Abstract
Background: Cardiovascular disease (CVD) contributes significantly to mortality in chronic obstructive pulmonary disease (COPD). Red blood cell distribution width (RDW), an automated measure of red blood cell size heterogeneity that is largely overlooked, is a newly recognized mortality marker in patients with established CVD. It is unknown whether RDW is associated with mortality in COPD patients.
Aims: To study the prognostic value of RDW in patients with COPD and to compare the value of this measurement with cardiac, respiratory, and hemotological status. Method: We performed retrospective analyses of 270 patients stable with COPD who were admitted to our hospital between January 2007 and December 2009. Demographic, clinical, echocardiographic, and laboratory characteristics were registered and recorded COPD deaths were registered as outcomes. Results: In the overall patients, the RDW level had a mean value of 15.1 ± 2.4. RDW was positively correlated with C-reactive protein (CRP) (p = 0.008, r = 0.21), right ventricular dysfunction (RVD) (p < 0.001, r = 0.25), and pulmonary arterial hypertension (PAH) (p = 0.03, r = 0.14). Variables (p < 0.1) included in the univariate survival analysis were forced expiratory volume in 1 second (FEV1% predicted), RDW levels, age, PaCO2, albumine and CRP levels, presence of CVD, presence of anemia, presence of RVD, and presence of PAH. Subsequent multivariate analysis suggested that RDW levels (1.12; 95% CI, 1.01 to 1.24; p = 0.01), and presence of RVD (2.6; 95% CI, 1.19 to 5.8; p = 0.01) were independently related to mortality. Conclusion: Elevated RDW levels were associated with increased mortality risk in stable COPD patients.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is the fourth-largest cause of death worldwide (Citation1). However, more patients with COPD die from cardiovascular causes than from respiratory failure (Citation2). Therefore, cardiovascular disease (CVD)-related morbidity and mortality is also high in COPD patients (Citation2). The most obvious explanation for the high cardiovascular morbidity and mortality rates seen in COPD patients is the high prevalence among this group of smoking and other known risk factors for CVD, such as a poor diet, sedentary lifestyle and low socioeconomic class (Citation2). In addition, COPD is associated in some patients with a “low grade systemic inflammation” that may also initiate or worsen co-morbid diseases, such as CVD (Citation3). Therfore, co-morbid diseases potentiate the morbidity of COPD, leading to increased mortality.
Red blood cell distribution width (RDW) is a numerical measure of the size variability of circulating erythrocytes and is routinely reported as a component of complete blood count in the differential diagnosis of anemia (Citation4). RDW is in a standard size, but disorders related to systemic inflammation, ineffective erythropoiesis, nutritional deficiencies, bone marrow dysfunction or increased destruction cause a higher RDW (Citation5–9). Very recently, researchers have reported higher mortality risk associated with higher RDW in patient populations with CVD (Citation10–12). RDW has been found to be a strong predictor of all-cause mortality in population cohorts not only for CVD (Citation13, 14). All of these studies hypothesized that higher RDW levels may reflect underlying chronic inflammation, which could result in an increased risk of CVD and increased mortality (Citation10–14).
Increased inflammation in the lungs, as well as a systemic inflammatory response, is now a well-established factor in COPD (Citation15, 16). A number of inflammatory markers have been shown to be elevated systemically in COPD including IL-6, IL-8, TNF α, C-reactive protein (CRP), and fibrinogen (Citation16–18). Inflammatory cytokines have been found to inhibit erythropoietin-induced erythrocyte maturation, which is reflected in part by an increase in RDW (Citation8). COPD-related inflammation may also impair erythropoiesis, as do other chronic inflammatory processes, and increase RDW. Although the relationship between RDW and survival is well recognized in CVD (Citation10–12, Citation19–25), it has not been previously demonstrated in COPD. Accordingly, we hypothesized that inflammatory activation may be a mechanistic link between higher RDW and increased mortality in patients with COPD. We therefore aimed to study whether RDW is effective as a prognostic marker in a population with COPD.
