Abstract
Indacaterol is an inhaled, once-daily, long-acting ®2-agonist for the treatment of COPD. Most previous studies were conducted with doses of 150 and/or 300 μg once-daily, and data with the 75 μg dose are limited. Two identically designed studies were, therefore, conducted to evaluate the efficacy and safety of the 75 μg once-daily dose. In two double-blind studies conducted in the USA, patients with moderate-to-severe COPD were randomized to treatment with indacaterol 75 μg once-daily (n = 163 and 159) or matching placebo (n = 160 and 159) for 12 weeks. The primary variable was forced expiratory volume in 1 s measured 24 h post-dose after 12 weeks (reported elsewhere). This report describes secondary efficacy endpoints, including transition dyspnea index (TDI) and St George's Respiratory Questionnaire (SGRQ) total scores, and the percentages of patients with improvements of or above the minimal clinically important difference (MCID; ≥1 in TDI score and ≥4 in SGRQ score). Differences between indacaterol and placebo for TDI total score at week 12 were 1.23 (p < 0.001) and 0.45 (p = 0.16), with odds ratios for achieving the MCID of 2.19 (p = 0.002) and 1.58 (p = 0.065). SGRQ total score decreased (improved) from baseline by 5.8 and 4.9 units with indacaterol at week 12 (2.0 and 0.9 with placebo), with odds ratios for achieving the MCID of 1.80 (p = 0.024) and 1.71 (p = 0.031). Patients receiving indacaterol had statistically significant or numerical improvements in diary-derived symptom variables compared with placebo. Treatment with indacaterol 75 μg may provide useful improvements in patient-reported outcomes in patients with moderate-to-severe COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) management guidelines recommend regular treatment with a long-acting inhaled bronchodilator for patients with moderate or more severe disease (Citation1). Indacaterol is a long-acting inhaled β2-agonist bronchodilator providing 24-hour bronchodilation on once-daily dosing in patients with moderate-to-severe COPD. The efficacy and safety of indacaterol in COPD have been previously demonstrated in placebo-controlled studies of 3–12 months’ duration using treatment with once-daily doses of 150 and/or 300 μg (Citation2–6).
One of the previous 6-month studies with indacaterol (Citation3) incorporated an initial dose-finding stage, which evaluated indacaterol doses of 75, 150, 300 and 600 μg once-daily. The 150 μg and 300 μg doses were carried forward into the remainder of the study based on pre-set efficacy criteria applied to a period of at least 2 weeks of treatment (Citation7). Although the lowest dose, 75 μg, did not meet the efficacy criteria that were set for that study, it did demonstrate 24-hour bronchodilator efficacy against placebo, and further evaluation of this dose against a range of outcomes and over a longer time was considered worthwhile. The 75 μg once-daily dose is now approved for use in the United States as a long-term maintenance treatment for patients with COPD.
To investigate further the potential utility of the 75 μg once-daily dose of indacaterol, 2 identically designed, placebo-controlled, 12-week studies were conducted (2 studies being required for drug approval in the United States). The primary objective of both studies was to evaluate the efficacy of indacaterol on ‘trough’ (24 hours post-dose) forced expiratory volume in 1 second (FEV1) after 12 weeks compared with placebo. These results, and those for the safety and tolerability assessments, have been reported elsewhere (Citation8). We report here the results from both studies on the efficacy of indacaterol on patient-reported outcomes, including dyspnea and health status.
Methods
Study design
The studies were randomized, double-blind, placebo-controlled studies of identical design (registered at ClinicalTrials.gov as NCT01072448 and NCT01068600). The studies comprised a pre-screening visit at which informed consent was obtained and current COPD therapy was adjusted (see later), followed by up to 14 days to permit medication withdrawal and then a 14-day run-in period when baseline data were collected, after which patients were randomized to study treatment for 12 weeks. Both studies were conducted at centers in the United States in respiratory outpatient clinics, physician's offices and clinical research centers. Signed approval from institutional review board and/or ethics committee was obtained for each center.
Patients
The studies recruited patients with moderate-to-severe COPD (defined according to GOLD 2008 criteria (Citation9)), aged ≥40 years and with a smoking history ≥10 pack-years. Their FEV1 was to be <80% and ≥30% of predicted normal and FEV1/forced vital capacity <70% at screening, both measured post-bronchodilator (albuterol 90 μg · 4 puffs).
