Abstract
Combined therapy with tiotropium and long-acting beta 2 agonists confers additional improvement in symptoms, lung function and aspects of health-related quality of life (QOL) compared with each drug alone in patients with COPD. However, the efficacy of combined therapy on walking distance, a surrogate measure of daily functional activity and morbidity remains unclear. The aim was, therefore, to quantify the benefit of this therapy on the six minute walk test. Secondary outcomes included change in lung function, symptoms, the BODE index and QOL. In a double-blind, crossover study, 38 participants with moderate to severe COPD on tiotropium were randomised to receive either formoterol or placebo for 6 weeks. Following a 2-week washout period, participants crossed over to the alternate arm of therapy for a further 6 weeks. Thirty-six participants, with an average age of 64.3 years and FEV1 predicted of 53%, completed the study. Combined therapy improved walking distance by a mean of 36 metres [95% CI: 2.4, 70.1; p = 0.04] compared with tiotropium. FEV1 increased in both groups (160 mL combination therapy versus 30 mL tiotropium) with a mean difference of 110 mL (95% CI: −100, 320; p = 0.07) between groups, These findings further support the emerging advantages of combined therapy in COPD. Australian New Zealand Clinical Trials.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive debilitating disorder characterised by worsening respiratory symptoms, ongoing airway inflammation and irreversible airflow limitation. It is a major cause of morbidity and mortality—currently predicted to be the fourth-leading cause of death in the world by 2030 (Citation1, 2). Quality-of-life studies demonstrate that COPD sufferers are mainly limited by physical function and up to 43% depend on family and friends for help with activities of daily living (Citation3).
Key goals of COPD management include preventing disease progression, relieving symptoms, reducing exacerbations and improving exercise tolerance and health status (Citation1, 2). Smoking cessation, annual influenza vaccination and pulmonary rehabilitation have been shown to improve these outcomes (Citation1) as have bronchodilators (Citation4–12), either alone or in combination with inhaled corticosteroids. Tiotropium, an anticholinergic bronchodilator, and long-acting beta 2 agonist bronchodilators (LABA) such as salmeterol, formoterol and indacaterol have been shown individually to significantly improve lung function, symptoms, health- related quality of life and exercise capacity in COPD compared with standard therapy (Citation4–14).
Although comparative trials have established tiotropium to be equivalent or superior to long-acting beta 2 agonists in COPD (Citation4, Citation14–16), studies have demonstrated that when these drugs are combined, there is further significant improvement in lung function and health-related quality of life measures (Citation9, 10, Citation12, Citation17). No studies to our knowledge, have primarily examined the effects of combined therapy on walking distance a functional outcome similar to quality of life, that is important and meaningful to patients. The six minute walk test (6MWT), measuring the distance walked in 6 minutes, is a validated tool which reflects functional exercise capacity for daily physical activities (Citation18). It has been shown to be responsive to interventions in COPD (Citation19) and is a predictor of morbidity and mortality (Citation20, 21).
The aim of this study was, therefore, to assess the therapeutic benefit of adding formoterol to tiotropium compared with tiotropium alone on the 6MWT in patients with COPD. We hypothesised that combined therapy would improve this patient outcome as well as lung function, symptoms and health-related quality of life measures.
Methods
Design
In this double-blind, crossover study, we randomised participants with clinically stable COPD on tiotropium for a minimum of 4 weeks, to receive either formoterol or placebo for six weeks, followed by a 2-week washout period. This washout period was considered clinically adequate to avoid drug carry over effects.
Participants then crossed over to the alternate arm of therapy for a further 6 weeks. The order of first intervention was randomised. Outcomes were measured at the beginning and end of the first 6 week treatment phase, and at the beginning and end of the second 6 week treatment phase of the study.
Participants
Participants were aged between 18 and 80 years, had COPD defined by American Thoracic Society/European Respiratory Society (ATS/ERS) criteria and were current or ex-smokers with a ≥ 10 pack-year smoking history (Citation22). They were clinically stable without a respiratory infection or exacerbation of COPD for 4 weeks prior to entry into the study. Inhaled corticosteroids were permitted. Patients with a history of asthma, myocardial infarction within 3 months of the study, symptomatic prostatic hypertrophy or glaucoma were excluded; participants on long-term long-acting beta-2 agonists, prednisone or theophylline were excluded. The study received approval from the regional Ethics Committee and was conducted in accordance with the Declaration of Helsinki International Conference on Harmonization (ICH)/Good Clinical Practice. Participants provided written informed consent. The study was registered with the Australian New Zealand Clinical Trials Registry, number: ACTRN12608000079347.