Methods
We retrospectively analyzed the dates of patients diagnosed with COPD by GOLD criteria (Citation26) between January 2007 and December 2009. These stable COPD patients who had no history of hospitalization or admission to an emergency department for at least 8 weeks were included in the study. Patients who had a history of cancer, sleep apnea syndrome, primary valvular heart disease, arthritis, connective tissue disorders, inflammatory bowel disease, renal failure, liver diseases, hematological system diseases, history of exacerbation during the last 2 months, blood transfusion and antiinflammatory drug (systemic steroids, immunosuppressive drugs) use in the last 2 months were excluded from the cohort. Of the 346 patients admitted during the study period, 270 patients were included in the study. Seventy-six patients were excluded because of systemic steroids drug use (n = 13), history of exacerbation during the last 2 months (n = 14), previous diagnoses of malignancies (n = 5), sleep apnea syndrome (n = 5), renal failure (n = 2), and inability to perform echocardiography (n = 37). The study was carried out in accordance with the Declaration of Helsinki (1989) developed by the World Medical Association. It was also approved by the Ethics Committee of our hospital.
All demographic, health behavior related factors [age, sex, BMI (body mass index), smoking history, total number of pack-years of smoking and alcohol intake], medical conditions [hypertension, diabetes mellitus, cardiovascular disease (heart failure, coronary artery disease, stroke, or arrhythmia)], electrocardiography, echocardiographic and biochemical data, and treatment modalities, re-admission to emergency units and/or hospitalizations were recorded (Tables , 2).
Table 1. Baseline characteristics of participants
Laboratory measurements
Blood samples were obtained after admission, following an overnight fast and 10 min of supine rest. Samples were processed immediately for the determination of all biochemical parameters. The RDW, hemoglobin level, white blood cell count and mean corpuscular volume were determined using ABX Pentra 120, with a differential count as part of the complete blood cell count. The normal range for RDW in our laboratory was 11.5% to 15.5%. Anaemia was defined according to World Health Organization criteria: hemoglobin, 13 g/dL for men and, 12 g/dL for women (Citation27). Serum albumin levels (N: 3.5- 5.5 g/dL) were measured using Abbot-labeled kits (catalog no: 30-3050/R2) and CRP levels (N: 0-1 mg/dL) were measured by using immunoturbidimetric methods in Beckman Coulter-Synchron LX- 20 chemistry autoanalyser in the biochemistry laboratory of our hospital.
Electrocardiographic findings
A 12-lead electrocardiogram (ECG) was evaluated by the cardiologist (OI) for heart rate and rhythm and for evidence of acute ischemia according to the following criteria: ST segment elevation > 0.5 mm, ST segment depression > 0.5 mm and horizontal or downsloping, T wave flattening or inversion, and conduction block (atrioventricular, left or right bundle branch). Heart rate and rhythm were obtained from the ECG. Coders were blinded to other clinical data.
Echocardiographic findings
Echocardiography was performed by SONOS 4,500 echocardiogram using 3.5 and 2.5 MHz transducers. (Sonos 5500, Philips, MA, USA). Standardized projections and measurements were performed for the evaluation of cardiac anatomy, ventricular function, and valve competence; left ventricular ejection fraction (LVEF) was measured by Simpson's method, using second harmonic imaging. Systolic pulmonary artery pressure was calculated by adding the estimated right atrial pressure onto the tricuspid regurgitation gradient. Pulmonary arterial hypertension (PAH) was defined as a higher than 30 mmHg systolic pulmonary artery pressure. Right ventrictular dysfunction (RVD) was diagnosed in the presence of any of the followings: dilatation of the RV (diastolic diameter >30 mm), abnormal motion of the interventricular septum, hypokinesis of RV, or tricuspid valve regurgitation (jet velocity, 92.5 m/s). Left ventricular dysfunction (LVD) was defined as a lower than%50 of LVEF and higher than 35 mm of diastolic diameter.
Pulmonary function tests
Spirometry dates, if performed in a stable state hospital admission or 8 weeks after discharge, were gathered from patient records. Spirometry was performed with equipment that met the American Thoracic Society performance criteria1 (Citation28). To adjust for height, age, sex, and race, we used published prediction equations for forced expiratory volume in 1 second (FEV1), FEV1–FVC ratio and forced vital capacity (FVC).
Study outcomes
The primer endpoint of the present study was mortality. Median follow-up was 36 (20–52) months, and maximum follow-up was 63 months. We used the hospital automation software which was integrated with the national population system to determine the deaths. The annual death rates were computed from the follow-up records.
