD in the individual patient depends on exposure to noxious particles and gases, primarily tobacco smoke (Citation3,4). A clinical diagnosis of COPD should be considered in any individual who has dyspnoea, chronic cough or sputum production and/or a history of exposure to risk factors for the disease. Spirometry is essential for the definite diagnosis of the disease (Citation1,2). However, many patients with COPD remain undiagnosed and unknown to the health care system at least until the more advanced stages of the disease (Citation5).
Delayed diagnosis of COPD may have significant impact on patients’ quality of life, not least due to symptoms and physical limitations; and suffering from impaired quality of life could in most cases be alleviated by treatment (Citation6). Smoking cessation is the most effective intervention, which delays the accelerated decline in lung function, and early diagnosis of COPD is therefore of utmost importance in current smokers (Citation7). In line with this, there is accumulating evidence that early intervention with medication may improve the natural history of the disease, i.e., the annual decline of lung function (Citation6,Citation8); and, in line with this, there is evidence that identification of COPD in smokers increase the success rate of smoking cessation (Citation9,10). The available evidence suggests that early diagnosis of COPD through spirometry may provide important opportunities for curbing the progressive nature of this disease.
Different approaches have been implemented in different countries for the early identification of COPD, including open spirometry programmes. However, in Denmark, as in many other countries, general practitioners and family physicians (GPs) are the primary health care providers for patients suffering from COPD. Developing, and subsequently implementing, an efficient primary care based programme for the early detection of COPD in Denmark, where >90% of the GPs have direct access to spirometry, may have major implications for the future burden of COPD.
The Danish National Board of Health recommends, in keeping with the International Primary Care Respiratory Group and the Danish Society for General Practice, case identification spirometry in all individuals over 35 years who present with at least one respiratory symptom and risk factors, such as prior or current smoking, or relevant occupational exposure (Citation11,12,Citation13) in order to facilitate early detection and management of COPD.
Thus, the aim of the present study was to evaluate the prevalence and severity of COPD by implementing a case-finding strategy providing spirometry, including diagnostic spirometry, to high-risk subjects identified through general practice using a non-interventional study design.
Material and Methods
On a voluntary basis, the aim was to engage at least 200 GPs (>5% of the Danish GPs) in the study from all over Denmark in order to ensure a representative sample. Written information about the project, as well as the invitation to participate, was distributed to the GP's by the sponsoring companies’ representatives. Each of the participating GPs was expected to assess at least 20 consecutive subjects who both attended their practice and fulfilled the eligibility criteria during the 6-month study period (March to August 2010).
Subjects were eligible for the study provided they fulfilled the following inclusion criteria: 1) Age ≥35 yrs, 2) Smoker/ex-smoker or relevant occupational exposure, and 3) ≥ One respiratory symptom (dyspnoea, cough, wheeze, sputum and/or recurrent chest infections); and not the following exclusion criteria: 1) Unable to perform spirometry, and 2) Previous diagnosis of obstructive lung disease (asthma and/or COPD) or other chronic respiratory disease.
All recruited subjects had spirometry performed in their GPs practice, performed with the GPs own spirometer, according to the spirometry guidelines from the Danish Respiratory Society (Citation14), including at least three forced expiratory manoeuvres and at least two measurements differing less than 5%. Participants with an initial spirometry showing airway obstruction, defined as FEV1/FVC ratio ≤0.7, had a bronchodilator reversibility test ().
Figure 1. Flow chart depicting the diagnostic evaluation of the recruited subjects having airflow obstruction (FEV1/FVC < 0.70) at initial spirometry. * COPD defined as post-treatment FEV1/FVC < 0.70 and no substantial reversibility.
