Abstract
COPD is progressive and typically begins many years before a definite diagnosis is made. As the rate of decline in lung function may be faster in the initial stages of the disease, early intervention could be beneficial to control symptoms and affect disease progression and outcomes. A systematic review of published literature relating to mild-to-moderate COPD (patients with FEV1 ≥50% predicted) was performed to evaluate the level of impairment and natural history or disease progression over time, and impact of interventions on the outcomes of patients with early-stage disease. Of the 79 published articles included in this analysis, 31 reported randomized controlled trials; the remaining 48 articles reported studies of non-randomized and/or observational design. Nine of the randomized controlled trials were ≥6 months’ duration, enabling assessment of outcomes over time. Most of the randomized controlled trials were in patients with moderate COPD (GOLD stage II); few included patients with the mildest stages of the disease (i.e., stage I). The results show that even patients with milder or moderate COPD can have substantial limitations and physical impairment, which worsen over time. Encouragement of smoking cessation, in conjunction with management of symptoms and treating activity limitation and exacerbations by appropriate non-pharmacologic and pharmacologic management at the earliest possible stage, could positively affect the impact and progression of the disease.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by chronic airflow limitation, which is usually progressive (Citation1). It is caused by an abnormal inflammatory response to noxious particles or gases, resulting in a combination of small airway disease (obstructive bronchiolitis) and parenchymal destruction (emphysema) (Citation1). Risk factors include smoking, indoor exposure to biomass fuels (Citation2), air pollution, genetic pre-disposition, advancing age, and poor lung health (e.g., previous infection, poor nutrition, and poor lung growth and development) (Citation1).
The impact of COPD on the individual patient depends on the degree of airflow limitation, the severity of symptoms and any systemic effects and co-morbidities (Citation1). The declining lung function results in progressively greater restrictions in daily activity and exercise tolerance, deconditioning, impaired quality of life (QoL), and an increasing number of symptoms and exacerbations. The reduction in daily activity is already present in mild disease (Citation3).
COPD has been classified by several authorities, largely based on spirometry using a simple classification score of airway limitation defined as a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio <0.7. The following Global Initiative for Chronic Obstructive Lung Disease (GOLD) system has been widely used to categorize COPD into 4 stages: stage I (mild), FEV1 ≥ 80% predicted; stage II (moderate), FEV1 ≥ 50% and < 80% predicted; stage III (severe), FEV1 ≥ 30% and <50%; and stage IV (very severe), FEV1 < 30% or <50% predicted with chronic respiratory failure (FEV1 < 30% predicted in the 2011 GOLD guideline update) (Citation1,Citation4). An additional category (GOLD stage 0) of symptomatic patients with FEV1/FVC > 0.7, who were considered to be “at risk” of developing COPD, was included in previous GOLD guidelines, but this category was discontinued due to lack of strong evidence for progression to COPD (Citation1).
Patients with COPD often do not seek medical attention until after they have developed chronic respiratory symptoms or have had an exacerbation (increase in, or development of new symptoms requiring a change in treatment and/or hospitalization) (Citation1). However, many patients with COPD are symptomatic long before this, often complaining of chronic cough and sputum production for several years before a diagnosis is made (Citation1). It is unclear what triggers the development of COPD in some individuals while others remain in a chronic non-obstructive bronchitic state.
Awareness of the relevance of these early symptoms is increasing amongst healthcare professionals. Several population-screening studies have also shown that patients with mild COPD can be identified early (Citation5–21). Overall, these studies suggest that early (GOLD stage I) disease (symptomatic but with FEV1 > 80% predicted) was found in approximately 2% to 10% of the adult population (Citation5,Citation7,Citation8,Citation19–21). In the international Burden Of Lung Disease (BOLD) study, the prevalence of GOLD stage I was shown to be 1.4% to 15.5% and GOLD stage II, 5.1% to 12.4% (Citation3). The importance of early diagnosis lies in the fact that important manifestations of COPD, such as exercise intolerance (Citation22) and reduced daily activity (Citation3) are already present at this stage of the disease. Also, the presence of mild airflow limitation is associated with increased mortality (Citation23).
As the rate of decline in lung function may be faster in the initial stages of the disease, early intervention could be beneficial (Citation24). Early detection, diagnosis, and management may also help to control symptoms, and potentially affect disease progression—and outcomes (Citation25). For example, although smoking cessation reduces the rate of decline in lung function at all stages of the disease (Citation26–29), the earlier it occurs in the natural evolution of COPD, the better the impact on lung function (Citation29). It is also possible that pharmacotherapy in the initial stages may reduce, or at least delay, decline in lung function, prevent exacerbations, improve symptoms, and QoL, as well as ultimately reduce morbidity and mortality (Citation30,31).
The objective of this study was to review systematically the published literature relating to mild-to-moderate COPD in order to evaluate: the level of impairment and the natural history or disease progression over time; and the impact of interventions on the outcomes of patients with early-stage disease.
Materials and Methods
A systematic review of the literature was conducted to identify data relating to patients with mild-to-moderate COPD. Literature published from January 1, 1980, to July 30, 2010, was searched using 4 databases (Medline, Embase, BIOSIS, and SciSearch) with the following defined search terms: “chronic obstructive pulmonary disease” OR “COPD” AND 1 or more of “earl(-y, -ier, -iest)”, “mild(-ly)”, “moderate”, “Stage 0”, “Stage 1”, OR “Stage I”, “Stage 2”, OR “Stage II”. The total hits from the search were assessed for relevance by a single researcher based on titles/abstracts, and those publications that were deemed potentially relevant were obtained in full and assessed.
Articles were included if they reported on patients with COPD who had a baseline FEV1 ≥ 50% predicted, where either the entire cohort or subgroups of patients were stratified according to this inclusion criterion. Also included were studies in which FEV1 ≥ 50% predicted was not a patient inclusion criterion, but in which the mean baseline FEV1 was ≥60% predicted (indicating a mild-to-moderate population). Any original clinical data published in English were eligible, including subgroup or secondary analyses and studies of experimental and observational design. There were no restrictions on the intervention (any drug treatment or none), study duration, or outcome measures. In vitro and ex vivo studies were excluded. Also excluded were unstratified data incorporating patients with FEV1 < 50% predicted; where mean baseline FEV1 was close to 50% predicted.
The most robust studies were considered to be the randomized controlled trials (RCTs) and, of those, studies of ≥6 months’ duration were identified as potential for providing information on change in disease course or outcomes over time. Supplementary data from shorter RCTs and non-randomized studies were also included to provide additional relevant information on aspects of the disease.
Results
Literature search results
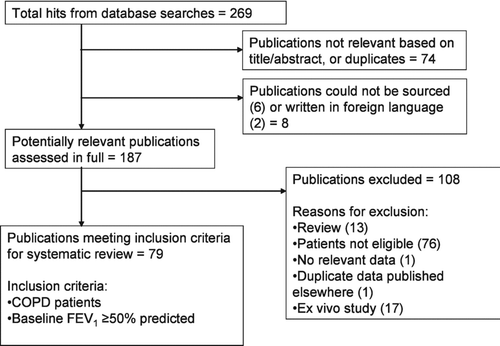
The search strategy returned a total of 269 hits (). Of these, 74 were excluded based on the title and/or abstract, 2 were available only in foreign languages, and 6 could not be sourced.