Statistical analyses
Variables were given as the mean or median with a standard deviation value. Student's t test was used to compare means and Mann–Whitney U-test was used to compare medians. Frequencies were compared with chi-square and Fisher's Exact test. Spearman and Pearson correlation tests were used for correlation analysis. The patients received study date was considered as zero-day, last check date or dead date was considered as last day on survival analysis. Kaplan-Meier survival analysis was performed for univariate survival analysis. Log-rank or Cox tests were used for comparison of survival rates of groups. Variables that were associated with survival at p < 0.10 in univariate analysis were included in multivariate analysis. The Cox relative risks model was used for multivariate analysis of these factors that were likely to affect the survival. Forward stepwise analysis method was preferred to show that the variable independently affects survival. A p-value of less than 0.05 was considered to be significant.
Results
The study population consisted of 270 patients, whose characteristics are given in . RDW ranged from 10.4% to 24.5% (median, 14.7%; interquartile range, 13.4% to 16.4%; mean, 15.1 ± 2.4%), and 111 (41%) had RDW levels out of the normal range of 11.5% to 15.5% [RDW < 11.5 (n = 3 [1%]) and RDW >15.5 (n = 108 [40%])].
In comparison patients with an elevated RDW (>15.5) value and with a nonelevated RDW (≤15.5) value were more likely to have a higher CRP levels, lower albumine and higher PaCO2 levels. In addition PAH, RVD, presence of CVD and mortality rate were significantly higher in patients with an elevated RDW (Tables and ).
Table 2. Follow-up dates and laboratory findings of the study participant
Absolute RDW levels positively correlated with CRP levels (r = 0.21, p = 0.08) () and inversely correlated with Hg concentration (r = –0.18, p = 0.04), mean corpuscular volume (MCV) (r = –0.37, p < 0.001), and serum albumin levels (r = –0.2, p = 0.02) (). First callout and . Among echocardiographic parameters, RDW levels correlated positively with right ventricular end-diastolic diameter (r = 0.17, p = 0.33), left ventriculer end-diastolic diameter (r = 0.15, p = 0.04), RVD (r = 0.25, p < 0.001), and PAH (r = 0.16, p = 0.03) (). But there were no significant interaction effects between RDW levels and ECG findings ().
Figure 1. Correlation coefficient between red cell distribution width (RDW) and the C-reactive protein (CRP). The scale is a logarithmic scale.
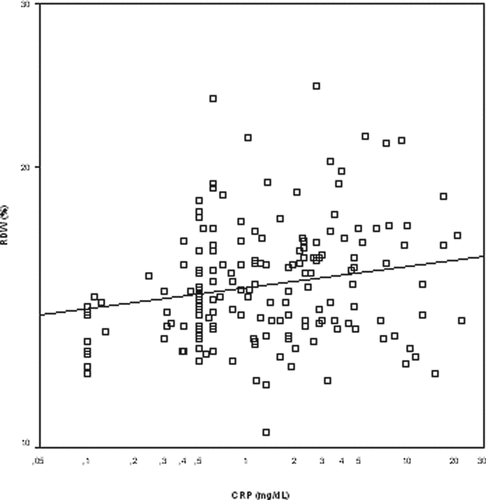
Table 3. Correlation coefficients of demografic and laboratory variables with RDW