Procedures
Patient visits were conducted at the same time throughout the study ±1 hour; all visits were conducted in the morning. Patients were advised to stop their respiratory medications as follows: tiotropium 24 hours; short-acting beta-agonists 6 hours; long-acting beta-agonists, 12 hours. Symptoms were recorded and dyspnoea was graded using the validated, modified Medical Research Council (MMRC) dyspnoea scale (Citation23, 24).The 6MWT was performed according to ATS guidelines (Citation18). Spirometry with reversibility was then measured according to published guidelines using predicted values from Crapo and colleagues (Citation25–27) The Body–mass index, Airflow obstruction Dyspnoea, Exercise capacity (BODE) index, a composite multidimensional functional grading system based on a 10-point scale, with higher scores indicative of poorer outcomes, was calculated (Citation28). Health-related quality of life was measured by the St George's Respiratory Questionnaire (SGRQ), which measures 56 items across 3 domains: symptoms, activity and impacts (psychosocial dysfunction)(Citation29). A total score is calculated from all three components, with zero indicating no health impairment and 100 representing maximum impairment, with a change in score of 4 units considered clinically significant (Citation30).
Medications, both drug and placebo inhalers, were provided by an independent, accredited compounding pharmacy. Compliance was measured by weighing the turbuhalers at the beginning of the study and at the end of each treatment period. Additionally, a count of the number of inhalations taken was calculated from the number dial. As tiotropium was delivered via the handihaler device, a capsule count was performed before and after each period. Patients also self-reported medication use at each visit. Adverse events were recorded.
Participants were randomly allocated to 1 of the 2 treatment arms using a randomisation schedule generated from a random numbers table. Randomisation tables and treatment allocation codes were generated and held off site by an independent monitor. Participants, research assistants, and investigators were masked to treatment allocation.
Statistical plan and analysis
We estimated that about 35 subjects would need to be enrolled to have 80% power to detect a mean difference of 25 meters in the 6-minute walk test between the groups, assuming a 10% dropout rate.
Analytical methods
Descriptive statistics were used to summarise the clinical characteristics of participants. Normality of the outcome data were tested and evaluated using the skewed statistics and visualised by box-plot and histogram. The changes in all outcome data were analysed with paired sample t-tests. Effects of treatment order, time period and carryover were assessed as per Hills and Armitage for a two-period crossover study (Citation31). All analyses were done by intention-to-treat. Significance was accepted at p < 0.05. Data analysis was performed using SAS/STAT software, Version 9.2 of the SAS System for Windows (Copyright © 2002–2008 SAS Institute Inc.) and R software, Version 2.11 (Citation20).
Results
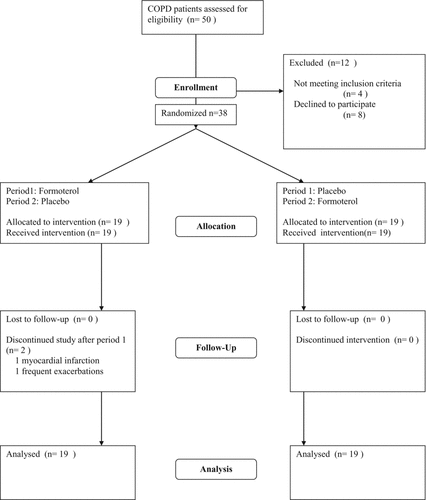
Thirty-eight subjects were enrolled. Two were withdrawn after completing period 1: one due to frequent exacerbations and 1 due to myocardial infarction On unblinding, both had been assigned to combination therapy (). Baseline characteristics are described in : participants had an average age of 64.3 (Citation8.Citation7) years and moderately severe COPD with a mean FEV1 predicted of 53(Citation16)% (Citation32). Seventy percent were on inhaled corticosteroids, 60% had been on tiotropium for longer than 4 weeks and none were on a LABA in the preceding 4 weeks prior to study entry.