The remaining 187 papers were deemed potentially relevant based on the title and/or abstract and were obtained in full. Of these, 108 were excluded (see Supplementary Appendix 1) and 79 were included. The reasons for exclusion were: patients were not eligible (n = 76); there were no relevant data (n = 1); the data were duplicated elsewhere (n = 1); the study was ex vivo (n = 17); the publication was a review article (n = 13).
Of the 79 papers included in this analysis, 31 reported on RCTs and the remaining 48 on studies of non-randomized and/or observational designs. The 31 RCT papers included 11 describing placebo-controlled investigations of pharmaceutical agents (Citation30–40), 6 investigations of pharmaceutical agents that had active controls (Citation41–46), 6 trials of non-pharmaceutical interventions (Citation47–52), and 6 describing combined pharmaceutical and non-pharmaceutical interventions (Citation29,Citation53–57). The remaining 2 papers contained only placebo data (Citation58, 59). Overall, 9 RCTs had the desired duration of ≥6 months (Citation30–33, Citation34, Citation38–40, Citation48, Citation59).
The 48 non-RCT papers included reports of 2 prospective, controlled, parallel-group studies, while the remainder were of less robust design. Of these, 17 papers were population-based COPD screening studies. For details of these 48 publications, please see Supplementary Appendix 2.
Disease progression in mild-to-moderate COPD
All 31 RCT papers provided baseline data on the status of patients with mild-to-moderate COPD (). Thirteen of the RCTs provided baseline data only (Citation29, Citation41–47, Citation53–57). Eighteen papers reported on RCTs that incorporated a suitable “no treatment” (or placebo) arm, thereby providing outcome data relevant to natural disease progression. These included 2 papers containing only placebo data (Citation58, 59), 11 papers on placebo-controlled investigations of pharmaceutical agents (Citation30–40) and 5 trials of non-pharmacologic interventions, which incorporated a suitable control arm (Citation48–52).
Table 1. Articles meeting the inclusion criteria for the systematic review, which reported baseline data and outcomes with no treatment.
Of the 18 papers reporting disease progression, 9 documented RCTs with ≥6 months’ duration (Citation30–32, Citation34, Citation38–40, Citation48, Citation59).
The majority of the RCTs were in patients with moderate COPD (GOLD stage II); few RCTs included patients with the mildest stages of COPD (i.e., GOLD stage I) (). The 2-year Detection, Intervention, and Monitoring Program of COPD and Asthma (DIMCA) trial provided data for patients with early signs of COPD (i.e., persistent symptoms and a moderately accelerated rate of lung decline) (Citation32,Citation40). The baseline values and outcomes, in terms of changes in aspects of the disease over the time period studied, in the placebo or control groups of patients are summarized in .
Table 2. COPD disease progression: summary of available outcomes data (baseline and over study period) in mild and moderate COPD patients in control groups.
Lung function—rate of decline in FEV1
Decline in FEV1 was reported from 4 RCTs of ≥6 months’ duration (Citation30–32, Citation38). For example, in 24 patients with mild COPD (mean FEV1 98% predicted) receiving placebo in the 2-year DIMCA trial, estimated post-bronchodilator FEV1 decline was 14 ml/year (Citation32). In a 3-year study in mild-to-moderate COPD (mean FEV1 76.9% predicted), the rate of decline in mean post-bronchodilator FEV1 was 65 ml/year in 643 patients receiving placebo (Citation38).
Among the trials in which patients with moderate COPD (GOLD stage II) were assessed, subgroup analyses of these patients in 2 large trials, Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT®) (n = 2375) and Towards a Revolution in COPD Health (TORCH) (n = 2156) (duration 4 years and 3 years, respectively, mean post-bronchodilator FEV1 59% predicted) showed rates of decline in FEV1 of –49 ml/year and –60 ml/year, respectively, in placebo/control patients (Citation30, 31).
Bridevaux et al. reported the results of a large (n = 6671) Swiss population-based study over 11 years, in which the annual rate of decline in symptomatic GOLD stage I patients (n = 224) was significantly greater than in asymptomatic control individuals (n = 3627) (44 ml/year versus 34 ml/year; p < 0.01) (Citation5). The corresponding comparison between asymptomatic GOLD stage I patients (n = 295) and the asymptomatic control individuals was numerically (37 versus 34 ml/year) but not significantly different (p = 0.08).
Rate of exacerbations
In total, 5 RCTs of ≥6 months’ duration reported the rate of exacerbations over their trial periods (Citation30–32, Citation34, Citation48). In a trial reported by Solèr et al., an annual rate of 1.72 exacerbations (0.86 over the 6-month trial period) was experienced by 131 GOLD stage I/II patients (mean FEV1 82.6% predicted) receiving placebo (Citation48). However, it is important to note that patients had to have a current exacerbation in order to enter the trial.
Among 24 patients with mild COPD (mean FEV1 98% predicted) receiving placebo in the 2-year DIMCA trial, 4 exacerbations (including mild) were reported among 3 patients (rates not reported) (Citation32). An annual severe exacerbation rate of 0.07 (based on oral steroid use) was reported for the placebo group in the 3-year European Respiratory Society Study on Chronic Obstructive Pulmonary Disease (EUROSCOP) trial (mean FEV1 76.9% predicted) (Citation34).
In the subgroup analysis of patients with moderate COPD (GOLD stage II, mean post-bronchodilator FEV1 59% predicted) in the UPLIFT® trial, mean annual exacerbation rates of 0.70 (moderate or severe) and 0.10 (severe) were reported in the control group (n = 1355) (Citation30). In the TORCH study, the annual rate of moderate/severe exacerbations was 0.82 in patients with GOLD stage II COPD (mean post-bronchodilator FEV1 59% predicted) receiving placebo (n = 535) (Citation31).
Respiratory symptoms
Overall, 5 studies have assessed aggravated respiratory symptoms, such as dyspnea, wheezing, and chronic cough in patients with mild-to-moderate COPD (Citation29, Citation32, Citation37, Citation54, Citation59). O'Donnell et al. described a small study of 16 patients with GOLD stage I COPD (mean post-bronchodilator FEV1 90% predicted), in which chronic activity-related dyspnea scores, measured on the Medical Research Council scale and the baseline dyspnea index, were 1.8 and 8.3, respectively (Citation37), indicating long-term activity-related dyspnea.
Among the 24 patients with mild COPD (mean FEV1 98% predicted) receiving placebo in the 2-year DIMCA trial, the mean symptom score at baseline was 1.3 (0 = no complaints to 3 = occurs every day) and there were 57 episodes of increased respiratory symptoms among 17 patients (Citation32).
In the 5-year Lung Health Study in 3926 smokers with moderate COPD (mean FEV1 78.1% to 79.4% predicted), respiratory symptoms were common at baseline, with wheezing in 74.4% to 76.9%, chronic cough in 39.7% to 44.3%, and dyspnea in 39% to 44.1% of patients (Citation29).
The 3-year EUROSCOP trial in 642 patients (median post-bronchodilator FEV1 79.2% predicted [men] and 81.5% predicted [women]) reported symptoms at baseline in most patients, especially wheezing (54.1% men; 60.5% women), dyspnea after activity (34.6% men; 39.9% women), and cough in winter (48.7% men; 58.4% women) (Citation58).
Quality of life
Overall, 4 RCTs assessed QoL using the St George's Respiratory Questionnaire (SGRQ) at baseline or during the study. The SGRQ includes 50 items (76 weighted responses) across 3 domains: symptoms, activity, and impact (range: 0–100) (Citation60). A 4-point decrease in score is considered to be a clinically meaningful improvement in a patient's health-related QoL. A small study by Kanehara et al. reported mean baseline SGRQ total scores of 34.0–35.7 in 26 patients with GOLD stage I or II COPD (mean FEV1 83.8% predicted) (Citation43).