RDW and survival
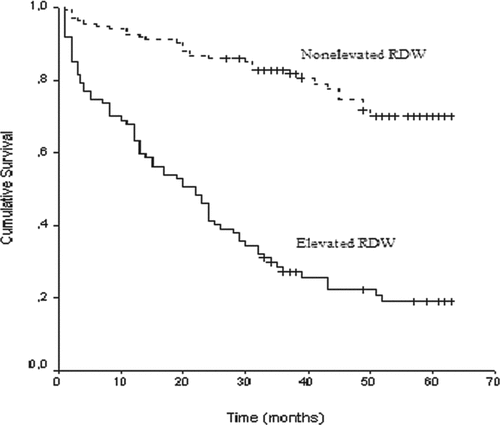
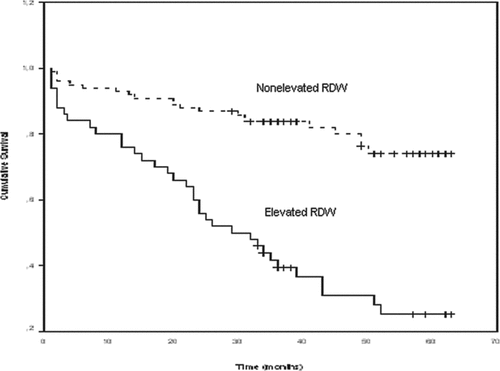
The overall 5-year survival rate of the patients was calculated as 49%. The 5-year survival rate was 70% for patients with a nonelevated RDW (≤15.5) and 19% for patients with an elevated RDW (>15.5) (p < 0.001) (, 3, and 4). First callout , 3, and 4. Factors that were found to affect the survival rate on the univariate analysis (p < 0.1) (absolute RDW levels, presence of CVD, presence of anemia, age, albumine and CRP levels, PaCO2, presence of RVD, the presence of PAH, and FEV1% predicted) were used in a Cox regression analysis for multivariate analysis of the factors that may affect the survival rate. The analysis showed that RDW levels (p = 0.01) and presence of RVD (p = 0.01) were both independent prognostic factors (). First callout .
Figure 2. Unadjusted- Kaplan-Meier curve for long-term survival according to red cell distribution width (RDW) groups in the entire cohort of patients (p < 0.001 by log-rank test).
Figures 3. Adjusted with right ventricular dysfunction (RVD) Kaplan-Meier curve for long-term survival according to red cell distribution width (RDW) groups in the entire cohort of patients (p < 0.001 by log-rank test).
Figures 4. Adjusted with right ventricular dysfunction (RVD) Kaplan-Meier curve for long-term survival according to red cell distribution width (RDW) groups in the entire cohort of patients (p < 0.001 by log-rank test).
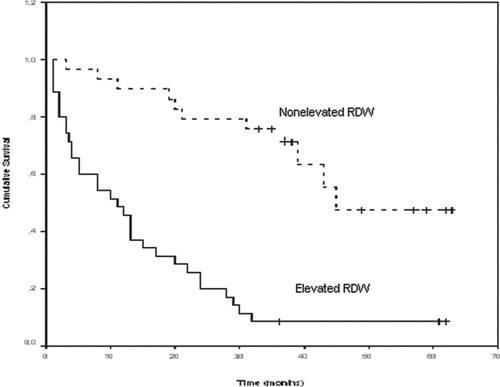
Table 4. Univariate and multivariate analysis of risk factors for mortality in patients with COPD
Discussion
In the last few years, there have been numerous studies consistently confirming the independent association between RDW and mortality across a broad spectrum of CVD patient populations, including patients with heart failure (Citation11, Citation19–21), with established coronary artery disease (CAD) (Citation12, Citation22), with pulmonary hypertension (Citation23), with acute pulmonary embolism (Citation24), and with acute myocardial infarction (25). COPD has a potential link with CVD from the point of inflammation, oxidative stress and endothelial dysfunction (Citation2, Citation29). In this context, a relationship between RDW levels and mortality may be considered in COPD. In the present study, we hypothesized that a chronic inflammatory state may act as a mechanistic link between RDW levels and increased mortality in patients with COPD. In a retrospective study of 270 patients, we found that RDW levels were independently associated with increased mortality in COPD.