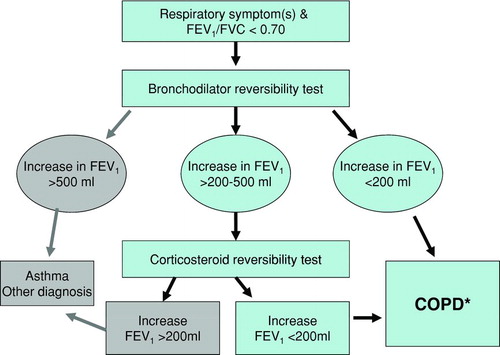
Spirometry was repeated 15 minutes after inhalation of 0.4 mg salbutamol (or equivalent). Subjects with an increase in FEV1 ≥500 ml were regarded as having asthma and excluded from the study, whereas subjects with an increase of more than 200 ml (but less than 500 ml) were offered a corticosteroid reversibility test. In the latter group of subjects, spirometry was repeated after either six weeks of treatment with inhaled corticosteroid (budesonide 1600 μg daily or equivalent) or oral prednisolone (37.5 mg daily for 14 days); although the latter is not recommended in the GOLD guidelines (Citation2), it was imperative to follow local Danish guidelines (Citation12), as the study was approved in Denmark as a non-interventional study. Subjects with a further increase in FEV1 following corticosteroid therapy were regarded as having asthma and excluded from the study, according to Danish guidelines (Citation12) ().
The case record form (CRF) consisted of 2 parts: 1) A questionnaire filled-in by the participant regarding age, gender, height, body weight, smoking status, daily tobacco consumption, years of smoking, airway symptoms, (cough, dyspnoea, wheezing, sputum, and recurrent lower airway infection), and severity of dyspnoea (graded according to MRC (Citation15), and 2) Spirometry data obtained by the GP (FEV1 expressed in absolute value), and FVC; and in some cases also spirometric values obtained after administration of bronchodilator and/or corticosteroid), and best-possible post-treatment values were used in the analysis for confirming a diagnosis of COPD.
All data from the individual CRF was entered into a consolidated web-based database; and upon registration the BMI, FEV1 percentage of predicted value (Citation16), FEV1/FVC ratio, severity of COPD (based on FEV1%predicted, and graded according to GOLD) (Citation2), and number of pack-years was automatically calculated. Consultants from the sponsoring companies performed quality control of the CRFs.
Data analysis
Data from individual participant's records were analysed focusing on the defined outcome variables; only data for participants with complete CRFs were included in the analyses. The FEV1 was expressed as the absolute value and also as a percentage of the predicted value (Citation16), and the FEV1/FVC ratio as the absolute value.
Predictors of an FEV1/FVC ratio below 0.7 were identified by means of logistic regression analysis with predictor variables gender, age, BMI, smoking status, and presence of respiratory symptoms (dyspnoea, cough, wheezing, sputum, and recurrent lower respiratory infections). Age, BMI and pack-years are all modelled by restricted cubic splines and, furthermore, the effects of these variables are reported as the effect of going from the 1st quartile to the 3rd, whereas the p-value is a global test. The 95% confidence intervals (CI) and p-values for odds ratios (OR) were obtained from multivariate logistic regression analyses. For all symptom factors, no symptoms were set as reference, whereas non-smokers and females were set as reference for smoking status and gender respectively. Pack-years for never smokers were set to the mean pack-years for ever smokers (former and current), which implies that OR for e.g., smokers vs. never smokers corresponds to comparing never smokers with a smoker with an average number of pack-years. The results were interpreted in terms of odds ratios and visualised using a nomogram. The statistical analyses were repeated after substitution the fixed FEV1/FVC ratio, i.e. FEV1/FVC < 0.7, with the lower limit of normal FEV1/FVC ratio as the cut-off value defining airway obstruction (Citation17).
The efficiency of the spirometric test was assessed by means of a logistic model including the previously identified factors associated with the presence of COPD, and the risk of COPD was calculated for each individual in the study by its predicted risk. Subsequently, by using different threshold values for the pre-test risk of COPD as a guideline for whom to test, we estimated the number of individuals with COPD identified as the number of individuals with COPD with a risk higher than the threshold; and, furthermore, the number of spirometric tests needed was estimated as the number of individuals with a risk higher than the threshold.
Numbers needed-to-screen (NNS) was calculated as the reciprocal values of the prevalence. In all the statistical analyses a significance level of 5% was adopted, and the statistical software R (Citation18) was used for the statistical analyses.
The TOP GOLD project was endorsed by the Danish College of General Practitioners. According to the EFPIA code (Citation19), and the Danish Association of the Pharmaceutical Industry (LiF) (Citation20), the present study was a non-drug, non-interventional study, and approval from the scientific ethical committee and the Danish Medicines Agency were not mandatory, but they were given all relevant study information. The study was approved by the Danish Data Protection Agency.