Patients were excluded if they had an exacerbation of COPD or respiratory tract infection within 6 weeks prior to screening. Patients were also excluded if they had a history of asthma or other pulmonary disease, diabetes Type I or uncontrolled diabetes Type II, or a clinically significant condition such as unstable ischemic heart disease, arrhythmia (excluding chronic stable atrial fibrillation) or other clinically significant ECG findings, uncontrolled hypertension and other significant cardiac disease or conduction defect.All patients gave their written informed consent.
Treatments
Patients received treatment with indacaterol 75 μg once-daily via single-dose dry powder inhaler or matching placebo, taken at the same time each morning (08.00–11.00). Patients receiving inhaled corticosteroids (ICS) at baseline continued this treatment (or the ICS component alone if taken as a fixed combination with a bronchodilator) at equivalent dose and regimen during the study. Albuterol was available for rescue use. All other bronchodilators were discontinued at a pre-screening visit with appropriate washout periods (tiotropium, 7 days; theophylline, 7 days; long-acting β2-agonists, 48 hours; short-acting anticholinergics, 8 hours; short-acting β2-agonists (other than study rescue albuterol), 6 hours).
Objectives, assessments and outcome measures
The results for the primary objective (the effect of indacaterol on ‘trough’ FEV1 after 12 weeks compared with placebo), and those for the safety and tolerability assessments, have been reported elsewhere (Citation8). This report focuses on previously unpublished secondary efficacy outcomes, including dyspnea (key secondary objective) and health status analyzed by change from baseline at week 12 and proportions of patients achieving the minimum clinically important differences (MCID), patient-reported symptoms and exacerbations of COPD. Dyspnea was measured by the transition dyspnea index (TDI), with a difference of ≥1 point between treatments in total score representing the MCID (Citation10–12). The TDI total score was measured after 4 and 12 weeks of treatment. The studies were designed to be large enough to have sufficient power to assess both the primary and key secondary objectives. The key secondary objective was the efficacy of indacaterol versus placebo on the 12-week TDI total score.
Health status was assessed by the St George's Respiratory Questionnaire (SGRQ), with a difference of ≥4 units relative to baseline (at an individual patient level) or relative to placebo (in group mean scores) representing the MCID (Citation13,14). The SGRQ, which is completed by the patient, contains 50 questions in 3 component areas: symptoms of disease, activity (i.e., limitations that the disease places on the patient), and impacts (of the disease). The component scores are summed to provide an SGRQ total score of between 0 (best) and 100 (worst). SGRQ total score was measured after 4 and 12 weeks of treatment.
Patients recorded their use of albuterol as rescue medication and their symptoms (cough, wheeze, sputum volume, sputum color, breathlessness) on a 0–3 scale each morning and evening in an electronic diary. Each morning, patients rated their respiratory symptoms during the night (0, no waking due to symptoms; 1, woke up once due to symptoms; 2, woke up more than once due to symptoms; 3, woke up frequently or could not sleep due to symptoms). Each evening, patients recorded whether respiratory symptoms had stopped them performing their usual daily activities (0, not at all; 1, a little; 2, quite a lot; 3, completely) and the activities that made them first feel breathless in the last 12 hours (0, never or only when running; 1, when walking uphill or up stairs; 2, when walking on flat ground; 3, at rest). The percentages of days without symptoms, days able to perform usual activities and nights without awakenings over 12 weeks were derived from the diary data.
COPD exacerbation frequency was measured as an exploratory efficacy endpoint (exploratory because these were only 12-week studies). An exacerbation was defined as worsening of 2 or more major symptoms (dyspnea, sputum volume or purulence), or worsening of 1 major symptom and 1 minor symptom (sore throat, cold, fever without other cause, increased cough, increased wheeze) for at least 2 consecutive days, and requiring treatment with systemic corticosteroids and/or antibiotic. Exacerbations were considered to be of moderate severity if they were treated with systemic corticosteroids and/or antibiotics, and severe if the patient was also hospitalized. Randomization (1:1 ratio) was stratified by smoking status (current/ex-smoker) and ICS use (yes/no).
Statistical methods
The analysis of all efficacy variables was conducted using the full analysis population, comprising all randomized patients who received at least 1 dose of study drug, analyzed according to the treatment to which they were randomized.