Spencer et al. summarized a 48-patient trial in GOLD stage II patients with a mean baseline SGRQ total score of 39 units in the control group (n = 24). (Citation47). Patients with moderate COPD (GOLD stage II) in the control group of UPLIFT® (n = 1355) (Citation30) had a mean SGRQ total score at baseline of 42 units, and this worsened by 0.99 units per year, corresponding to a further deterioration in health status. In GOLD stage II patients involved in the TORCH trial, the mean baseline SGRQ total score for the placebo group (n = 535) was 43.9, and this decreased (improved) by –1.3 points per year (Citation31).
In a sub-analysis of the non-randomized SAlute Respiration nell'Anziano (Respiratory Health in the Elderly) (SaRA) study (n = 381) that used the former GOLD classification system (Citation61), worse baseline SGRQ scores (impacts and total) correlated with worse disease severity: total mean scores were 30, 36, and 38 units in GOLD stage 0, I, and IIa patients, respectively (n = 266) (Citation62).
Exercise capacity
Exercise capacity in patients with mild-to-moderate COPD has been evaluated in several studies, and using different endpoints. O'Donnell et al. examined exertion symptoms in 16 GOLD stage I patients (mean post-bronchodilator FEV1 90% predicted). At baseline, symptom-limited peak work rate and oxygen consumption were reduced; 72% and 79% predicted, respectively (Citation37).
Clark et al. compared muscle strength and endurance in 43 patients with mild COPD (mean FEV1 76–79% predicted) with healthy but sedentary subjects (Citation49). Patients with mild COPD showed a significant reduction in isokinetic muscle strength compared with healthy controls (no p-value reported). Baseline BORG scores for breathlessness at peak exercise were 2.4–3.5 among the patients with mild COPD.
AkkocaYildiz et al. reported that in a small study of 10 patients with GOLD stage I or II COPD (mean FEV1 68.95% predicted), baseline mean exercise time (symptom-limited incremental cycle exercise test) was 7.03 minutes, and work rate was 121.5 Watts (Citation46). In 48 patients with GOLD stage II COPD (FEV1 57% to 60% predicted), Spencer et al. found that the mean distance covered during the 6-minute walk distance (6MWD) test at baseline was 523–530 meters (Citation47).
These RCT data are supported by non-randomized studies. For example, a trend towards a decreased number of steps taken per day and decreased time spent in moderately intense physical activity for GOLD stage I patients (n = 9) was reported compared with healthy controls (n = 30) (Citation3). For GOLD stage II patients (n = 28), all physical activity-related outcomes were significantly reduced compared with controls (p < 0.05).
A study by Ofir et al. examined exercise capacity in GOLD stage I patients (mean FEV1 91% predicted) compared with healthy controls (n = 21 in each group) (Citation63). Among the COPD patients, significant reductions in peak oxygen consumption and power output (>20%) were observed and dyspnea ratings were higher for a given work rate and ventilation (p < 0.05) compared with healthy controls. Changes in end-expiratory lung volume during exercise were also greater in COPD patients compared with controls (0.54 L versus 0.06 L; p < 0.05) and their breathing was more shallow and rapid.
In a further non-randomized study, Antonelli-Incalzi et al. assessed physical performance by the 6MWD test (0–100); the test values were 78, 75, and 72% predicted in GOLD stage 0, I, and IIa patients, respectively (Citation62).
Functional status
Functional status (Citation6 domains of general health) was only reported in 1 RCT, the 2-year DIMCA trial in patients with mild COPD using the Dartmouth COOP Functional Health Assessment Charts/World Organization of General Practice/Family Physicians (COOP/WONCA) scores (range: 1 = very good to 5 = very bad). Among the 24 patients (mean baseline FEV1 98% predicted) receiving placebo, baseline mean scores ranged from not impaired (1.2 for social activities) to slightly impaired (2.4 for general health), and most patients perceived no change in their functional status during the trial period (Citation32).
Mortality
No information was available regarding mortality rates in patients with GOLD stage I disease among the included articles. However, an overall mortality rate of 10% was reported in GOLD stage II patients receiving placebo over 4 years during the UPLIFT® trial (n = 1355) (Citation30). Similarly, an overall mortality rate of 11.4% was recorded in patients at GOLD stage II who were given placebo over 3 years in the TORCH trial (n = 535) (Citation31). Pauwels et al. reported a 1.6% mortality rate over 3 years in the placebo-group patients with mild-to-moderate COPD (mean baseline FEV1 76.8−76.9% predicted, n = 643) (Citation38).
Risk factors
Several studies have assessed risk factors for disease progression. Data from the EUROSCOP study indicated that gender, body mass index, and smoking may be associated with worsening of COPD (Citation59). For example, in men, symptoms were associated with lower baseline FEV1 and obesity was associated with a reduced decline in FEV1. In women, more severe airway obstruction was associated with an accelerated decline in FEV1. An increased number of cigarettes smoked was also associated with an accelerated decline in FEV1. In the non-randomized SaRA study (266 patients with GOLD stage 0–IIa COPD), female gender, comorbidity, and older age was associated with worse QoL (Citation62).
Effects of interventions in mild-to-moderate COPD
A total of 29 RCTs provided data on the effect of intervention on mild-to-moderate COPD (). These included 11 placebo-controlled investigations of pharmaceutical agents (Citation30–40), 6 trials of pharmacologic interventions without a placebo control (Citation41–46), 6 trials of non-pharmacologic interventions (Citation47–52), and 6 papers from the Lung Health Study with mixed interventions (Citation29,Citation53–57). Of these, 16 had a duration ≥6 months (Citation29–32,Citation34,Citation38–41,Citation47,48,Citation53–57). provides a top-line summary of the most commonly-reported efficacy outcomes associated with different interventions from the selected studies of patients with mild-to-moderate COPD.
Table 3. Articles meeting the inclusion criteria for the systematic review and which reported outcomes with intervention (the effect of treatment on mild-to-moderate COPD).
Table 4. Top-line summary of the effects of different interventions on selected efficacy outcomes in studies of patients with mild-to-moderate COPD.
Lung function—absolute improvements
In a group of 16 GOLD stage I patients (mean post-bronchodilator FEV1 90% predicted), O'Donnell et al. demonstrated that ipratropium bromide provided moderate improvements in lung function outcomes (FEV1, residual volume) and in selected variables during constant work-rate cycle exercise (inspiratory capacity, tidal volumes, and dyspnea) compared with placebo (p < 0.05) (Citation37).
In the 3-year TORCH trial, 2156 patients who were GOLD stage II or above (including 28 GOLD stage I; mean post-bronchodilator FEV1 59% predicted) received salmeterol monotherapy (n = 522), fluticasone monotherapy (n = 537), salmeterol and fluticasone (n = 562), or placebo (n = 535) (Citation31). Sub-group analysis of GOLD stage II patients revealed that, compared with placebo, salmeterol and fluticasone improved FEV1 (by 101 ml) at 3 years (p-value not reported).
The sub-group analysis of GOLD stage II patients (mean post-bronchodilator FEV1 59% predicted) in the 4-year UPLIFT® trial showed that mean pre- and post-bronchodilator FEV1 values were significantly higher in the tiotropium group compared to the control group at all time points (with differences between groups ranging from 101–119 ml and 52–82 ml, respectively; p < 0.0001) (Citation30).
