Abstract
Interleukin (IL)-17A, IL-22 and IL-10 have been implicated in the development of chronic obstructive pulmonary disease (COPD), but their expression in COPD is uncertain. Here we investigate the expression of IL-17A, IL-22 and IL-10 in the serum and sputum of COPD patients. Blood samples and induced sputum samples were collected from 94 patients with COPD, 23 healthy smokers, and 22 healthy control non-smokers. IL-17A, IL-22 and IL-10 were measured by enzyme-linked immunosorbent assay (ELISA). We found that: 1) serum and sputum IL-17A were higher in COPD compared to healthy smokers and non-smokers; 2) serum IL-17A increased with COPD stages, it was inversely correlated with percentage of forced expiratory volume in the first second (FEV1%) reference and positively correlated with C-reactive protein (CRP), Sputum IL-17A levels in the severe COPD patients were positively correlated with sputum neutrophils, and reversely correlated with sputum macraphages (p < 0.01); 3) serum and sputum IL-22 were significantly higher in COPD and healthy smokers than those in the non-smoker group, sputum IL-22 was similar in severe COPD (stage III and IV), which were higher than those in the other groups (p < 0.05); and, 4) serum and sputum IL-10 were similiar in COPD and healthy smokers, which were decreased compared to non-smokers. These data suggest that the increased level of IL-17A in serum and sputum plays important roles in the pathogenesis of COPD. The increased sputum IL-22 might also play important roles in the pathogenesis of COPD, while IL-10 secretion might be not only affected by COPD but also by cigarette smoke.
Introduction
Chronic obstructive pulmonary disease (COPD), is the third largest cause of respiratory death, accounting for more than one fifth (23%) of all respiratory deaths (Citation1). However, its exact etiology and pathogenesis is not clear. COPD is usually characterised by irreversible airflow obstruction and chronic airway inflammation, predominantly caused by smoking. Chronic inflammation observed in COPD is characterized by recruitment of several inflammatory cell types to the lungs and pro-inflammatory cytokines production.
It is now well established that T lymphocytes participate actively in the COPD. In recent years, A distinct T-cell lineage, called T-helper (Th) 17 cells, has been identified, which were characterized by the production of interleukin (IL)-17A, IL-17F, and IL-22.
Some previous studies (Citation2–4) found increase of the number of IL-17A+ immunoreactive cells in the COPD patients compared to control non-smokers. Vargas-Rojas et al. (Citation5) found that the increase of Th17 response and the lost of balance between CD4(+) T cell subsets happened in the peripheral blood from COPD patients. Shen et al. (Citation6) injected anti-IL-17 antibody to tobacco-smoke-exposed mice and found the concentration of IL-17 in lung tissue and neutrophils in the bronchoalveolar lavage fluid were significantly decreased. However, Barczyk et al. (Citation7) measured the IL-17 levels in induced sputum via ELISA method and found that it was not involved in pathogenesis of stable COPD. Doe et al. (Citation8) found the median IL-17A+ cells/mm2 submucosa in COPD was similar to smoking control subjects and increased IL-17A expression was not associated with increased neutrophilic inflammation.
As to IL-22, Di Stefano found that the number of IL-17A (+) and IL-22 (+) immunoreactive cells is increased in the bronchial submucosa of stable COPD compared with control non-smokers (Citation2). However, a recent study reported by Paats revealed that IL-22 expressed by circulating CD4+ T-cells were similar in COPD patients and healthy controls (Citation9). It is necessary to produce a detailed analysis of the expression of the Th17-related cytokines IL-17A and IL-22 with stable COPD of different severity [Global Obstructive Lung Disease Initiative (GOLD) stages] and age-matched control groups by using more samples.