The TDI total score was analyzed using a mixed-model analysis of covariance (ANCOVA) containing treatment as a fixed effect and BDI (baseline dyspnea index) score and baseline FEV1 reversibility as covariates. The model also included baseline smoking status, ICS use and country as fixed effects and center as a random effect. A similar model was used to analyze rescue albuterol use, with appropriate baseline measurements as covariates. SGRQ scores are summarized as raw mean changes from baseline. For TDI and SGRQ total scores, missing week 12 values were imputed using the week 4 observation carried forward. The percentages of patients achieving clinically important improvements in TDI total score and SGRQ total score were analyzed using logistic regression with the same covariates as the ANCOVA. Additionally, the week 12 TDI and SGRQ results are presented in subgroups of patients according to COPD severity (moderate, GOLD II; severe or worse, GOLD III–IV). These subgroup data, exacerbation frequency (without imputation) and diary symptom data were summarized descriptively.
To allow for multiplicity, a hierarchical testing procedure was used to maintain an alpha level of 0.05 for the primary and key secondary variables (trough FEV1 and TDI total score at week 12). As described elsewhere (Citation8), superiority for the primary efficacy variable was demonstrated in each study. Other secondary analyses reported here were not adjusted for multiplicity. Results are shown as least squares means with standard errors for group mean values and 95% confidence intervals (CIs) for differences between treatments. The TDI and SGRQ total score responder analyses are presented as odds ratios and associated 95% CI and two-sided p-values.
The studies were sized to have sufficient power for the primary and key secondary objectives. With an estimated standard deviation in TDI total score of 2.76 at 12 weeks (Citation3), 138 evaluable patients per treatment group were required to detect a difference of 1 unit at week 12, using a 5% significance level (2-sided) with 85% power. With a dropout rate of 15%, a sample size of 326 randomized patients (163 per treatment group) was required. This sample size gave >99% power for the primary objective.
Results
Results are described in the order of study 1 (NCT01072448) and study 2 (NCT01068600).
Patients
Patient disposition is shown in . Common reasons for screening failure were failure to meet diagnostic/severity criteria (42% and 27% of those screened), unacceptable test procedure result (16% and 25%) and withdrawal of consent (14% and 15%). The studies were completed by 88.3% and 93.1% of indacaterol-treated patients and by 81.3% and 89.3% of placebo patients. The increased number of dropouts in the placebo group was due mainly to insufficient therapeutic effect and withdrawal of consent. The studies and treatment groups were generally well matched in terms of the patients’ baseline characteristics (); there were, however, some small differences between studies in the proportions of patients with severe COPD, of ICS users and of current smokers. There were small differences between treatment groups in baseline FEV1 and, in study 2, in the proportion of patients with moderate COPD.
Table 1. Patient flow-through studies
Table 2. Demographics and baseline characteristics
Efficacy
In both studies the results for the primary efficacy variable demonstrated the superiority of indacaterol over placebo, with statistically significant and clinically relevant differences in trough FEV1 at week 12 (Citation8). Detailed spirometry results were reported previously (Citation8).
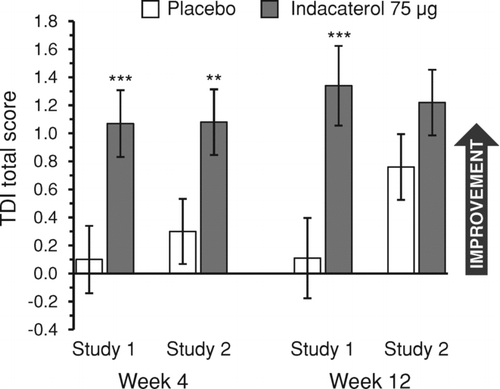
Dyspnea as measured by the TDI total score improved relative to placebo by a statistically significant margin at week 4 in both studies and at week 12 in study 1 only (). The week 12 least-squares mean TDI total scores in the indacaterol and placebo groups were 1.34 (SE 0.284) and 0.11 (SE 0.287) respectively in study 1, and 1.22 (SE 0.234) and 0.76 (SE 0.235) respectively in study 2. The improvements compared with placebo at both time points in study 1 were either very close to (0.97 [95% CI 0.39, 1.55] at week 4) or above (1.23 [95% CI 0.57, 1.89] at week 12) the 1-point MCID.
Figure 1. TDI total score at weeks 4 and 12. Data are least squares means ± standard errors. **p < 0.01, ***p ≤ 0.001 versus placebo. TDI = transition dyspnea index.