RDW is a quantitative measure of anisocytosis. It is routinely measured by automated hematology analyzers and has been reported as a component of the complete blood count (Citation4). RDW is typically elevated in conditions of ineffective red cell production (such as iron deficiency, B12 or folate deficiency, and hemoglobinopathies), increased red cell destruction (such as hemolysis), or after blood transfusion (Citation4–9,Citation29). Oxidative stress was also shown to be associated with RDW. Antioxidants, including total serum carotenoids, a-tocopherol, and selenium, were shown to be significantly associated with a decrease in RDW (Citation30). In patients with conditions characterized by increased levels of oxidative stress, such as Down syndrome (Citation31), poor pulmonary function (Citation32), and dialysis (Citation33), RDW values are elevated. Additionally, higher RDW levels may result from ineffective erythropoiesis due to chronic inflammation. Inflammatory cytokines have been found to suppress the maturation of erythrocytes, allowing juvenile erythrocytes to enter into the circulation and lead to an increase in RDW (Citation8). COPD is associated with a chronic inflammatory state and increased levels of inflammatory cytokines (Citation16–18). As with inflammation, the pulmonary oxidative stress associated with COPD (Citation3). Oxidative stress increases with severity of airflow obstruction as measured by lipid peroxidation products in induced sputum from COPD patients (Citation34). Finally, RDW is increased in patients with poor respiratory function in relation with oxidative stress. We speculate that RDW may be high due to inflammation and oxidative stress in COPD, and therefore higher levels of RDW may reflect an underlying inflammatory state in COPD. In the present study, we found that RDW levels were high in almost half of stable COPD patients (44%). In addition, the correlation between RDW levels and CRP (indicator of inflammation) and albumin levels (suppressed due to inflammation) suggests that the high RDW levels may be seen as a marker of systemic inflammation in COPD. In addition, there was no relationship between RDW levels and the stage of COPD. However the relationship between RDW levels and PAH, indicated that RDW is an inflammatory marker that suggests the severity of COPD.
Patients with stable COPD, particularly when the disease is severe, show evidence of systemic inflammation, measured either as increased circulating cytokines, and acute phase proteins, or as abnormalities in circulating cells (Citation6, Citation16). The most studied of these markers is CRP, an acute-phase reactant secreted by the liver. Mounting evidence now suggests that increased serum CRP levels are also associated with lung inflammation in stable COPD (Citation6, Citation20). Two epidemiologic studies (Citation19, Citation35) showed that increased CRP levels were independently associated with global and cardiovascular mortality in COPD patients. Recently, in a large unselected cohort of patients, RDW showed a strong and graded association with inflammatory markers (Citation36). The relationship between CRP and RDW has been shown in previous reports (Citation37). In this regard, we found a correlation between RDW and CRP levels. It is therefore possible that RDW as a systemic marker of ongoing lung inflammation could be used as a predictor of future COPD outcomes. Even though hCRP levels were not examined in the present study, no relationship was observed between CRP levels and survival in multivariate analyses, nor were RDW levels significant predictors of outcome. The main finding of this study was that systemic inflammation, as evaluated by RDW, appeared to be the best prognostic indicator of survival.
Although chronic obstructive pulmonary disease (COPD) is “traditionally” associated with polycythemia, the systemic inflammation that is now recognized as a feature of COPD makes it a possible cause of anemia of chronic disease (ACD). Although reports have shown very strong evidence of an association between anemia and mortality in COPD patients (Citation38), in the present study, higher levels of RDW were associated with mortality in cases without anemia. Our analyses concerning the effect of the interaction between RDW categories and anemia status on mortality in univariate models produced significant results; however the effect disappeared after multivariate adjustment. The most interesting finding from our study is that RDW was a better prognostic marker than hemoglobin regardless of anemia status. In this case, RDW may be an earlier prognostic marker than haemoglobin, as it may reflect early steps in the complex processes of anemia, when ineffective production and increased destruction of red cells occur, but hemoglobin is still within the normal range.
Although recent studies showed that RDW was a prognostic factor for CVD, RDW levels have been shown to be associated with other chronic conditions, (Citation29–33, Citation39) and recently RDW has been found to be a strong predictor of all-cause mortality in two large population cohorts (Citation13, 14). These studies showed that this association was not specific to cardiovascular disease, as RDW also predicted mortality from other chronic conditions, including cancer, kidney disease, and chronic lower respiratory tract disease. Possible mechanisms may include the fact that higher levels of RDW may reflect an underlying inflammatory state and oxidative stress, which is related to CVD and morbidity with poor outcomes. These conditions, which cause oxidative stress or chronic inflammation and are often present in patients with COPD, are associated with a worse prognosis (Citation2). Patients with COPD show evidence of increased systemic inflammation, and oxidative stress has been implicated in the pathogenesis of CVD in patients with COPD (Citation29). In addition, systemic inflammation in COPD is a risk factor for CVD-related mortality (Citation3). In the present study, RDW level is the most important factor to affect mortality and a significant correlation was found between RDW levels and the presence of CVD, RVD, LVEF. According to these findings, RDW level is considered as a marker of mortality due to the CVD in patients with COPD. However, in the present study, these dates contradict the view that the presence of CVD does not affect the mortality rate in multivariate analyses. This may be due to the retrospective design of the study; also there may be cases of CVD, which were not detected from file dates. This also applies to the investigation of CVD in COPD patients with a high RDW level.