Results
Characteristics of enrolled subjects
A total of 241 GPs participated in the study, and 4.049 individuals (51% males) with complete data were included in the analyses. A total of 59% of the study population (58% males and 60% females) were current smokers, and 4% declared themselves as lifelong non-smokers. The mean number of pack-years for males and females, respectively, was 37 (range 0–193) and 27 (range 0–168) (p < 0.01). Passive tobacco exposure was not investigated. The mean age was 58 and 57 years, respectively for males and females. The youngest subject was 35 years old, and the oldest subject was 91 years old with normal distribution for both men and women; and mean body mass index (BMI) was 28 (range 16–56) and 27 (range 14–53).
Prevalence and severity of respiratory symptoms
The most common reported respiratory symptoms were dyspnoea (71.4% of the recruited subjects) and cough (48.2%), whereas sputum, wheeze, and recurrent lower airway infections were reported by 30.7%, 18.3%, and 10.3%, respectively. A total of 2021 subjects (49.9%) reported only one respiratory symptom; and 283 subjects (7.0%) reported at least 4 out 5 respiratory symptoms. The majority of subjects (89.8%) reported dyspnoea score 1 and 2, whereas 414 subjects (10.2%) reported a dyspnoea score of at least MRC 3.
Prevalence and severity of COPD
Airway obstruction, defined as FEV1/FVC < 0.7, was found at the initial spirometry in 23.1% (n = 937) of the enrolled subjects. All subjects with airway obstruction were tested for bronchodilator reversibility (), whereas only 3.2% of the recruited subjects proceeded to the corticosteroid reversibility test. Based on these diagnostic spirometry tests, the diagnosis of COPD was confirmed in 878 (21.7%) subjects; of whom 35% had mild COPD (GOLD 1), 50% moderate COPD (GOLD 2), and 15% severe or very severe COPD (GOLD 3-4).
Of the subjects diagnosed with COPD, 19% changed disease severity status following administration of bronchodilator compared to the screening spirometry (correlation between COPD severity at screening and after bronchodilator rs = 0.8, p < 0.001). The number needed to assess for a new diagnosis of COPD was 4.6. Substituting the fixed FEV1/FVC ratio with the lower limit of normal FEV1/FVC ratio, as the definition of airway obstruction, slightly reduced the number of subjects identified with COPD, primarily in the older age groups, but did not have substantial impact on the findings.
Predictors of COPD
Although the prevalence of COPD increased with increasing age, COPD was diagnosed in 8.3% of the subjects younger than 48 yrs. Furthermore, apart from the association with age, the analysis also showed that gender, BMI, number of pack-years, and self-reported cough, wheezing, and sputum production were significant predictors of COPD. The prevalence of COPD was higher for increasing age and decreasing BMI.
The 95% confidence intervals for odds ratios of these risk factors are given in , and a nomogram for the analysis obtained by the multiple regression models is given in . From the nomogram, individual predictions can be obtained by adding the partial effect on each risk factor and using the sum to calculate the risk of having COPD; and, furthermore, the nomogram also shows directly the relative importance of the risk factors. The interaction between smoking status and number of pack-years was non-significant.
Figure 2. Nomogram used for predicting the probability of FEV1/FVC < 70%. The model reported in Table 2 has been used for constructing the nomogram. For each variable the number of points read of in the top line ‘point’, e.g., being af smoker gives approximately 33 points, and then ‘total points’ is computed by summing these individual points. From the ‘total points’ the risk of COPD is found by drawing a vertical line at the total points achieved, e.g., 150 points corresponds to a risk of 0.1.
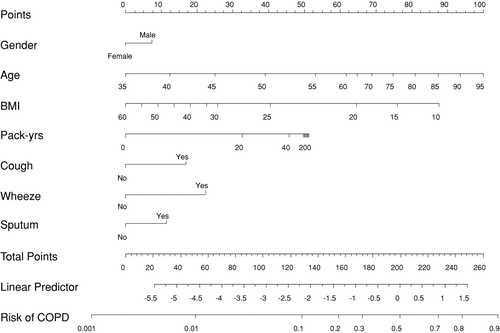
Table 1. Distribution of FEV1/FVC<70 stratified on Gender, Smoking Status, Age and BMI
Substituting the fixed FEV1/FVC ratio with the lower limit of normal FEV1/FVC ratio, as the definition of airway obstruction, did not have substantial impact on the findings in relation to predictors of COPD.