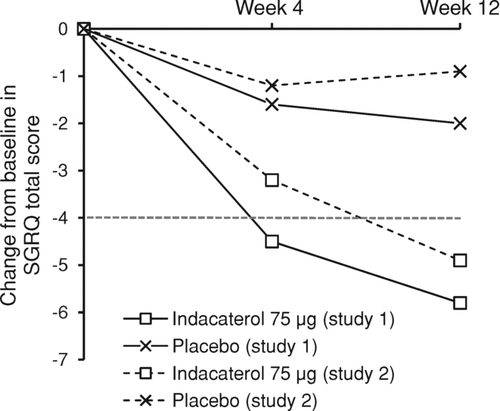
Health status measured by the SGRQ total score improved with indacaterol by a clinically relevant decrease from baseline at week 12 in both studies (). Indacaterol improved the week 12 individual SGRQ component scores from baseline across all domains: symptoms −8.8 (SD 17.78) and −8.5 (SD 17.86) (placebo: −2.0 [SD 14.57] and −1.9 [SD 16.52]); activity −5.7 (SD 13.84) and −4.3 (SD 16.01) (placebo: −2.2 [SD 13.52] and −1.6 13.07), and impacts –4.8 (SD 12.84) and −4.2 (SD 15.63) (placebo: −1.8 [SD 12.55] and −0.1 [SD 12.35]).
Figure 2. Change from baseline in SGRQ total score at weeks 4 and 12. Data are unadjusted (raw) means (week 4 data are without imputation). Broken line indicates level of clinically relevant improvement. SGRQ = St George's Respiratory Questionnaire.
Higher proportions of indacaterol-treated patients achieved the MCID in TDI and SGRQ total scores at week 12 compared with placebo in both studies (). Odds ratios were statistically significantly in favor of indacaterol for TDI total score in study 1 and for SGRQ total score in both studies. Indacaterol had broadly similar effects in subgroups of patients with moderate COPD (GOLD II) or with severe-to-very severe COPD (GOLD III–IV) ().
Figure 3. Percentages of patients with a clinically important improvement in TDI total score and SGRQ total score after 12 weeks of treatment, with associated odds ratios (OR) and p-values for the indacaterol–placebo differences (*p < 0.05, **p < 0.01 versus placebo). TDI = transition dyspnea index; SGRQ = St George's Respiratory Questionnaire.
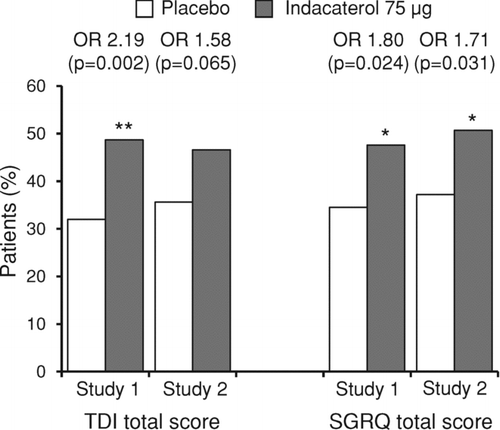
Table 3. TDI and SGRQ results in patient subgroups according to COPD severitya
Supportive analyses without imputation for missing values of the indacaterol–placebo differences at week 12 in TDI and SGRQ total scores and in the analyses of patients achieving the MCIDs showed closely similar results and p-values, with only 1 loss of statistical significance (in study 2, the odds ratio for patients achieving the MCID in SGRQ total score was 1.63 [p = 0.052] without imputation, compared with 1.71 [p = 0.031] with imputation).
Patients receiving indacaterol had statistically significant or numerical improvements in diary-derived symptom variables compared with placebo. The percentage of days able to perform usual activities increased with indacaterol versus placebo numerically in study 1 (38.7% [SE 2.07] versus 33.6% [SE 2.09], p = 0.082), and significantly in study 2 (39.0% [SE 1.97] versus 30.3% [SE 1.94], p < 0.001). The percentage of days with no daytime symptoms increased significantly with indacaterol versus placebo in study 1 (9.5% [SE 1.13] versus 5.0% [SE 1.14], p = 0.003) and numerically in study 2 (8.0% [SE 1.20] versus 5.2% [SE 1.19], p = 0.091). The percentage of nights with no awakenings was similar to and not statistically significantly different from placebo in either study (69.4% [SE 1.88] versus 66.8% [SE 1.88] and 63.4% [SE 1.82] versus 61.5% [SE 1.81]). The use of rescue albuterol (results also reported separately) was significantly reduced with indacaterol compared with placebo in both studies (Citation8). The overall numbers of COPD exacerbations were low in both studies; a small numerical difference in favor of indacaterol in study 1 was not observed in study 2 ().