RVD and PAH are common in patients with COPD (Citation40). When present, they can reduce exercise tolerance, increase dyspnea and contribute to an overall decrease in functional status. Most importantly, however, its presence in the setting of COPD portends a higher mortality rates (Citation40). In some reports (Citation41, 42) high values of RDW were the only parameters independently associated with RV failure and in patients with COPD. In a recent study, Hampole et al. showed that RDW value was an independent predictor of mortality in patients with pulmonary hypertension due to any reason (Citation23). In our study, we demonstrated that RDW was correlated with RVD and PAH in patients with stable COPD which was supported to previous reports (Citation23, Citation41). Hence, we think that high RDW levels may be a an indicator of RVD in COPD patients.
There are several limitations of this study. First, laboratory dates including RDW measurements were only available at baseline, thus it is unknown whether changes or improvement in RDW is associated with differences in mortality. Second, the findings are restricted to the population included in the study: patients from pulmonary clinics with moderate-to-very-severe COPD (a small number of patients with stage I (5%) and II (14%). It is possible that these results may not apply to individuals without COPD or to those with mild to moderate disease. Third, this study is a retrospective study and the patinets were evaluated by file records for CVD. Finally, the patients were evaluated by CRP instead of hCRP.
In conclusion, we demonstrated that elevated RDW levels in patients with COPD was associated with an increased risk for mortality. An elevated RDW may be an indicator of underlying inflammation and oxidative stres in COPD and it could be a useful, inexpensive and CVD associated prognostic factor in COPD patients. However, it is clear that more prospective studies are needed in this area.
Declaration of Interest Statement
The authors declare no conflicts of interest. The authors had no financial assistance in the writing of this manuscript.
References
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997; 349:1436–1442.
- Maclay JD, McAllister DA, Macnee W. Cardiovascular risk in chronic obstructive pulmonary disease. Respirology 2007; 12(5):634–641.
- Agustı´ A. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2:367–370.
- Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med 1991: 9:71–74.
- Romero AJ, Carbia CD, Ceballo MF, Diaz NB. Red cell distribution width (RDW): its use in the characterization of microcytic and hypochromic anemias. Medicina (B Aires) 1999; 59:17–22.
- Williams WJ. Examination of the blood. In: Williams WJ, Beutler E, Erslev AJ, Lichtman MA, eds. Hematology, 3rd ed. New York: McGraw-Hill, 1983: 9–14.
- Seppa K, Sillanaukee P, Saarni M. Blood count and hematologic morphology in nonanemic macrocytosis: differences between alcohol abuse and pernicious anemia. Alcohol 1993; 10:343–347.
- Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory asist device. Perfusion 2005; 20:83–90.
- Ozkalemkas F, Ali R, Ozkocaman V, Ozan U, Oztürk H, Kurt E, Evrensel T, Yerici O, Tunalı A. The bone marrow aspirate and biopsy in the diagnosis of unsuspected nonhematologic malignancy: a clinical study of 19 cases. BMC Cancer 2005; 5:144.
- Anderson JL, Ronnow BS, Horne BD, Carlquist JF, May HT, Bair TL, Jensen KR, Muhlestein JB. Usefulness of a complete blood count-derived risk score to predict incident mortality in patients with suspected cardiovascular disease. Am J Cardiol 2007; 99:169–174.
- Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol 2007; 50:40–47.
- Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008; 117:163–168.
- Patel KV, Ferrucci L, Ershler WB, Longo DL, GuralnikJM. Red blood cell distribution widih and the risk of death in middle-aged and older adults. Arch lntern Med 2009; 169:515–523.
- Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a communiİy-based prospective cohort. Arch lntern Med 2009; 169:588–594.
- Oudijk EJ, Lammers JW, Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Eur Respir J Suppl 2003; 22:5–13.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a metaanalysis. Thorax 2004; 59:574–580.