Efficiency of using screening spirometry
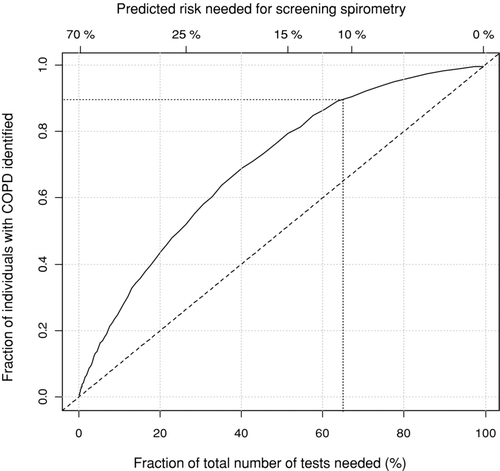
The efficiency of the spirometric test was assessed by means of a logistic model including the previously identified factors, (i.e., age, gender, BMI, number of pack-years, and presence of cough, wheezing and/or sputum production), associated with the presence of COPD. It can be seen from that using a threshold of 10% pre-test risk of COPD for doing spirometry would have reduced the number of necessary spirometry tests by 35% although 90% of the individuals suffering from COPD would still have been identified. Using the 10% pre-test risk of COPD as the threshold, revealed a number needed to screen of 3.9 for a new diagnosis of COPD.
Figure 3. The figure shows the association between predicted risk for COPD needed for doing screening, fraction of total number of spirometry tests needed, and the fraction of all individuals with COPD identified. First, the predicted risk of COPD for each individual in the study was calculated based on a multivariate logistic regression model based on gender, age, BMI, pack-years, and respiratory symptoms (cough, wheeze and sputum). Second, each individual is classified as relevant for screening or not relevant based on a threshold value for the predicted risk of COPD. Finally, the fraction of individuals with COPD that was relevant for screening was found. For example, a threshold value of 11% means that all individuals with a predicted risk of COPD of at least 11% are assigned to the screening group; this group corresponds to around 65% of all screenings done, but identifies around 90% of all individuals with COPD, which is indicated by the dotted line.
Discussion
In the present study, we have shown that a significant proportion of patients suffering from COPD can be diagnosed by the implementation of a case-finding strategy providing both questionnaire assessment and diagnostic spirometry to high-risk subjects in a primary care-setting. Our study additionally showed that 86% of the newly diagnosed patients with COPD could be classified as GOLD-stages I and II. The identified factors associated with a new diagnosis of COPD may facilitate further improvement in the case finding strategy, and by that the diagnostic yield.
The overall prevalence of COPD in our study was 21.7%, and the majority of patients had mild to moderate disease. These percentages are comparable to those reported by Minas et al. from a study conducted in 15 primary care centres in Greece (Citation21), as well as from studies conducted in England and Poland (Citation22,23), in spite of substantial differences between the populations studied.
However, previous studies have shown a higher prevalence of airflow limitation, defined as FEV1/FVC ratio <0.7, in smokers, most likely due to differences in study populations, including age range of screened subjects (Citation24,25). Our study included eligible subjects, i.e., subjects with relevant exposure and at least one respiratory symptom, attending their GP for any reason, and does, therefore, not fulfil the criteria for a screening study. However, in keeping with previous studies, our data highlight the problem with under-diagnosis of COPD in the primary care setting.
Several respiratory diseases may present with symptoms and signs similar to COPD, including congestive heart failure and bronchiectasis, and these diseases may also co-exist with COPD. The differential diagnosis of COPD may therefore be complex, but asthma remains the most common differential diagnosis. It may be difficult to distinguish asthma from early stages of COPD, but testing for bronchodilator reversibility and defining COPD on the basis of post-bronchodilator FEV1/FVC ratio make it unlikely that we have misclassified a significant number of subjects (Citation2,Citation26,Citation27). Although the reversibility testing with corticosteroid is not recommended in the GOLD guidelines (Citation2), it is still recommended in the Danish COPD guidelines (Citation12), but as only very few patients went on to be tested according to the outlined procedure, it is unlikely that this has had significant impact on our findings.