Table 4. Number and rate of exacerbations of COPD
Safety and tolerability
Adverse events were reported for similar proportions of patients in the two treatment groups overall in both studies, and have been reported in detail previously (Citation8). Briefly, the most common adverse event was COPD worsening, occurring in similar proportions of patients (8–12%) in both active and placebo treatment groups. Headache and nasopharyngitis were more common in the indacaterol treatment group (3–6% and 5–6%, respectively) than with placebo (1–4% and 1–2%); nearly all the cases during indacaterol treatment were mild or moderate in severity. Two patients died during the studies, both from the placebo treatment group.
Discussion
Treatment with indacaterol 75 μg once-daily improved patient-reported outcomes in patients with moderate-to-severe COPD. The improvements in dyspnea in 1 study and in health status in both studies were statistically significant and were often improved by a margin close to, or greater than, the MCID. These are important outcomes for patients with COPD. Dyspnea is the most troublesome symptom of COPD, and is often frightening for the patient (Citation15,16). With increasing severity of the disease, dyspnea increasingly restricts patients’ activities and is one aspect of the impaired health status that is common among COPD patients (Citation17).
Some variations in efficacy were observed between the 2 present studies, notably in terms of the findings for TDI, where statistically significant improvements with indacaterol relative to placebo at week 12 were observed in study 1 but not in study 2. There are no obvious differences in the patient populations to explain the discrepancy. A greater placebo effect on dyspnea in study 2 is one possibility, given that week 12 TDI scores in the placebo groups were higher in study 2 than in study 1, and indacaterol scores were more closely matched between the studies. The consistency in effect of indacaterol in the 2 studies is also borne out by the similar proportions of patients who responded with a clinically important improvement from baseline in TDI total score: 48.7% and 46.6% of patients receiving indacaterol. The exploratory data collected on exacerbations follow a similar pattern in suggesting a greater placebo effect in study 2 than in study 1.
The 75 μg once-daily dose of indacaterol has not been directly compared over 12 weeks with the 150 μg dose approved for use in patients with COPD in countries outside the USA and Canada. Studies have shown that the 150 μg dose generally provides statistically significant and clinically relevant improvements in bronchodilation, dyspnea and health status at week 12, with some variability in results between different studies (Citation2–4; Citation18). Good overall safety and tolerability have been reported with use of once-daily doses higher than 75 μg, with little indication of any dose-response (Citation19).
The efficacy of indacaterol 75 μg on the symptoms of COPD was reflected in other outcomes in the present study, including the symptoms component of the SGRQ score. Improved symptom control with indacaterol relative to placebo was also demonstrated by the statistically significant reductions in use of albuterol as rescue bronchodilator in both studies, as reported previously (Citation8). The higher completion rates with indacaterol provide support for the overall efficacy and acceptability of this treatment. The closely similar outcomes for the TDI and SGRQ results when analyzed with and without imputation for missing values demonstrates that the higher drop-out rate in the placebo group had little if any effect on these outcomes.
Health status, as measured by the SGRQ total score, improved from baseline by a clinically relevant amount with indacaterol in both studies, but not with placebo. The changes from baseline in the SGRQ component scores suggest that indacaterol not only improved the symptoms of COPD but also reduced some of the limitations that the disease puts upon patients’ lives. In addition, health status improvements with indacaterol were observed consistently in patients in GOLD II and GOLD III severity. However, for unknown reasons, results for both SGRQ and TDI for placebo patients in these subgroups were variable.
A number of issues deserve consideration. Our statistical model adjusted for multiplicity in the analysis of the primary and key secondary outcomes (trough FEV1 and TDI total score), allowing for the possibility of type 1 error in the statistically significant p-values attached to other secondary outcomes, including health status. Nevertheless, we believe that the preponderance of p-values of less than 0.05, showing significant efficacy of indacaterol relative to placebo, are unlikely to have arisen by chance. There were no available validated daily symptom diaries available at the time of the study design, so the lack of formal validation of the symptomatic measures based on patient diary records may result in less robust data than the validated TDI and SGRQ instruments also used in these studies. The duration of these studies was 12 weeks, which may be short for chronic diseases such as COPD. However, previous studies with indacaterol, although at higher doses, have shown that the efficacy observed at 12 weeks in terms of trough FEV1, dyspnea and health status was maintained over periods of up to 1 year, with acceptable profiles of tolerability and safety (Citation5,6).