- Palange P, Testa U, Huertas A, Calabrò L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, Vignola MA, Bonsignore MR. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J 2006; 27:529–541.
- Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996; 51:819–824.
- Borne Y, Smith JG, Melander O, Hedblad B, Engstrom G. Red cell distribution width and risk for first hospitalization due to heart failure: a population-based cohort study. Eur J Heart Fail 2011; 13:1355–1361.
- Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, Lenihan DJ, Oren RM, Wagoner LE, Schwartz TA, Adams KF Jr. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail 2010;16: 230–238.
- Van Kimmenade RR, Mohammed AA, Uthamalingam S, van der Meer P, Felker GM, Januzzi Jr JL. Red blood cell distribution width and 1-year mortality in acute heart failure. Eur J Heart Fail 2010; 12:129–136.
- Lappe JM, Horne BD, Shah SH, May HT, Muhlestein JB, Lappé DL, Kfoury AG, Carlquist JF, Budge D, Alharethi R, Bair TL, Kraus WE, Anderson JL. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta 2011; 412:2094–2099.
- Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol 2009; 104:868–872.
- Zorlu A, Bektasoglu G, Guven FM, Gucuk E, Ege MF, Altay H, Cınar Z, Tandogan I, Yilmaz MB. Usefulness of admission red cell distribution width as a predictor of early mortality in patients with acute pulmonary embolism. Am J Cardiol 2012; 109:128–134.
- Dabbah S, Hammerman H, Markiewicz W, Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol 2010; 105: 312–317.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163:1256–1276.
- World Health Organization. Nutritional Anemias: Report of a WHO Scientific Group. Geneva: World Health Organization, 2009.
- American Thoracic Society. Standardization of spirometry: 1987 update. Am Rev Respir Dis 1987; 136:1285–1298.
- Agusti A, Soriano JB. COPD as a systemic disease. COPD 2008; 5:133–138.
- Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, Fried LP (2010) Serum antioxidants and inflammation predict red cell distribution width in older women: The Women's Health and Aging Study I. Clin Nutr 29: 600–604.
- Garcez ME, Peres W, Salvador M. Oxidative stress and hematologic and biochemical parameters in individuals with Down syndrome. Mayo Clin Proc 2005; 80:1607–1611.
- Grant BJ, Kudalkar DP, Muti B McCann SE, Trevisan M, Freudenheim JI, Relation between lung function and RBC distribution width in a population-based study. Chest 2003; 124(2):494–500.
- Kobayashi S, Moriya H, Aso K, Ohtake T. Vitamin E-bonded hemodialyzer improves atherosclerosis associated with a rheological improvement of circulating red blood cells. Kidney Int 2003; 63:1881–1887.
- Paredi P, Kharitonov SA, Leak D, Ward S, Cramer D, Barnes PJ. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 162:369–373.
- Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in COPD. Am J Respir Crit Care Med 2007; 175:250–255.
- Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009; 133:628–632.
- Fukuta H, ohte N, Mukai S, Saeki T, Asada K, Wakami K, Kimura G. Elevated plasma levels of B-type natriuretic Peptide but not c-reactive protein are associated with higher red cell distribution width in patients with coronary artery disease. Int Heart J 2009; 50:301–312.
- Chambellan A, Chailleux E, Similowski T, and the ANTADIR observatory group. Prognostic value of the hematocrit in patients with severe COPD receiving longterm oxygen therapy. Chest 2005; 128:1201–1208.
- Spell DW, Jones J, Harper WF, Bessman DJ. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prevent 2004; 28:37–42.
- Klinger JR, Hill NS. Right ventricular dysfunction in chronic obstructive pulmonary disease. Evaluation and management. Chest 1991 Mar; 99(3):715–723.
- Sincer I, Zorlu A, Yilmaz MB, Dogan OT, Ege MR, Amioglu G, Aydin G, Ardic I, Tandogan I. Relationship between red cell distribution width and right ventricular dysfunction in patients with chronic obstructive pulmonary disease. Heart Lung 2012 May; 41(3):238–243.
- Terzano C, Conti V, Di Stefano F, Petroianni A, Ceccarelli D, Graziani E, Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung 2010; 188:321–329.