In a 1-year study of patients with GOLD stage II COPD (FEV1 50−80% predicted), Di Lorenzo et al. compared salmeterol (n = 91) with theophylline (n = 87) (Citation40). FEV1 improved in both groups; the improvements with salmeterol persisted over 12 months, while those with theophylline were not maintained at 9 months or at 12 months.
In a 1-year study reported by Spencer et al., supervised exercise (n = 24) and unsupervised exercise (n = 24) in GOLD stage II patients (FEV1 57–60% predicted) failed to show an improvement from baseline in lung function, with no difference between groups (Citation47).
Lung function—rate of decline in FEV1
During the 2-year DIMCA trial in mild COPD (mean FEV1 95% to 99% predicted), the annual rate of post-bronchodilator FEV1 decline was significantly greater for patients receiving fluticasone compared with placebo: –93 versus –14 ml/year (p = 0.001), although the sample size was small (n = 24 in each group) (Citation32).
Pauwels et al. conducted a 3-year comparison of budesonide (n = 634) with placebo (n = 643) in patients with moderate COPD (mean pre-bronchodilator FEV1 77% predicted), and found that FEV1 initially improved with budesonide, then declined at a similar rate to placebo (Citation38). Overall, the median decline in post-bronchodilator FEV1 over the 3-year period was 140 ml for budesonide versus 180 ml for placebo (p = 0.05), equating to an annual rate of decline of 47 ml and 60 ml, respectively.
In the 3-year TORCH trial, salmeterol + fluticasone reduced the rate of FEV1 decline by 16 ml/year (95% confidence interval [CI], 0–32) compared with placebo in the subgroup of GOLD stage II patients (p-value not reported).
The sub-group analysis of GOLD stage II patients (mean post-bronchodilator FEV1 59% predicted) in the 4-year UPLIFT® trial reported a lower rate of decline of post-bronchodilator FEV1 for tiotropium (n = 1218) compared with placebo (n = 1157) (43 versus 49 ± 2 ml/year; p = 0.024) (Citation30).
A 5-year follow-up of patients with mild-to-moderate COPD (mean post-bronchodilator FEV1 78% to 79% predicted) who received smoking cessation as an intervention in the Lung Health Study revealed a rate of FEV1 decline approximately 50% of that observed in patients who continued to smoke: 31 versus 62 ml/year (Citation29).
Rate of exacerbations
During the 2-year DIMCA trial in 48 patients with mild COPD (mean post-bronchodilator FEV1 98% to 99% predicted), a low rate of exacerbations was reported in both groups; 6 episodes in 5 fluticasone patients, compared with 4 episodes in 3 placebo patients (Citation32).
A 37% reduction in estimated annual severe exacerbation rate (based on oral steroid use) was reported for budesonide (n = 593) compared with placebo (n = 582): 0.05 versus 0.07 per year; rate ratio 0.63; p = 0.002) in the 3-year EUROSCOP trial in patients with mild-to-moderate COPD (mean baseline FEV1 76.9% predicted) (Citation34).
In the GOLD stage II subgroup of the TORCH study, rates of moderate or severe exacerbations were 31% lower for salmeterol plus fluticasone (n = 562) versus placebo (n = 535) groups: mean 0.57 versus 0.82 exacerbations/year (p-value not reported) (Citation31). In the subgroup analysis of 2739 GOLD stage II patients in the UPLIFT® trial, a 20% reduction in the mean number of moderate or severe exacerbations (0.56 versus 0.70 per patient year; p < 0.0001) was reported for tiotropium compared with placebo (Citation30).
Solèr et al. demonstrated that exacerbations were reduced by 29% in GOLD stage I or II patients (mean FEV1 83% to 85% predicted) receiving a bacterial extract (n = 142) compared with placebo (n = 131): 0.61 versus 0.86 exacerbations over 6 months (p = 0.03), equivalent to annual exacerbation rates of 1.22 versus 1.72, respectively (Citation48).
Spencer et al. showed that in a 1-year study in GOLD stage II patients (FEV1 57% to 60% predicted), exacerbation rates following pulmonary rehabilitation were similar for the supervised and unsupervised exercise groups (mean 2.3 and 1.4 exacerbations per year, respectively), as were hospital admissions and length of hospital stay (Citation47).
Respiratory symptoms
During the 2-year DIMCA trial in patients with mild COPD (mean post-bronchodilator FEV1 98% to 99% predicted), there were 127 episodes of increased respiratory symptoms in 18 patients treated with fluticasone compared with 57 episodes in 17 placebo patients (Citation32). Among the 16 GOLD stage I patients enrolled in the study by O'Donnell et al., ipratropium bromide moderately improved dyspnea during exercise compared with placebo (Citation37).
The Lung Health Study assessed the effect of smoking cessation plus ipratropium (n = 1961), smoking cessation plus placebo (n = 1962), and advice to quit smoking (usual care) (n = 1964) on respiratory symptoms (mean pre-bronchodilator FEV1 74% to 76% predicted) (Citation56). At the 5-year follow-up, the prevalence of symptoms (cough, phlegm, wheezing, shortness of breath) was significantly lower in both smoking cessation groups (p < 0.0001) than with usual care, although ipratropium conferred no additional effect.
QoL
In the GOLD stage II subgroup of the 3-year TORCH study, salmeterol plus fluticasone (n = 562) improved SGRQ total score compared with placebo (n = 535), with a difference between groups of -2.3 units (p-value not reported) (Citation31).
In the subgroup analysis of GOLD stage II patients in the UPLIFT® trial, QoL with tiotropium (n = 1179) was superior to placebo (n = 1119) at all time points (p ≤ 0.006) (Citation30). Differences in SGRQ scores between groups ranged from 2.7–4.0 (total score), 2.3–3.9 (impact), 2.7–4.1 (symptoms), and 3.1–4.4 (activity) units for tiotropium and placebo, respectively. However, the slope of decline in SGRQ total score did not improve significantly.
In a 1-year study of patients with GOLD stage II COPD, Di Lorenzo et al. found that salmeterol (n = 91) improved Short Form-36 Health Survey (physical functioning) domains to a greater extent than theophylline (n = 87) (change in health perception at 9 months, p = 0.03; social functioning at 12 months, p = 0.04) (Citation41).
In GOLD stage II patients, Rennard et al. showed that infliximab showed no effect at 3 mg/kg (n = 31) or 5 mg/kg (n = 28) compared with placebo (n = 39) on the chronic respiratory questionnaire at 44 weeks (Citation39).
The 1-year study of supervised exercise (n = 24) versus unsupervised exercise (n = 24) in GOLD stage II patients (FEV1 57% to 60% predicted) by Spencer et al. showed no difference in the change from baseline of SQRQ total score (3 versus -3 and there was also no difference in hospital anxiety and depression) scores (Citation47).
Exercise capacity
Exercise capacity was reported in 1 interventional study (Citation37, Citation47). Spencer et al. showed, in 48 GOLD stage II patients following pulmonary rehabilitation, that there was no difference between patient groups receiving supervised versus unsupervised exercise in the change from baseline of 6MWD (-11 [95% CI, -21, 10] meters versus -6 [95% CI, -34, 11] meters) (Citation47); there was also no difference in incremental and endurance shuttle walk test, although patients in both groups successfully maintained the 6MWD (Citation47). Symptom-limited exercise endurance time during constant work rate exercise (an index of exercise capacity) in 16 GOLD stage I patients (O'Donnell et al.) was comparable after ipratropium or placebo (8.2 minutes in both groups), and the reasons for stopping exercise also did not differ between groups (Citation37).