The function of IL-10 is opposite to IL-17A and IL-22. IL-10 is an anti-inflammatory cytokine. It has the ability to suppress Th1 cytokines such as IFN-γ and TNF-α and has anti-inflammatory effects on neutrophils by inhibiting IL-8 and MIP-1 (macrophage inflammatory protein-1) (Citation10–12). However, the results of the production of IL-10 in COPD was also conflicts (Citation13–17).
It is necessary to do more researches in a large number of COPD patients, in order to increase the power of the present analyses and detect intergroup differences and minimize type II (β) errors. In this study, cytokines IL-10, 17A, and 22 were to analysize in serum and sputum. We also analysized the correlation between the findings with COPD severity, smoking status.
Materials and Methods
Study subjects
Ninety-four COPD patients and 45 healthy volunteers were recruited (demographics shown in ). Recruitment of cases and controls occurred between April 2008 and April 2012 in our outpatient pulmonary clinic. Ethical approval for the study was granted by the local Institutional Ethics Committee (Wuhan Zhongnan Hospital Ethics Committee Institutional, Wuhan University, China), and written informed consent was obtained from all participants.
Table 1 Characteristics of all subjects .
COPD was diagnosed and severity categorized by using Global Initiative for Chronic Obstructive Lung Disease criteria. All COPD patients were in stable phase and free from acute exacerbation for at least 4 weeks. Three additional inclusion criteria were used for the smoker group: there were all current smokers, smoking history of at least 10 pack-years, and smoking habit of at least 10 cigarettes per day in recent years.
Exclusion criteria in this study were: associated with other diseases (such as relevant cardiopulmonary morbidities, atopy diseases, autoimmune diseases, cancer); with acute infectious disease in 4 weeks prior to enrollment; refused to sign an informed consent. Sixty-two COPD patients were dropped out for acute exacerbation during the 4 weeks.
Laboratory methods
A detailed medical history was obtained from all participants. All participants underwent spirometric tests according to the international standards. Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were measured.
All study subjects were invited to undergo the examination of the count of blood total neutrophils, total lymphocytes, and the examination of C-reactive protein (CRP). They were finished by the Wuhan Zhongnan hospital's Examination Center.
Blood samples were collected at the time of pulmonary evaluation, serum was obtained and aliquots were stored at –80°C until analysis.
Sputum was induced by inhalation of 3% sterile hypertonic saline by a De Vilbiss Ultraneb 99 ultrasonic nebulizer (Healthcare Inc, Somerset, PA) as previously described (Citation18). Sputum was processed according to the studies. Briefly, the selected plugs were diluted with 4 volumes of phosphate-buffered saline (PBS 1*; Gibco). Then, it was filtered through a 50-μm nylon mesh to remove any mucus and detritus without removing cells. The resulting suspension was centrifuged at 1000 g for 20 minutes. The supernatant was collected and stored at –80°C until analysis.
IL-22 enzyme-linked immunosorbent reaction (ELISA) kit was purchased from BD Biosciences, USA; IL-10 and IL-17A ELISA kits were purchased in the United States R & D system. The sensitivity of the IL-17A, IL-22 and IL-10 kits were 10 pg/mL, 5 pg/mL, and 1 pg/mL, respectively. The serum levels of interleukins were detected, following the instructions provided by the ELISA kits manufacturers. Each sample was detected and duplicated, and the mean value of the two measures was used for the analyses.
Statistical methods
Group data were expressed as mean ± standard deviation (SD) for functional data or median (range) for morphological data. Differences between groups were analysed using analysis of variance (ANOVA) for functional data. A p-value below 0.05 was considered statistically significant. Correlations were assessed by Spearman rank (rs) and Pearson (r) correlation coefficients. Statistical analysis was performed using SPSS Software (SPSS Inc., Chicago, Ill., USA) and GraphPad Prism 5 (GraphPad Software, La Jolla, California, USA).