Previous studies suggest that using a fixed cut-off ratio for FEV1/FVC of 0.70 as the diagnostic criterion may overestimate the prevalence of COPD in the elderly and underestimate the prevalence in the younger subjects (Citation28). It has, therefore, been suggested, and much debated (Citation29), to use the lower limit of normality, defined as 1.645 standard deviations below predicted, to define airflow limitation (Citation17), and by that for the diagnosis of COPD. In the present study, we repeated all analyses using LLN FEV1/FVC ratio instead of the fixed FEV1/FVC ratio, but in keeping with most published COPD-guidelines, we decided to use the fixed FEV1/FVC ratio, not least because this appears to be easier to handle as the diagnostic criterion in general practice, but also because the overall findings were very similar.
In contrast to a number of recent studies (Citation21,Citation23,Citation30) subjects with a previous diagnosis of obstructive lung disease, i.e., COPD or asthma, were not eligible for the present study. However, in spite of that the NNS was only 4.6 for a new diagnosis of COPD; and, furthermore, offering screening spirometry only to individuals with an a priori risk of at least 10% for COPD would have reduced the NNS to as low as 3.9. The present study, therefore, clearly reveal that the strategy described here for early identification of COPD is highly efficient.
Buffels et al. (Citation26) have previously confirmed the crucial importance of spirometry in improving the early diagnosis of COPD; and screening spirometry performed at an acceptable level to exclude a diagnosis of COPD may reduce the number of necessary diagnostic spirometric tests (Citation13,Citation31). However, although it is possible to achieve a spirometry quality in primary care comparable to the levels achieved in pulmonary function laboratories, it is well-known that there may be lack of device calibration and quality control, as well as formal and accurate technical training of the staff in primary care (Citation27).
Another possible limitation of the present study may therefore be related to the quality of the spirometric tests performed. The IPCRG guidelines (Citation13) suggest a threshold of FEV1/FVC ratio > 0.8 for excluding COPD at the screening spirometry. The spirometry data obtained in the present study revealed a relative high prevalence of subjects having a FEV1/FVC ratio > 0.9 (data not shown), which may suggest at least some variability in the quality of the spirometric tests performed. In most cases probably related to the problem of obtaining the best possible FVC, especially in the elderly (Citation32). However, by only doing diagnostic spirometry in subjects with an FEV1/FVC ratio < 0.7 at screening spirometry, it is unlikely that bias related to the quality of spirometry have overestimated the prevalence of COPD in the present study.
In conclusion, the present showed that 22% of the subjects at-risk studied were diagnosed with COPD. Spirometry programmes should, therefore, be implemented in routine primary care for the identification of patients with COPD, since newly diagnosed patients in the present study in the majority of cases had mild to moderate disease, and early identification of cases is likely to provide a unique opportunity for early intervention.
Declarations of Interest
As members of the steering committee of the TOP GOLD study, AL, CSU, and RD have received consulting fees from Boehringer Ingelheim Denmark and Pfizer Aps, Denmark. LP is employed by Boehringer Ingelheim Denmark, and JD by Pfizer Aps, Denmark. LCK, PHC and CD declare that they have no conflicts of interests. As principal investigators AL, CSU, RD, LP, JD, LCK, PHC, and CD had full access to all the study data, and take responsibility for their integrity and the accuracy of their analysis. AL, CSU, RD, LP, and JD participated in the study design, and supervised the study. LCK, PHC and CD analysed the data and provided the statistical expertise. AL and CSU drafted the report, and the report was revised for important intellectual content by RD, LP, JD, and CD.
References
- Celli BR, McNee W, ATS/ERS committee members. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management and Prevention of COPD. Guidelines available from the GOLD website www.goldcopd.com (accessed October 2011).
- Editors, Lancet. COPD – more than just tobacco smoke (editorial). Lancet 2009; 374:663.
- Blanc PD, Menezes AM, Plane E, Mannino DM, Hallal PC, Toren K, Eisner MD, Zock JP. Occupational exposures and COPD: an ecological analysis of international data. Eur Respir J 2009; 33:298–304.
- Celli BR. Chronic obstructive pulmonary disease: from unjustified nihilism to evidence-based optimism. Proc Am Thorac Soc 2006; 3:58–65.
- Ferguson GT. Maintenance pharmacotherapy of mild and moderate COPD: What is the evidence? Respir Med 2011; 105:1268–1274.
- Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med 2000; 161:381–390.
- Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP for the UPLIFT investigators. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009; 374:1171–1178.