Functional status
Functional status was reported in only 1 RCT, the 2-year DIMCA trial (Citation32) in patients with mild COPD (mean post-bronchodilator FEV1 98% to 99% predicted). Overall, there was no significant difference between fluticasone and placebo in COOP/WONCA scores.
Mortality
The reviewed data showed no significant effect on mortality for budesonide (Citation38) or tiotropium (Citation30); although salmeterol plus fluticasone reduced the risk of mortality by 33% in GOLD stage II patients, no p-value was reported (Citation31).
Compliance
Only a small number of studies reported compliance rates. In patients with early COPD in a 2-year trial, mean compliance was 72% with fluticasone (Citation40).
Discussion
This systematic review revealed a wealth of data in patients with moderate COPD (e.g., GOLD stage II). However, only 2 RCTs of exclusive populations with mild COPD (e.g., GOLD stage I) were identified; 1 conducted over 2 years (Citation32) and 1 of a short duration (Citation37). In most of the other trials, the patients were GOLD stage II or more severe, or were an unstratified combination of GOLD stage I and II patients. Some of the non-randomized studies also used exclusive or stratified populations of GOLD stage I patients, but they were generally very small and usually observational (Citation63–69).
The results from this review show that even patients with milder or moderate COPD (FEV1 ≥ 50% predicted) can have substantial limitations and physical impairment relative to normal individuals, and these impairments worsen over time. The progressive deterioration in lung function over the course of a patient's lifetime is likely to be greatest in those diagnosed with mild-to-moderate COPD at a younger age than in elderly patients (e.g., > 65 years) with more severe COPD. Although the effect of patient age was not addressed by the current systematic review, evidence suggests that intervening early with effective respiratory therapy may not only improve patient outcomes, but also modify the course of disease (Citation70).
Among the RCTs identified in our analysis, FEV1 decline in patients with mild COPD was reported at a rate of 14 ml/year (Citation32), however this result should be interpreted with caution due to the small number of patients (n = 24), and the relatively large standard error (17 ml/year). In much larger groups of patients with moderate (GOLD stage II) disease in the UPLIFT® and TORCH RCTs, rate of decline in FEV1 was in the range of 49–60 ml/year (Citation30,31). In a large population-based study, rates of decline in FEV1 of 44 ml/year were reported for symptomatic GOLD stage I patients compared with 34 ml/year in individuals with normal lung function (Citation6). As a comparison, decline in FEV1 was 17.6–19.6 ml/year in healthy never-smokers in the Framingham Cohort Study (Citation71).
Even patients with mild disease experience exacerbations (Citation32, Citation34). For GOLD stage II patients, the annual rate of exacerbations leading to hospitalization (severe exacerbations) appears to be in the region of 0.7% to 0.9%, taken from 2 studies (Citation30, 31).
Patients with mild (Citation32, Citation37) and more moderate COPD (Citation29, Citation58, Citation59) also frequently experienced aggravated respiratory symptoms, and these were also shown to worsen over time (Citation32). It is possible that patient selection in the EUROSCOP study may have been biased towards those with symptoms (all were current smokers at the start of the study) (Citation59).
Additionally, QoL was shown to be reduced in patients with mild or moderate COPD (Citation43). The GOLD stage II subgroup analyses from the 2 large trials, UPLIFT® and TORCH, demonstrated conflicting trends for SGRQ scores over time (Citation30, 31), although the absolute changes were small and not clinically significant. A recently published paper by Jones et al. reported impaired SGRQ total scores at baseline in patients with GOLD stage I and stage II disease (38.5 and 40.4 units, respectively), which are comparable to those reported in the studies analyzed in this review (Citation72).
One study suggested that patients with mild COPD do not experience much functional impairment (Citation32), but several studies showed that exercise capacity was reduced in patients with mild or moderate COPD (Citation37,Citation46,Citation47,Citation49). However, the findings by O'Donnell et al. (Citation37) that symptom-limited peak work rate and oxygen consumption was reduced in 16 GOLD stage I patients is not a universal finding (Citation73), and this discrepancy between studies illustrates the need to study a larger number of individuals randomly selected from the general population to obtain an unbiased study population.
Among the papers identified, there were no data available for mortality rates in GOLD stage I patients. However, in 1 study that did not meet our search criteria GOLD stage I and II patients had a significantly higher risk of mortality compared to control subjects with normal lung function (hazard ratios of 1.4 [95% CI, 1.1–1.6] and 2.4 [95% CI, 2.0–2.9]) (Citation74), highlighting that, even in patients with the earliest stages of COPD, mortality is increased compared to patients without COPD. Among the RCTs reporting on GOLD stage II patients, there were mortality rates of 10% to 11% over 3–4 years in the placebo groups (Citation30,31), and a further study reported 0.8% mortality in mild-to-moderate COPD patients (mean pre-bronchodilator FEV1 76.8% to 76.9% predicted) (Citation38).
It is important to note that, when considering disease progression in placebo groups, the data may be confounded by the use of different concomitant medications permitted during some of the trials. For example, in the UPLIFT® study, all concomitant respiratory drugs, apart from inhaled anticholinergic drugs, were allowed during the trial (Citation30). In the TORCH study, patients could receive concomitant short-acting bronchodilators, short-term oral corticosteroids, theophyllines, and oxygen <12 hours per day, but no other medications (such as long-acting anticholinergics) (Citation31).
In terms of pharmaceutical interventions, the longer-term (≥6 months) RCTs suggest that certain medications such as long-acting bronchodilators, alone or in combination, can slow the rate of decline in lung function and improve exacerbations and QoL or health status outcomes of GOLD stage II patients, whilst other medications showed little benefit. In the UPLIFT® study, tiotropium reduced the decline in lung function, exacerbation rates and QoL in GOLD stage II patients compared with placebo (Citation30). Similar findings were reported for the TORCH trial where the rate of decline in FEV1 appeared to be reduced by salmeterol plus fluticasone and, to a lesser extent, salmeterol monotherapy (Citation31); salmeterol plus fluticasone also improved exacerbations and health status compared with placebo (Citation31). In a further study, salmeterol was shown to be better than theophylline for improving lung function and QoL, and theophylline appeared not to be effective at preventing lung function decline (Citation41).
In terms of ICS monotherapy, budesonide has been shown to reduce the exacerbation rate (Citation34) and decline in lung function (Citation38) compared with placebo in GOLD stage I/II patients. However, in the EUROSCOP study, the pattern of lung function improvement with budesonide showed the “inverted hockey stick” phenomenon (Citation75), whereby an early increase in FEV1 (over the first 6 months of the trial) was followed by a decline approximately parallel to that of the placebo arm (from 9 months until the end of treatment) (Citation38). This “inverted hockey stick” effect suggests that, although corticosteroid therapy treats inflammation in COPD, the course of disease progression remains unaltered (Citation75). Another 3-year, parallel-group, randomized, double-blind, placebo-controlled trial of budesonide including patients with mild COPD (mean FEV1 86% predicted) demonstrated no difference in outcomes versus placebo for rate of decline in FEV1, respiratory symptoms or exacerbations (Citation76). However, this study was excluded from the current systematic analysis because the outcomes reported for the patient population were not stratified by disease severity, and a sizeable proportion had severe or very severe COPD (FEV1 < 50% predicted).