Results
Clinical findings
The clinical characteristics of the subjects were reported in . The three groups of subjects examined were similar with regard to age, percentage of female, and BMI. Smoking history was similar in different stage COPD patients and healthy smokers. Compared with controls, the levels of CRP were significantly higher in COPD patients (p < 0.05). The numbers of neutrophils and lymphocytes in peripheral blood were similar in the different groups. The total cells counts, the percent of neutrophils and lymphocytes in the sputum were higher in COPD patients than those in the controls (p < 0.05).
The Detection of Cytokine IL-17A Levels
The serum levels of IL-17A were significantly higher in COPD than those in the smoker and non-smoker group (p < 0.05, ). The serum levels of IL-17A increased with COPD stages (p < 0.05, ) and were inversely correlated with FEV1% reference (p < 0.01, ). Sputum IL-17A was most below the limit of detection in the samples from COPD I and II, healthy smokers and non-smokers. Sputum IL-17A was similar in severe COPD stage III (28.07 ± 8.62 pg/mL) and COPD stage IV (29.94 ± 10.52 pg/mL), and higher than those in the other groups.
Figure 1. IL-17A in different groups. Data were presented as mean ± standard deviation (SD). The left picture shows The serum level of IL-17A in non-smokers was 63.73 ± 20.37 pg/mL. The serum level of IL-17A in healthy smokers was 89.26 ± 26.82 pg/mL. The serum level of IL-17A in COPD stage 1 was 107.20 ± 24.45 pg/mL. The serum level of IL-17A in COPD stage 2 was 150.30 ± 32.38 pg/mL. The serum level of IL-17A in COPD stage 3 was 208.80 ± 27.98 pg/mL. The serum level of IL-17A in COPD stage 4 was 229.20 ± 29.52 pg/mL. The serum levels of IL-17A increased gradually from non-smoker group to COPD stage 4 group (p < 0.05). The right picture shows Sputum IL-17A were similar in severe COPD (stage III and IV), which were higher than those in the other groups (p < 0.05).
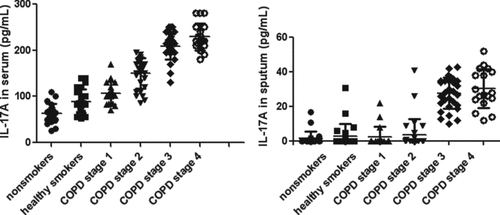
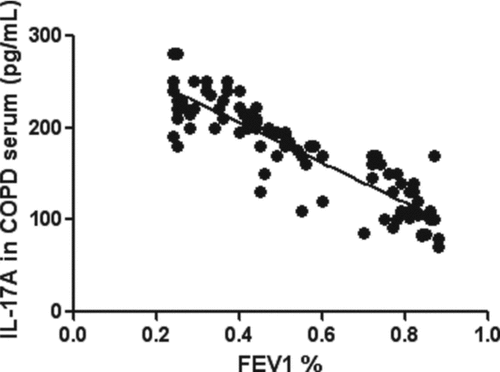
Figure 2. Correlations between serum IL-17A in subjects with COPD and FEV1% reference. The serum levels of IL-17A was inversely correlated with FEV1% reference (r = –0.88, p < 0.01).
By correlation analysis we found that serum IL-17A levels in the COPD patients were positively correlated with CRP (p < 0.01, ). Because most of IL-17A in COPD stage I and II was undetectable, we only analyzed them in the severe COPD sputum. Sputum IL-17A levels in the COPD patients were positively correlated with sputum neutrophils, and reversely correlated with sputum macraphages (p < 0.01, ).
Figure 3. Correlations between serum IL-17A in subjects with COPD and CRP. The serum IL-17A levels in the COPD patients were positively correlated with CRP (r = 0.78, p < 0.01).