Table 1. Baseline characteristics
Combined therapy (tiotropium plus formoterol) improved 6-minute walking distance after six weeks compared with tiotropium alone () by a mean of 36.3 metres [95% CI: 2.4, 70.1]. Although an improvement in FEV1 was observed in both treatment groups over 6 weeks, greater with combined therapy, it did not achieve statistical significance (160 mL versus 30 mL; mean difference between groups: 110 mL [95% CI, −100, 320; p = 0.07] (). Inspiratory capacity and symptom scores were similar in both groups (). Health-related quality of life outcomes were also similar in both groups. (). The improvement in the BODE index was greater with combined therapy compared with tiotropium alone (mean −0.27 [95% CI, −0.65, 0.11] versus 0.34 [95% CI, 0.11,0.66]; p = 0.04). No significant treatment period, or carry-over effect was present. Compliance with medications was 80%. Treatment with combined therapy was generally well tolerated, and no adverse events defined as ocular, oropharyngeal, cardiac, respiratory, gastrointestinal, urological, or dermatological were reported during the study. The data for the subject who was withdrawn due to a myocardial infarction were reviewed by an independent monitoring committee and not considered to be related to the study medications.
Table 2. Outcome variables: Exercise capacity, lung function and symptoms
Table 3. Health-related quality of life (SGRQ)
Discussion
This study demonstrates that adding formoterol to tiotropium therapy further increases walking distance in patients with COPD compared with tiotropium alone. The improvement in 6-minute walking distance of 36 metres obtained with combined therapy although modest, was clinically and statistically relevant; with a change of more than 25 to 30 metres considered to be the minimal clinically important difference (Citation33, 34). Previously, tiotropium alone has been shown to improve the 6-minute walking distance by 15 to 26 metres, and the incremental shuttle walk test (SWT) by 36 metres compared with placebo, in patients with COPD (Citation35–37); findings that are comparable to the 36 m increase noted in this study. More recently, 2 crossover studies adding tiotropium for 2 weeks in patients with COPD receiving formoterol demonstrated a significant improvement in exercise endurance measured by treadmill and cycle ergometry compared with placebo (Citation38, 39).
There is increasing evidence that a “walking test” is of clinical relevance, with implications for maintaining patients’ independence with daily activities and reflecting disease burden (Citation20, Citation40, Citation41). Several walking tests are utilised both clinically and in research: The SWT assesses peak walking distance or capacity; the endurance SWT (ESWT) assesses endurance time or the time taken to walk at a fixed speed (calculated as the speed corresponding to 80% of peak VO2, predicted from the SWT), and the 6MWT assesses the distance walked over a fixed period of time (Citation39). Although the SWT and ESWT may be more responsive to pharmacological interventions than the 6-minute walk test (Citation38) they examine different, complimentary aspects of walking. The distance walked in 6 minutes, however, remains the easiest test to perform, and translates well into clinical practice and real life when performed with attention to the learning effect and reproducibility of the test (Citation17, Citation40).
The improvement in exercise capacity was reflected by the BODE index, which incorporates the 6 MWT, lung function and symptoms into its multi-dimensional functional measurement. Worsening BODE scores have, similar to the 6-minute walk test, been shown to correlate with increasing morbidity and mortality (Citation28, Citation42–44).
Significant changes were not noted in lung function, symptom scores or quality of life. The magnitude of the treatment effect obtained with these outcomes, however, was similar to that documented in previous larger, studies combining tiotropium with LABA (Citation37). In a meta-analysis of 8 studies comparing therapy with formoterol and tiotropium to tiotropium alone, combined therapy improved lung function (FEV1) by a mean difference (MD) of 105 mL and symptom scores assessed by the transitional dyspnoea index(TDI) by a MD of 1.5 units (Citation12).
A recent Cochrane collaboration meta-analysis (n = 5 studies) examining the clinical benefits of combining therapy with LABA and tiotropium versus tiotropium alone showed that health-related quality of life and lung function outcomes also improved significantly with combined therapy, although the treatment effects were small: SGQR (MD) −1.61 units and FEV1 (MD) 0.07 L (Citation45). Last, in a crossover trial, combined therapy with tiotropium 18mcg daily and formoterol 12 mcg daily for 6 weeks increased FEV1 by a further 100 mL, compared with formoterol or tiotropium alone (Citation9).
Although FEV1, reflecting airflow limitation, improved with the addition of formoterol to tiotropium therapy, inspiratory capacity (IC) and forced vital capacity (FVC), measures of dynamic hyperinflation did not. This appears to be contrary to current literature demonstrating that these variables correlate well with the 6 MWT (Citation46) and that IC improves with bronchodilator therapy (Citation39, Citation47). Possible explanations for the lack of improvement in IC include the timing of spirometric measurements to exercise and inhaled bronchodilator therapy. Studies demonstrating improvement in IC with bronchodilator therapy have assessed change immediately pre- and post-treadmill and cycle cardiopulmonary tests and at peak drug concentrations (1 to 2 hours after medication) (Citation39, Citation47).