- Bednarek M, Gorecka D, Wielgomas J Smokers with airway obstruction are more likely to quit smoking. Thorax 2006; 61:869–873.
- Stratelis G, Molstad S, Jakobsson P, Zetterstrom O. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scand J Prim Health Care 2006; 24:133–139.
- KOL: Anbefalinger for tidlig opsporing, opfølgning, behandling, og rehabilitering (COPD: Recommendations for early detection, monitoring, treatment and rehabilitation; in Copenhagen Danish). Danish National Board of Health 2007.
- Nielsen LM, Brorson S, Gorlen T, Jakobsen M, Lange P, Heebøll-Nielsen NC, Pallesen R, Smith M. KOL i Almen Praksis (COPD in General Practice; in Copenhagen Danish). Danish College of General Practitioners 2008.
- Levy ML, Fletcher M, Price DB, Hausen T, Halbert RJ, Yawn BP. International Primary Care Respiratory Group (IPCRG) Guidelines: diagnosis of respiratory diseases in primary care. Prim Care Resp J 2006; 15:20–34.
- Madsen F, Maltbæk N, Mortensen J, Pedersen OF. Lungefunktionsstandard (in Danish). Danish Society of Respiratory Medicine 2007; available from www.lungemedicin.dk (accessed October 2011).
- Bestall JC, Paul EA, Garrod R, Granham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:581–586.
- Quanjer PH, Tammeling GJ. Summary of recommendations. Standardized lung function testing. Report Working Party, European Community for Coal and Steel. Bull Eur Physiopathol Respir 1983; 19(suppl 5):7–20.
- Pellegrin R, Viegi G, Brisasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CPM, Gustafsson P, Hankinson J, Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968.
- Ihaka I, Gentleman RR. A language for data analysis and graphics. J Computational Graphical Statistics 1996; 5:299–314.
- EFPIA code on the promotion of prescription-only medicines to, and interactions with, healthcare professionals. European Federation of Pharmaceutical Industries and Associations (Council Directive 2001/83/EC). www.efpia.eu [last accessed 05.10.11].
- Lægemiddelindustriforeningen www.lifdk.dk [last accessed 05.10.11].
- Minas M, Hatzoglou C, Karetsi E, Papaioannou AI, Tanou K, Tsaroucha R, Gogou E, Gourgoulianis KI, Kostikas K. COPD prevalence and the differences between newly and previously diagnosed COPD patients in a spirometry program. Prim Care Respir J 2010; 19:363–70.
- Shahab L, Jarvis MJ, Britton J, West R. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative sample. Thorax 2006; 61:1043–1047.
- Bednarek M, Maciejewski J, Wozniak M, Kuca P, Zielinski J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008; 63:402–407.
- Stratelis G, Jacobsson P, Molstad S, Zetterstrom O. Early detection of COPD in primary care: screening by invitation of smokers aged 40 to 55 years. Br J Gen Pract 2004; 54:201–206.
- Vanvoorde J, Verbanck S, Gijssels L, Early detection of COPD: a case finding study in general practice. Respir Med 2007; 101:525–530.
- Buffels J, Degryse J, Heyman J, Decramer M. Office spirometry significantly improves early detection of COPD in general practice: the DIDASCO study. Chest 2004; 125:1394–1399.
- Price D, Freeman D, Cleland J, Kaplan A, Cerasoli F. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J 2011; 20:15–22.
- Vollmer WM, Gislason T, Burney P, Enright PL, Gulsvik A, Kocabas A, Buist AS. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J 2009; 34:588–597.
- Miller MR, Pedersen OF, Pellegrino R, Brusasco V. Debating the definition of airflow obstruction: time to move on? Eur Respir J 2009; 34:527–528.
- Hvidsten SC, Storesund L, Wentzel-Klarse T, Gulsvik A, Lehmann S. Prevalence and predictors of undiagnosed chronic obstructive pulmonary disease in a Norwegian adult general population. Clin Respir J 2010; 4:13–21.
- Price D, Crockett A, Arne M, Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J 2009; 18:216–223.
- Vandevoorde J, Verbanck S, Schuermans D, Kartounian J, Vincken W. FEV1/FEV6 and FEV6 as an alternative for FEV1/FVC and FVC in the spirometric detection of airway obstruction and restriction. Chest 2005; 127:1560–1564.