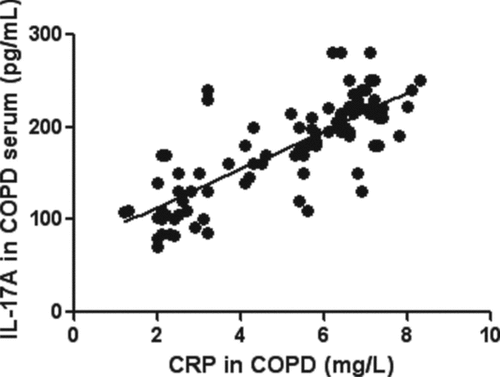
Table 2 Correlations between cytokines and inflammatory . cells in sputum of severe COPD patients
The Detection of Cytokine IL-22 Levels
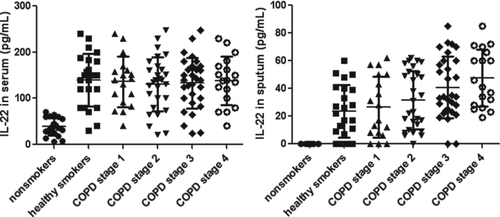
The serum levels of IL-22 were significantly higher in COPD and healthy smokers than those in the non-smoker group (p < 0.01, ). There was no difference of IL-22 in serum among the different COPD stages and smokers, though a little higher IL-22 observed in severe COPD (stage III and IV). Sputum IL-22 was below the limit of detection in the samples from all non-smokers, 5 healthy smokers, and 12 subjects with COPD (stage I and II). Sputum IL-22 was similar in healthy smokers and COPD (stage I and II). Sputum IL-22 was similar in severe COPD (stage III and IV), and higher than those in the other groups. Sputum IL-22 was not correlated with sputum inflammatory cells (p > 0.05).
Figure 4. IL-22 in different groups. Data were presented as mean ± standard deviation (SD). The left picture shows the serum levels of IL-22 were significantly higher in COPD and smokers than those in the non-smoker group (p < 0.01). There was no difference of IL-22 in serum among the different COPD stages and smokers (p > 0.05). The right picture shows Sputum IL-22 was below the limit of detection in the samples from all non-smokers. Sputum IL-22 was similar in severe COPD (stage III and IV), which were higher than those in the other groups (p < 0.05).
The Detection of Cytokine IL-10 Levels
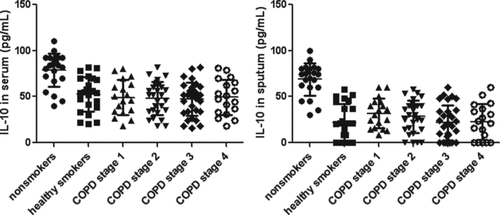
The serum levels of IL-10 were similiar in COPD and healthy smokers (p > 0.05), which was lower than non-smokers group (p < 0.01, ). Sputum IL-10 was below the limit of detection in the 7 samples from healthy smokers and 16 COPD. There was no significant difference between COPD and healthy smokers. Sputum IL-10 were significantly higher in the non-smoker group than COPD and healthy smokers (p < 0.01). Sputum IL-10 was not correlated with sputum inflammatory cells.
Figure 5. IL-10 in different groups. Data were presented as mean ± standard deviation (SD). The left picture shows the serum levels of IL-10 were similiar in COPD and healthy smokers (p > 0.05), which was lower than non-smokers group (p < 0.01). The right picture shows there was no significant difference between COPD and healthy smokers. Sputum IL-10 was significantly higher in the non-smoker group than COPD and healthy smokers (p < 0.01).
Discussion
IL-17A was secreted by Th17. Recent studies found that IL-17 also associated with γδT cells (Citation19), natural killer T cells (Citation20), Tc17 cells (Citation21), neutrophils. Th17 and IL-17A has been identified to be involed in COPD inflammation by many studies (Citation2, Citation3-6, Citation22–26). In our present study we report a significant increase serum IL-17A and sputum IL-17A in severe COPD (stage III and IV) compared to smokers with normal lung function and non-smokers by detecting much more samples compared to previous studies. In this study, the serum and sputum IL-17A were similar in COPD stage III and IV.