It is also unclear if the degree of clinical improvement achieved by adding tiotropium to established LABA therapy is interchangeable and comparable to that of adding a LABA such as formoterol or salmeterol to patients on regular tiotropium. As all our patients were on tiotropium, additional improvements in lung volumes with formoterol compared with placebo may have been too small to detect given the sample size. Finally, there is increasing evidence that not all patients with severe COPD experience hyperinflation at rest and dynamic hyperinflation with exercise. Other mechanical factors such as respiratory and leg muscle fatigue and “neuromuscular uncoupling” may play a role (Citation48, 49).
Potential limitations of this study include the sample size, which likely precluded some of the results from reaching statistical significance. However, given that the treatment effect documented with combined long-acting bronchodilator therapy on the 6MWT and absolute FEV1 is of a similar magnitude to previous studies, we propose that the benefit is genuine and clinically significant. These findings further endorse the emerging role of combined therapy in enhancing patient centred outcomes in moderate-to-severe COPD.
Declaration of Interest Statement
This study was supported by a grant from the Auckland Medical Research Foundation of New Zealand. LJ, SM, HR, CO and IZ have no conflict of interest to declare. CW is a member of an independent scientific committee for an annual Respiratory Workshop held in New Zealand that is sponsored by Boehringer Ingelheim.
The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors would like to thank the participants in this study, Andrew Nelson, Angela Smith and the Centre for Clinical Research and Effective Practice for providing technical and personnel assistance for this study, and Shailen Ramjee for pharmaceutical support.
NOTICE OF CORRECTION:
A previous version of this paper was published early online.
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013. Available from: http://www.goldcopd.org/.
- DK McKenzie, M Abramson, AJ Crockett, E Dabscheck, N Glasgow, S Jenkins, C McDonald, R Wood-Baker, I Yang, Frith PA on behalf of The Australian Lung Foundation. The COPD-X Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease V2.32, 2012.
- Broad J, Jackson R. Chronic Obstructive Pulmonary Disease and Lung Cancer in New Zealand. Auckland: UniServices; 2003.
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003; 58(5):399–404. Epub 2003/05/03.
- Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J 2002; 19(2):209–216. Epub 2002/03/02.
- Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19(2):217–224. Epub 2002/02/28.
- Grove A, Lipworth BJ, Reid P, Smith RP, Ramage L, Ingram CG, Effects of regular salmeterol on lung function and exercise capacity in patients with chronic obstructive airways disease. Thorax 1996; 51(7):689–693. Epub 1996/07/01.
- Casaburi R, Kukafka D, Cooper CB, Witek TJ, Jr., Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest 2005; 127(3):809–817. Epub 2005/03/15.
- van Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J 2005; 26(2):214–222. Epub 2005/08/02.
- Cazzola M, Centanni S, Santus P, Verga M, Mondoni M, di Marco F, The functional impact of adding salmeterol and tiotropium in patients with stable COPD. Respir Med 2004; 98(12):1214–1221. Epub 2004/12/14.
- Cazzola M, Biscione GL, Pasqua F, Crigna G, Appodia M, Cardaci V, Use of 6-min and 12-min walking test for assessing the efficacy of formoterol in COPD. Respir Med 2008; 102(10):1425–1430. Epub 2008/07/16.
- Wang J, Jin D, Zuo P, Wang T, Xu Y, Xiong W. Comparison of tiotropium plus formoterol to tiotropium alone in stable chronic obstructive pulmonary disease: a meta-analysis. Respirology 2010; 16(2):350–358. Epub 2010/12/09.
- Beier J, Beeh KM. Long-acting beta-adrenoceptor agonists in the management of COPD: focus on indacaterol. Int J Chron Obstruct Pulmon Dis 2011; 6:237–243.
- Donohue JF, Fogarty C, Lotvall J, Mahler DA, Worth H, Yorgancioglu A, Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010; 182(2):155–162.
- Donohue JF, van Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ, Jr., A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002; 122(1):47–55. Epub 2002/07/13.
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MP, Beeh KM, Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364(12):1093–1103. Epub 2011/03/25.