The evidence for fluticasone monotherapy in early COPD was more variable. For example, fluticasone monotherapy had a detrimental effect on lung function, exacerbations and symptoms in early COPD compared with placebo, but this may be confounded by the small size of the study (Citation32). The larger TORCH study found small benefits for fluticasone monotherapy versus placebo in GOLD stage II patients, although a higher rate of pneumonia was also reported with fluticasone (Citation31). A shorter-term RCT in 592 patients found a significant improvement in lung function with a combination of tiotropium and formoterol compared with salmeterol with fluticasone at 6 weeks (p = 0.0006) (Citation42).
The Lung Health Study showed that predictors of change in lung function include responsiveness to a β2-agonist, baseline FEV1, methacholine reactivity, age, sex, race, and baseline smoking rate—but not baseline respiratory symptoms (Citation29). In women, more severe airway obstruction was associated with an accelerated decline in FEV1 (Citation59).
In terms of mortality, current data show no significant effect for budesonide (Citation38) or tiotropium (Citation30) on mortality rates in GOLD II stage patients. In a further study, a combination of salmeterol plus fluticasone reduced the risk of mortality by 33% in GOLD stage II patients (Citation31). A reduction in mortality was seen in the UPLIFT® overall population with tiotropium compared with control, although it was not observed in the GOLD stage II patients (Citation30). This may be because fewer patients died in the GOLD stage II group. Therefore, other endpoints may be more useful in this milder population. Overall, mortality is generally reduced (at least numerically) by long-acting bronchodilators, which have a good safety profile.
Several studies have shown that smoking cessation is beneficial in COPD. For example the Lung Health Study showed that smoking cessation decreased the rate of decline in FEV1 by 50% compared with continued smoking (Citation29). Smoking cessation is still the most effective way to modify the natural course of the disease and as such, is a fundamental aspect of COPD treatment (Citation1).
Little benefit was observed for both supervised exercise and unsupervised exercise in 1 study (Citation47), although a shorter-term study found that exercise capacity increased with training (Citation48). In a further short-term study, improvement in exercise capacity correlated with an increase in FEV1 (Citation46). However, there are many benefits to maintaining physical activity in all stages of the disease, including early COPD (Citation77). Indeed, there is also evidence that higher levels of physical activity even in normal individuals lead to slower decline in lung function and reduced mortality (Citation78).
This review highlights the challenges faced in the management of patients with milder COPD (e.g., GOLD stage I), for whom few evidence-based guidelines exist and in whom few RCTs have been performed. One limitation is that patients with COPD may discontinue their treatment program early, although this can vary depending on the treatment (Citation74).
Despite its systematic nature, the present literature review has some limitations. Due to wide scope of the subject matter, search criteria were limited in a relatively stringent manner to identify a manageable number of publications to assess and review; therefore some relevant articles may have been unintentionally omitted. Additionally, no statistical methods were employed to analyze the results from the included studies, therefore the results can be considered descriptive only. Nonetheless, the review covers comprehensively the available literature relating to mild-to-moderate COPD up until July 30, 2010, and the benefits of interventions in patients with this earlier-stage disease.
Interesting results from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study have become available since the literature search was conducted for the current systematic review. This large observational study included patients with COPD (n = 2164), smokers with normal lung function (n = 337), and never smokers (n = 245), and was conducted at 46 centers in 12 countries (Citation79). The aim was to define COPD phenotypes and identify parameters that help to predict disease progression. The results showed that comorbidities were more prevalent in COPD patients than in controls, but occurred to the same extent, irrespective of GOLD stage.
For the 954 patients with moderate (GOLD stage II) COPD, mean FEV1 was 63.6% predicted in women and 62.8% in men. The mean distances covered on the 6MWD test were 391 and 415 meters, and the mean SGRQ total score was 43.8 and 41.6, respectively. The COPD exacerbation rate in the year prior to the study was 0.8 in women and 0.5 in men. As expected, despite considerable heterogeneity within each GOLD category, symptoms and exercise capacity generally deteriorated with advancing disease stage and the number of exacerbations increased. Supporting the conclusions of our literature review, even patients with moderate (GOLD stage II) COPD reported symptoms, exercise limitation and frequent exacerbations; a more detailed analysis of exacerbations in ECLIPSE over 3 years (Citation80) found that 22% of patients with GOLD stage II COPD (n = 945) experienced two or more exacerbations in the first year of follow-up. The occurrence of exacerbations was related to a susceptibility phenotype, independent of disease severity, and associated with factors including exacerbation history and impaired health status (Citation80).
Conclusions
Patients with mild-to-moderate COPD experience considerable morbidity and mortality. Early intervention could reduce this and may potentially affect disease progression. Further RCTs are needed in GOLD stage I and II patients, with complete stratification to assess disease progression and outcomes fully, compared with more severe COPD. It would also be important to determine the prognosis of different patient subgroups, for example, gender, age, and smoking status.
Declaration of interest
FM has received fees for speaking at conferences sponsored by Boehringer Ingelheim, Pfizer, and GlaxoSmithKline, and has served on advisory boards for GlaxoSmithKline and Boehringer Ingelheim. He has received research grants for participating in multicenter trials sponsored by GlaxoSmithKline, Boehringer Ingelheim, Altana Pharma, Merck, Astra Zeneca, Nycomed, and Novartis, and has received unrestricted research grants from Boehringer Ingelheim and GlaxoSmithKline. He holds a CIHR/GSK research chair on COPD. ND is employed by PAREXEL, and her work was funded jointly by Boehringer Ingelheim and Pfizer. CC has received honoraria or consultancy fees from Abbott, Actelion, AstraZeneca, Bayer, Bioavail, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, InterMune, Janssen-Ortho, Merck, Novartis, Nycomed, Ortho-McNeill, Pfizer, and Sanofi-Aventis. He has also received grants from Actelion, AstraZeneca, Biogen, Gilead, InterMune, and Janssen-Ortho, and speaker bureau fees from VitalAire. All authors were involved in the drafting and editing of the manuscript, and approved the final article.
Acknowledgments
CC and FM were responsible for the overall concept of the study; ND was responsible for design of the literature search strategy and for preliminary analysis of results. The authors would like to thank PAREXEL for assistance with literature searches, figure preparation, and data checking, work that was jointly funded by Boehringer Ingelheim International GmbH & Co. KG and Pfizer Inc.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2010. http://www goldcopd com.
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E; BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007 Sep 1; 370(9589):741–750.
- Troosters T, Sciurba F, Battaglia S, Langer D, Valluri SR, Martino L, Benzo R, Andre D, Weisman I, Decramer M. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med 2010 Jul; 104(7):1005–1011.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2011. http://www.goldcopd.com.
- Benfield T, Lange P, Vestbo J. COPD stage and risk of hospitalization for infectious disease. Chest 2008 Jul; 134(1):46–53.
- Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz JM, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax 2008 Sep; 63(9):768–774.
- de Marco R, Accordini S, Cerveri I, Corsico A, Sunyer J, Neukirch F, Künzli N, Leynaert B, Janson C, Gislason T, Vermeire P, Svanes C, Anto JM, Burney P; European Community Respiratory Health Survey Study Group. An international survey of chronic obstructive pulmonary disease in young adults according to GOLD stages. Thorax 2004 Feb; 59 (2):120–125.
- de Marco R, Accordini S, Anto JM, Gislason T, Heinrich J, Janson C, Jarvis D, Künzli N, Leynaert B, Marcon A, Sunyer J, Svanes C, Wjst M, Burney P. Long-term outcomes in mild/moderate chronic obstructive pulmonary disease in the European community respiratory health survey. Am J Respir Crit Care Med 2009 Nov 15; 180(10):956–963.