The decline of lung function may be partly due to the damage of lung structure as well as airway inflammation. Thus, we had the result that IL-17A was similar in COPD stage III and IV. However, another recent study (Citation26) found that IL-17A was decreased in severe COPD compared to GOLD stage I. In that study (Citation26), the patients’ smoking history was shorter than those in our study, and the macrophage inflammation was also more than those in our study. All those might induce the discrepancy. Meanwhile, We found that the serum levels of IL-17A were correlated with FEV1%.
Our results suggested that IL-17A might play an important role in airway inflamation. Maybe IL-17A secretion triggers production of numerous chemokines, resulting in neutrophil and macrophage recruitment, which contribute to airway inflammation. By analyzing the correlation of IL-17A and inflammatory cells, we found that sputum IL-17A levels in the COPD patients were positively correlated with sputum neutrophils, and reversely correlated with sputum macraphages.
IL-17A induces the release of CXCL1 (GRO-a), CXCL8 (IL-Citation8) and granulocyte-macrophage colony-stimulating factor (GM-CSF) from airway epithelial cells and smooth muscle cells and thereby may orchestrate neutrophilic inflammation (Citation27,28). IL-17A also potently stimulates lung microvascular endothelial cells to produce CXCL8, and induces the expression of E-selectin, VCAM-1, and ICAM-1. Thus, IL-17A promotes neutrophilic inflammation (Citation29).
COPD is also a systemic inflammatory disease and CRP is a predictive indicator to monitor COPD (Citation30). Thus, we studied the correlation between CRP and IL-17A and found positive correlation. More studies need to reveal the mechanism that IL-17A might have some effect on the increasement of CRP.
Th17 cells also produce IL-22, which has been linked to chronic inflammatory disease such as asthma (Citation31, 32). Di Stefano (Citation2) found the number of IL-22+ immunoreactive cells increased in the COPD patients compared to control non-smokers. However, Paats (Citation9) only found that the proportions of IL-22+ cells in the CD4+ memory T-cell population were significantly increased in active smokers compared to past smokers, although it was of no significance in COPD.
Recently, a new T cells, named Th22 cells were discovered (Citation33). Thus, we were interested to study the IL-22 in COPD. In this study, the serum IL-22 were significantly higher in COPD and smokers compared to the non-smokers. There was no difference of IL-22 in serum among the different COPD stages and smokers.
Sputum IL-22 was below the limit of detection in the samples from all non-smokers, 5 smokers with normal lung function, and 12 subjects with COPD (stage I and II). Sputum IL-22 was similar in severe COPD (stage III and IV), and higher than those in the other groups. It is therefore conceivable that cigarette smoke affect IL-22 production and the increased sputum IL-22 might also play important roles in the pathogenesis of COPD. Previous studies (Citation34–36) also found cigarette smoke affect IL-22 production via aryl hydrocarbon receptor (AHR). Future studies should also examine Th22 in more severe COPD and mild COPD patients to see if they can contribute to disease progress.
IL-10 is an important agent in the inflammation, which has the ability to inhibit IFN-gamma and IL-2 production in Th2 cells (Citation36). Takanashi (Citation13) found that IL-10 levels and a small number of IL-10-expressing cells decreased in the sputum obtained from COPD and smokers was demonstrated compared to healthy non-smokers. Moermans et al. (Citation14) found that sputum IL-10 was lower in COPD compared to healthy subjects. Pelegrino et al. (Citation15) found that serum and induced sputum concentrations of IL-10 was similar in COPD and smokers without COPD. Hackett et al. (Citation16) also found IL-10 dysregulation in COPD.
On the contrary, Barcelo et al. (Citation17) found that IL-10+ CD8+ T cells and IL-10+ CD4+ T cells in COPD alveolar lavage fluid increased compared non-smokers and smokers with normal lung function. Because IL-10 is secreted by monocytes, macrophages, mast cells, T and B lymphocytes, and dendritic cells (DCs). This might be contributing to the difference results in the previous study. Our data showed that IL-10 might be mainly affected by cigarette smoke because it was similar in healthy smokers and different COPD patients.