Elsewhere, we have reported that treatment with indacaterol 75 μg once-daily was well tolerated and had a good safety profile and provided effective 24-hour bronchodilation that was associated with significant reductions in rescue albuterol use over the 12 weeks of these studies (Citation8). Taken together with the improvements in patient-reported outcomes demonstrated here, we can conclude that indacaterol provided useful benefits for patients with COPD and is an appropriate choice for physicians when selecting a long-acting bronchodilator for the maintenance treatment of patients with moderate-to-severe COPD.
Declaration of Interests
The studies were funded by Novartis. Dr. Gotfried has received research grants from Novartis, GSK, Boehringer Ingelheim, Forest, Pearl Therapeutics and TEVA, and is on the Speaker Bureau for GSK, Merck, Dey and Pfizer. Dr. Kerwin has received consulting fees from Dey Laboratories, GlaxoSmithKline, Ironwood Pharmaceuticals, MAP Pharma (AstraZeneca), and Sepracor (Sunovion) and speaking fees from AstraZeneca, GlaxoSmithKline, Merck and Teva. Mr. Lawrence, Dr. Lassen and Dr. Kramer were employees of the studies’ sponsor, Novartis, and held shares in Novartis at the time these studies were conducted.
Sarah Filcek (CircleScience), a professional medical writer funded by Novartis, and David Young (Novartis) assisted in the preparation of the manuscript. Authors’ contributions: DL, CL and BK made substantial contributions to the concept and design of the studies, MHG and EMK to the acquisition of data and DL to the analysis of data. All authors had full access to original study data and were involved in the interpretation of the data. All authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published.
References
- Global Initiative For Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of chronic obstructive pulmonary disease. Updated 2011. At: www.goldcopd.com [accessed 15 March 2012].
- Feldman G, Siler T, Prasad N, Jack D, Piggott S, Owen R, Efficacy and safety of indacaterol 150 μg once-daily in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med 2010; 10:11.
- Donohue JF, Fogarty C, Lötvall J, Mahler DA, Worth H, Yorgancioglu A, Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010; 182(2):155–162.
- Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 2011; 37(2):273–279.
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, Efficacy of a new once-daily long-acting inhaled β2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010; 65(6):473–479.
- Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B, Long-term safety and efficacy of indacaterol, a long-acting β₂-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest 2011; 140(1):68–75.
- Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Kramer B, Higgins M, Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulm Pharmacol Ther 2010; 23(3):165–171.
- Kerwin EM, Gotfried MH, Lawrence D, Lassen C, Kramer B. Efficacy and tolerability of indacaterol 75 μg once daily in patients aged ≥40 years with chronic obstructive pulmonary disease: results from 2 double-blind, placebo-controlled 12-week studies. Clin Ther 2011; 33(12):1974–1984.
- Global Initiative For Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Updated 2008. At: www.goldcopd.com [accessed 1 February 2012].
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984; 85(6):751–758.
- Witek TJ Jr, Mahler DA. Meaningful effect size and patterns of response of the transition dyspnea index. J Clin Epidemiol 2003; 56(3):248–255.
- Witek TJ Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J 2003; 21(2):267–272.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145(6):1321–1327. PubMed PMID: 1595997.
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005; 2(1):75–79.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10):1005–1012.
- Williams M, Cafarella P, Olds T, Petkov J, Frith P. Affective descriptors of the sensation of breathlessness are more highly associated with severity of impairment than physical descriptors in people with COPD. Chest 2010; 138(2):315–322.
- Ries AL. Impact of chronic obstructive pulmonary disease on quality of life: the role of dyspnea. Am J Med 2006; 119(10 Suppl 1):12–20.
- Jones PW, Barnes N, Vogelmeier C, Lawrence D, Kramer B. Efficacy of indacaterol in the treatment of patients with COPD. Prim Care Respir J 2011; 20(4):380–388.
- Donohue JF, Singh D, Kornmann O, Lawrence D, Lassen C, Kramer B. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis 2011; 6:477–492.