- van Noord JA, Aumann JL, Janssens E, Smeets JJ, Zaagsma J, Mueller A, Combining tiotropium and salmeterol in COPD: Effects on airflow obstruction and symptoms. Respir Med 2010; 104(7):995–1004. Epub 2010/03/23.
- Barker AF. Bronchiectasis. N Engl J Med 2002; 346(18):1383–1393. Epub 2002/05/03.
- de Torres JP, Pinto-Plata V, Ingenito E, Bagley P, Gray A, Berger R, Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest 2002; 121(4):1092–1098. Epub 2002/04/12.
- Spruit MA, Polkey MI, Celli B, Edwards LD, Watkins ML, Pinto-Plata V, Predicting Outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc 2012; 13(3):291–297. Epub 2011/07/23.
- Cote CG, Pinto-Plata V, Kasprzyk K, Dordelly LJ, Celli BR. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest 2007; 132(6):1778–1785. Epub 2007/10/11.
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6):932–946. Epub 2004/06/29.
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959; 2(5147):257–266. Epub 1959/08/29.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93(3):580–586. Epub 1988/03/01.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Standardisation of spirometry. Eur Respir J 2005; 26(2):319–338. Epub 2005/08/02.
- Crapo RO. The role of reference values in interpreting lung function tests. Eur Respir J 2004; 24(3):341–342. Epub 2004/09/11.
- Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981; 123(6):659–664. Epub 1981/06/01.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10):1005–1012. Epub 2004/03/05.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145(6):1321–1327. Epub 1992/06/01.
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002; 19(3):398–404. Epub 2002/04/09.
- Hills M, Armitage P. The two-period cross-over clinical trial. 1979. Br J Clin Pharmacol 2004; 58(7):S703–5716; discussion S17-9. Epub 2004/12/15.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176(6):532–555. Epub 2007/05/18.
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010; 91(2):221–225.
- Polkey MI, Spruit MA, Edwards LD, Watkins ML, Pinto-Plata V, Vestbo J Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med 2013; 187:382–386.
- Fujimoto K, Kitaguchi Y, Kanda S, Urushihata K, Hanaoka M, Kubo K. Comparison of efficacy of long-acting bronchodilators in emphysema dominant and emphysema nondominant chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2011; 6:219–227. Epub 2011/06/11.
- Okudan N, Gok M, Gokbel H, Suerdem M. Single dose of tiotropium improves the 6-minute walk distance in chronic obstructive pulmonary disease. Lung 2006; 184(4):201–204. Epub 2006/09/29.
- Verkindre C, Bart F, Aguilaniu B, Fortin F, Guerin JC, Le Merre C, The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration 2006; 73(4):420–427. Epub 2006/02/18.
- Berton DC, Reis M, Siqueira AC, Barroco AC, Takara LS, Bravo DM, Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med 2010; 104(9):1288–1296.
- Canto ND, Ribeiro JP, Neder JA, Chiappa GR. Addition of tiotropium to formoterol improves inspiratory muscle strength after exercise in COPD. Respir Med 2012; 106(10):1404–1412.
- Garcia-Rio F, Lores V, Mediano O, Rojo B, Hernanz A, Lopez-Collazo E, Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med 2009; 180(6):506–512. Epub 2009/06/23.
- Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest 2001; 119(1):256–270. Epub 2001/02/07.
- Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J 2005; 26(4):630–636. Epub 2005/10/06.
- Cote CG, Pinto-Plata VM, Marin JM, Nekach H, Dordelly LJ, Celli BR. The modified BODE index: Validation with mortality in COPD. Eur Respir J 2008; 32(5):1269–1274. Epub 2008/06/27.
- Cote CG, Celli BR. BODE index: a new tool to stage and monitor progression of chronic obstructive pulmonary disease. Pneumonol Alergol Pol 2009; 77(3):305–313. Epub 2009/07/11.
- Karner C, Cates CJ. Long-acting beta(2)-agonist in addition to tiotropium versus either tiotropium or long-acting beta(2)-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 4:CD008989. Epub 2012/04/20.
- Marin JM, Carrizo SJ, Gascon M, Sanchez A, Gallego B, Celli BR. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163(6):1395–1399.
- O'Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23(6):832–840.
- Calverley PM. Dynamic hyperinflation: is it worth measuring? Proc Am Thorac Soc 2006; 3(3):239–244.
- Guenette JA, Webb KA, O'Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J 2012; 40(2):322–329.