In summary, the present data suggest that IL-17A and IL-22 play an important role in COPD inflammation. Future studies should focus on the mechenism of IL-17A and IL-22 in COPD, and discuss the roles of IL-10+ cells in COPD.
Conclusions
These data suggest that the increased level of IL-17A in serum and sputum plays important roles in the pathogenesis of COPD. The increased sputum IL-22 might also play important roles in the pathogenesis of COPD, yet IL-10 secretion might be not only affected by COPD but also by cigarette smoke.
Declaration of Interest Statement
The authors have declared that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589):765–773.
- Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D'Anna SE, Zanini A, Brun P, Casolari P, Chung KF, Barnes PJ, Papi A, Adcock I, Balbi B. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol 2009; 157(2):316–324.
- Eustace A, Smyth LJ, Mitchell L, Williamson K, Plumb J, Singh D. Identification of cells expressing IL-17A and IL-17F in the lungs of patients with COPD. Chest 2011; 139(5):1089–100.
- Chang Y, Nadigel J, Boulais N, Bourbeau J, Maltais F, Eidelman DH, Hamid Q. CD8 positive T cells express IL-17 in patients with chronic obstructive pulmonary disease. Respir Res 2011; 12: 43.
- Vargas-Rojas MI, Ramírez-Venegas A, Limón-Camacho L, Ochoa L, Hernández-Zenteno R, Sansores RH. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir Med 2011; 105(11):1648–1654.
- Shen N, Wang J, Zhao M, Pei F, He B. Anti-interleukin-17 antibodies attenuate airway inflammation in tobacco-smoke-exposed mice. Inhal Toxicol 2011; 23(4):212–218.
- Barczyk A, Pierzchala W, Sozañska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med 2003; 97(6):726–733.
- Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, McCormick M, Woods J, May R, Sleeman MA, Anderson IK, Brightling CE. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest 2010; 138(5):1140–1147.
- Paats MS, Bergen IM, Hoogsteden HC, van der Eerden MM, Hendriks RW. Systemic CD4+ and CD8+ T cell cytokine profiles correlate with GOLD stage in stable COPD. Eur Respir J 2012; 40(2):330–337.
- Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol 2008; 180(9):5771–5777.
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011; 29:71–109.
- Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol 2009; 9(4):447–453.
- Takanashi S, Hasegawa Y, Kanehira Y, Yamamoto K, Fujimoto K, Satoh K, Okamura K. Interleukin-10 level in sputum is reduced in bronchial asthma, COPD and in smokers. Eur Respir J 1999; 14(2)309–314.
- Moermans C, Heinen V, Nguyen M, Henket M, Sele J, Manise M, Corhay JL, Louis R. Local and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary disease. Cytokine 2011; 56(2):298–304.
- Pelegrino NR, Tanni SE, Amaral RA, Angeleli AY, Correa C, Godoy I. Effects of active smoking on airway and systemic inflammation profiles in patients with Chronic Obstructive Pulmonary Disease. Am J Med Sci 2012; Aug 7.
- Hackett TL, Holloway R, Holgate ST, Warner JA. Dynamics of pro-inflammatory and anti-inflammatory cytokine release during acute inflammation in chronic obstructive pulmonary disease: an ex vivo study. Respir Res 2008; 9:47.
- Barceló B, Pons J, Fuster A, Sauleda J, Noguera A, Ferrer JM, Agustí AG. Intracellular cytokine profile of T lymphocytes in patients with chronic obstructive pulmonary disease. Clin Exp Immunol 2006; 145(3):474–479.