- Ekberg-Aronsson M, Pehrsson K, Nilsson JA, Nilsson PM, Löfdahl CG. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res 2005 Aug 25; 6:98.
- Geijer RM, Sachs AP, Verheij TJ, Lammers JW, Salome PL, Hoes AW. Are patient characteristics helpful in recognizing mild COPD (GOLD I) in daily practice? Scand J Prim Health Care 2006 Dec; 24(4):237–242.
- Kogler H, Metzdorf N, Glaab T, Welte T. Preselection of patients at risk for COPD by two simple screening questions. Respir Med 2010 Jul; 104(7):1012–1019.
- Kornmann O, Beeh KM, Beier J, Geis UP, Ksoll M, Buhl R; Global Initiative for Obstructive Lung Disease. Newly diagnosed chronic obstructive pulmonary disease. Clinical features and distribution of the novel stages of the Global Initiative for Obstructive Lung Disease. Respiration 2003 Jan-Feb; 70(1):67–75.
- Lange P, Mogelvang R, Marott JL, Vestbo J, Jensen JS. Cardiovascular morbidity in COPD: A study of the general population. COPD 2010 Feb; 7(1):5–10.
- Maleki-Yazdi MR, Lewczuk CK, Haddon JM, Choudry N, Ryan N. Early detection and impaired quality of life in COPD GOLD stage 0: a pilot study. COPD 2007 Dec; 4(4):313–320.
- Pedone C, Scarlata S, Sorino C, Forastiere F, Bellia V, Antonelli Incalzi R,. Does mild COPD affect prognosis in the elderly? BMC Pulm Med 2010 Jun 7; 10:35.
- Stav D, Raz M. Prevalence of chronic obstructive pulmonary disease among smokers aged 45 and up in Israel. Isr Med Assoc J 2007 Nov; 9(11):800–802.
- Stratelis G, Jakobsson P, Molstad S, Zetterstrom O. Early detection of COPD in primary care: screening by invitation of smokers aged 40 to 55 years. Br J Gen Pract 2004 Mar; 54(500):201–206.
- Toljamo T, Kaukonen M, Nieminen P, Kinnula VL. Early detection of COPD combined with individualized counselling for smoking cessation: a two-year prospective study. Scand J Prim Health Care 2010 Mar; 28(1):41–46.
- Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2002 Aug 1; 166(3):329–332.
- Zielinski J, Bednarek M; Know the Age of Your Lung Study Group. Early detection of COPD in a high-risk population using spirometric screening. Chest 2001 Mar; 119(3):731–736.
- Zielinski J, Bednarek M, Górecka D, Viegi G, Hurd SS, Fukuchi Y, Lai CK, Ran PX, Ko FW, Liu SM, Zheng JP, Zhong NS, Ip MS, Vermeire PA. Increasing COPD awareness. Eur Respir J 2006 Apr; 27(4):833–852.
- Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O'Donnell DE. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med 2009 Nov 15; 180(10):964–971.
- Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med 2006 Jan; 100(1):115–122.
- Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax 2010 Sep; 65(9):837–841.
- Price D, Freeman D, Cleland J, Kaplan A, Cerasoli F. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J 2011 Mar; 20(1):15–22.
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA Jr, Enright PL, Kanner RE, O'Hara P, Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994 Nov 16; 272(19):1497–1505.
- Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002 Sep 1; 166(5):675–679.
- Burchfiel CM, Marcus EB, Curb JD, MacLean CJ, Vollmer WM, Johnson LR, Fong KO, Rodriguez BL, Masaki KH, Buist AS. Effects of smoking and smoking cessation on longitudinal decline in pulmonary function. Am J Respir Crit Care Med 1995 Jun; 151(6):1778–1785.
- Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med 2000 Feb; 161(2 Pt 1):381–390.
- Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP; UPLIFT Investigators. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009 Oct 3; 374(9696):1171–1178.
- Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, Yates JC, Willits LR, Vestbo J. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009 Jun 30; 10:59.
- van Grunsven P, Schermer T, Akkermans R, Albers M, van den Boom G, van Schayck O, van Herwaarden C, van Weel C. Short- and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respir Med 2003 Dec; 97(12):1303–1312.
- Kryger M, Roth T, Wang-Weigand S, Zhang J. The effects of ramelteon on respiration during sleep in subjects with moderate to severe chronic obstructive pulmonary disease. Sleep Breath 2009 Mar; 13(1):79–84.
- Löfdahl CG, Postma DS, Pride NB, Boe J, Thorén A. Possible protection by inhaled budesonide against ischaemic cardiac events in mild COPD. Eur Respir J 2007 Jun; 29(6):1115–1119.
- Dal Negro R, Visconti M, Trevisan F, Bertacco S, Micheletto C, Tognella S. Erdosteine enhances airway response to salbutamol in patients with mild-to-moderate COPD. Ther Adv Respir Dis 2008 Oct; 2(5):271–277.
- Kryger M, Wang-Weigand S, Zhang J, Roth T. Effect of ramelteon, a selective MT(1)/MT (2)-receptor agonist, on respiration during sleep in mild to moderate COPD. Sleep Breath 2008 Aug; 12(3):243–250.
- O'Donnell DE, Laveneziana P, Ora J, Webb KA, Lam YM, Ofir D. Evaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPD. Thorax 2009 Mar; 64(3):216–223.
- Pauwels RA, Löfdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, Ohlsson SV; European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med 1999 Jun 24; 340(25):1948–1953.
- Rennard SI, Fogarty C, Kelsen S, Long W, Ramsdell J, Allison J, Mahler D, Saadeh C, Siler T, Snell P, Korenblat P, Smith W, Kaye M, Mandel M, Andrews C, Prabhu R, Donohue JF, Watt R, Lo KH, Schlenker-Herceg R, Barnathan ES, Murray J; COPD Investigators. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007 May 1; 175(9):926–934.
- van Grunsven PM, van Schayck CP, van Deuveren M, van Herwaarden CL, Akkermans RP, van Weel C. Compliance during long-term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease (COPD): results of the Detection, Intervention, and Monitoring Program of COPD and Asthma (DIMCA) Study. J Asthma 2000 May; 37(3):225–234.
- Di Lorenzo G, Morici G, Drago A, Pellitteri ME, Mansueto P, Melluso M, Norrito F, Squassante L, Fasolo A; SLMT02 Italian Study Group. Efficacy, tolerability, and effects on quality of life of inhaled salmeterol and oral theophylline in patients with mild-to-moderate chronic obstructive pulmonary disease. Clin Ther 1998 Nov-Dec;20(6):1130–1148.
- Rabe KF, Timmer W, Sagkriotis A, Viel K. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest 2008 Aug; 134(2):255–262.
- Kanehara M, Yokoyama A, Tomoda Y, Shiota N, Iwamoto H, Ishikawa N, Taooka Y, Haruta Y, Hattori N, Kohno N. Anti-inflammatory effects and clinical efficacy of theophylline and tulobuterol in mild-to-moderate chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2008 Dec; 21(6):874–878.
- D'Elia C, Sechi F. Neltenexine versus carbocysteine in the treatment of exacerbations of mild chronic obstructive pulmonary disease: a randomized, controlled, open-label study. Curr Ther Res. Clin Exp 2001; 62:851–861.