- Cosio BG, Iglesias A, Rios A, Noguera A, Sala E, Ito K, Barnes PJ, Agusti A. Low-dose theophylline enhances the anti-inflammatory effects of steroids during exacerbations of COPD. Thorax 2009; 64(5):424–429.
- Murdoch JR, Lloyd CM. Resolution of allergic airway inflammation and airway hyperreactivity is mediated byIL-17-producing {gamma}{delta}T cells. Am J Respir Crit Care Med 2010; 182(4):464–476.
- Lee KA, Kang MH, Lee YS, Kim YJ, Kim DH, Ko HJ, Kang CY. A distinct subset of natural killer T cells produces IL-17, contributing to airway infiltration of neutrophils but not to airway hyperreactivity. Cell Immunol 2008; 251(1):50–55.
- Kondo T, Takata H, Matsuki F, Takiguchi M. Cutting edge: Phenotypic characterization and differentiation of human CD8 +Tcells producing IL-17. J Immunol 2009; 182(4):1794–1798.
- Cazzola M, Matera MG. IL-17 in chronic obstructive pulmonary disease. Expert Rev Respir Med 2012; 6(2): 135-8.
- Vanaudenaerde BM, Verleden SE, Vos R, De Vleeschauwer SI, Willems-Widyastuti A, Geenens R, Van Raemdonck DE, Dupont LJ, Verbeken EK, Meyts I. Innate and adaptive interleukin-17-producing lymphocytes in chronic inflammatory lung disorders. Am J Respir Crit Care Med 2011; 183(8):977–986.
- Hong SC, Lee SH. Role of th17 cell and autoimmunity in chronic obstructive pulmonary disease. Immune Netw 2010; 10(4):109–114.
- Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol 2009;183(10):6236–6243.
- Zhang X, Zheng H, Zhang H, Ma W, Wang F, Liu C, He S. Increased interleukin (IL)-8 and decreased IL-17 production in chronic obstructive pulmonary disease (COPD) provoked by cigarette smoke. Cytokine 2011; 56(3):717–725.
- Rahman MS, Yang J, Shan LY, IL-17R activation of human airway smooth muscle cells induces CXCL-8 production via a transcriptional-dependent mechanism. Clin Immunol 2005; 115:268–276.
- Vanaudenaerde BM, Wuyts WA, Dupont LJ, Van Raemdonck DE, Demedts MM, Verleden GM. Interleukin-17 stimulates release of interleukin-8 by human airway smooth muscle cells in vitro:a potential role for interleukin-17 and airway smooth muscle cells in bronchiolitis obliterans syndrome. J Heart Lung Transplant 2003; 22:1280–1283.
- Roussel L, Houle F, Chan C, Yao Y, Bérubé J, Olivenstein R, IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol 2010; 184(8):4531–4537.
- Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175(3): 250–255.
- Zhu J, Cao Y, Li K, Wang Z, Zuo P, Xiong W, Xu Y, Xiong S. Increased expression of aryl hydrocarbon receptor and interleukin 22 in patients with allergic asthma. Asian Pac J Allergy Immunol 2011; 29(3): 266–272.
- Nakagome K, Imamura M, Kawahata K, Harada H, Okunishi K, Matsumoto T, Sasaki O, Tanaka R, Kano MR, Chang H, Hanawa H, Miyazaki J, Yamamoto K, Dohi M. High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism. J Immunol 2011; 187(10): 5077–89.
- Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009; 119(12): 3573–85.
- Rico de Souza A, Zago M, Pollock SJ, Sime PJ, Phipps RP, Baglole CJ. Genetic ablation of the aryl hydrocarbon receptor causes cigarette smoke-induced mitochondrial dysfunction and apoptosis. J Biol Chem 2011; 286(50): 43214–28.
- Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, Dayer JM, Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol 2010; 40(9): 2450–9.
- Ogawa Y, Duru EA, Ameredes BT. Role of IL-10 in the resolution of airway inflammation. Curr Mol Med 2008; 8(5): 437–45.