- Santra CK. Treatment of moderate chronic obstructive pulmonary disease (stable) with doxofylline compared with slow release theophylline–a multicentre trial. J Indian Med Assoc 2008 Dec; 106(12):791–2, 794.
- AkkocaYildiz O, Onen ZP, Demir G, Eriş Gülbay B, Saryal S, Karabiyikoğlu G. Is there any difference between effects of ipratropium bromide and formoterol on exercise capacity in moderate COPD patients? Tuberk Toraks 2006; 54(2):105–113.
- Spencer LM, Alison JA, McKeough ZJ. Maintaining benefits following pulmonary rehabilitation: a randomised controlled trial. Eur Respir J 2010 Mar; 35(3):571–577.
- Solèr M, Mütterlein R, Cozma G; Swiss-German OM-85 Study Group. Double-blind study of OM-85 in patients with chronic bronchitis or mild chronic obstructive pulmonary disease. Respiration 2007; 74(1):26–32.
- Clark CJ, Cochrane LM, Mackay E, Paton B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J 2000 Jan; 15(1):92–97.
- Haider T, Casucci G, Linser T, Faulhaber M, Gatterer H, Ott G, Linser A, Ehrenbourg I, Tkatchouk E, Burtscher M, Bernardi L. Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J Hypertens 2009 Aug; 27(8):1648–1654.
- Faager G, Stâhle A, Larsen FF. Influence of spontaneous pursed lips breathing on walking endurance and oxygen saturation in patients with moderate to severe chronic obstructive pulmonary disease. Clin Rehabil 2008 Aug; 22(8):675–683.
- Fujimoto K, Matsuzawa Y, Yamaguchi S, Koizumi T, Kubo K. Benefits of oxygen on exercise performance and pulmonary hemodynamics in patients with COPD with mild hypoxemia. Chest 2002 Aug; 122(2):457–463.
- Harber P, Tashkin DP, Simmons M, Crawford L, Hnizdo E, Connett J; Lung Health Study Group. Effect of occupational exposures on decline of lung function in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007 Nov 15; 176(10):994–1000.
- Kanner RE, Anthonisen NR, Connett JE; Lung Health Study Research Group. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001 Aug 1; 164(3):358–364.
- Kanner RE, Connett JE, Altose MD, Buist AS, Lee WW, Tashkin DP, Wise RA. Gender difference in airway hyperresponsiveness in smokers with mild COPD. The Lung Health Study. Am J Respir Crit Care Med 1994 Oct; 150(4):956–961.
- Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med 1999 Apr; 106(4):410–416.
- Tashkin DP, Altose MD, Connett JE, Kanner RE, Lee WW, Wise RA. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. The Lung Health Study Research Group. Am J Respir Crit Care Med 1996 Jun; 153(6 Pt 1):1802–1811.
- Magnussen H, Watz H, Zimmermann I, Macht S, Greguletz R, Falques M, Jarreta D, Garcia Gil E. Peak inspiratory flow through the Genuair inhaler in patients with moderate or severe COPD. Respir Med 2009 Dec; 103(12):1832–1837.
- Watson L, Vonk JM, Löfdahl CG, Pride NB, Pauwels RA, Laitinen LA, Schouten JP, Postma DS; European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. Predictors of lung function and its decline in mild to moderate COPD in association with gender: results from the Euroscop study. Respir Med 2006 Apr; 100(4):746–753.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure for chronic airflow limitation –the St George's Respiratory Questionnaire. Am Rev Respir Dis 1992 Jun; 145(6):1321–1327.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive disease. Am J Respir Crit Care Med 2001 Apr; 163(5):1256–1276.
- Antonelli-Incalzi R, Imperiale C, Bellia V, Catalano F, Scichilone N, Pistelli R, Rengo F; SaRA Investigators. Do GOLD stages of COPD severity really correspond to differences in health status? Eur Respir J 2003 Sep; 22(3):444–449.
- Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008 Mar 15; 177(6):622–629.
- Bernardi L, Casucci G, Haider T, Brandstätter E, Pocecco E, Ehrenbourg I, Burtscher M. Autonomic and cerebrovascular abnormalities in mild COPD are worsened by chronic smoking. Eur Respir J 2008 Dec; 32(6):1458–1465.
- de Torres JP, Pinto-Plata V, Casanova C, Mullerova H, Córdoba-Lanús E, Muros de Fuentes M, Aguirre-Jaime A, Celli BR. C-reactive protein levels and survival in patients with moderate to very severe COPD. Chest 2008 Jun; 133(6):1336–1343.
- Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K. Six-minute walking and pulmonary function test outcomes during the early period after lung cancer surgery with special reference to patients with chronic obstructive pulmonary disease. Jpn J Thorac Cardiovasc Surg 2004 Mar; 52(3):113–119.
- Ohno Y, Iwasawa T, Seo JB, Koyama H, Takahashi H, Oh YM, Nishimura Y, Sugimura K. Oxygen-enhanced magnetic resonance imaging versus computed tomography: multicenter study for clinical stage classification of smoking-related chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008 May; 177(10):1095–1102.
- Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, Moore AJ, Moxham J, Polkey MI. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007 Feb; 62(2):115–120.
- Torchio R, Gulotta C, Parvis M, Pozzi R, Giardino R, Borasio P, Greco Lucchina P. Gas exchange threshold as a predictor of severe postoperative complications after lung resection in mild-to-moderate chronic obstructive pulmonary disease. Monaldi Arch Chest Dis 1998 Apr; 53(2):127–133.
- Morice AH, Celli B, Kesten S, Lystig T, Tashkin D, Decramer M, COPD in young patients: a pre-specified analysis of the four-year trial of tiotropium (UPLIFT). Respir Med 2010 Nov; 104(11):1659–1667.
- Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 2009 Jul 1; 180(1):3–10.
- Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, Perez T, Soler Cataluña JJ, van der Molen T, Adamek L, Banik N. Properties of the COPD Assessment Test (CAT) in a cross-sectional European study. Eur Respir J 2011 Jul; 38(1):29–35.
- Pinto-Plata VM, Celli-Cruz RA, Vassaux C, Torre-Bouscoulet L, Mendes A, Rassulo J, Celli BR. Differences in cardiopulmonary exercise test results by American Thoracic Society/European Respiratory Society-Global Initiative for Chronic Obstructive Lung Disease stage categories and gender. Chest 2007 Oct; 132(4):1204–1211.
- Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk inCommunities (ARIC) study. Respir Med 2006 Jan; 100(1):115–122.
- van den Boom G, Rutten-van Mölken MP, Molema J, Tirimanna PR, van Weel C, van Schayck CP, The cost effectiveness of early treatment with fluticasone propionate 250 microg twice a day in subjects with obstructive airway disease. Results of the DIMCA program. Am J Respir Crit Care Med 2001 Dec 1; 164(11):2057–2066.
- Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999 May 29; 353(9167):1819–1823.
- Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, Wouters E, Sethi S, Cooper CB. COPD as a lung disease with systemic consequences–clinical impact, mechanisms, and potential for early intervention. COPD 2008 Aug; 5(4):235–256.
- Pelkonen M, Notkola IL, Lakka T, Tukiainen HO, Kivinen P, Nissinen A. Delaying decline in pulmonary function with physical activity: a 25-year follow-up. Am J Respir Crit Care Med 2003 Aug 15; 168(4):494–499.
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK, Tal-Singer R, Wouters E, Yates JC, Vestbo J. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators, Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010 Sep 10; 11:122.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators, Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010 Sep 16; 363(12):1128–1138.