Abstract
Background: Intravenous alpha-1 antitrypsin protein (AAT) augmentation is a prescribed therapy for severe, genetically determined, alpha-1 antitrypsin deficiency (AATD), a genetic basis for pulmonary emphysema. AAT, a predominant systemic inhibitor of neutrophil elastase thus far has not been shown to decrease elastin degradation in a significant number of patients on this therapy. The objective of this study was to compare levels of biomarkers of elastin degradation in plasma, bronchoalveolar lavage (BALF) fluid and urine before and after beginning AAT augmentation therapy in patients with AATD. Methods: Desmosine and isodesmosine (DI), which occur only in elastin, are amino acid cross-links in mature elastin. Levels of DI in body fluids measure degradation of elastin and can be measured more specifically by mass spectrometry. This method was used to measure DI levels in plasma, bronchoalveolar lavage fluid and urine in cohorts of severe AATD patients on augmentation, not on augmentation and before and after the initiation of augmentation therapy. Results: Statistically significant reductions in plasma DI and in BALF DI were demonstrated in AATD patients receiving intravenous (IV) augmentation therapy as compared with those not receiving it. Administration by aerosol also produced statistically significant reductions in levels of DI in BALF. Conclusions: Results indicate that the currently prescribed doses of AAT augmentation inhibit neutrophil elastase adequately to reduce elastin degradation, both systemically and in the lung per se. The currently prescribed doses did not reduce elastin degradation to control levels, which may be possible with higher doses.
Introduction
Augmentation of circulatory levels of alpha-1 antitrypsin (AAT) protein has been a prescribed therapy for the long-term treatment of severe alpha-1 antitrypsin deficiency (AATD) for over 25 years (Citation1). The hypothesis is that maintaining higher levels of alpha-1 protein in blood and tissues should be protective against the effect of neutrophil elastase, for which AAT is the major systemic inhibitor. However, attempts to demonstrate a reduction in elastin degradation by augmentation therapy have been inconsistent.
Stone and colleagues studied two AATD patients, a 63-year-old female and a 41-year-old male. They received monthly infusions of 260 mg/kg of AAT and were followed for 18 months. Mean values of post-treatment urinary desmosine values determined by the isotope-dilution high performance liquid chromatography (HPLC) method show a sustained drop that exceeded 35% in both subjects from pre-treatment levels (Citation2). Measurements of desmosine in body fluids other than urine were not available in this study.
A study was carried out by the American-Italian study group in 2000 (Citation3). This trial was unblinded and open label and studied 12 AATD subjects with severe to moderate emphysema who received supplementation with Prolastin (Bayer Company, Shawnee Mission, KS, USA) with a weekly regimen of 60 mg/kg for 4 weeks. Spot urine samples were collected weekly for 4 weeks during the run in and then weekly prior to each of the weekly infusions. Urine specimens were collected 2 days after weeks 2 and 4. Urinary desmosine values were determined by the isotope-dilution HPLC method (Citation4). During supplementation, the urinary desmosine excretion was unchanged in comparison with the run-in.
In this study the AATD subjects with emphysema never receiving supplementation therapy excreted more desmosine than healthy smokers or COPD patients with normal AAT, a result consistent with higher plasma levels of DI in AATD patients than in non-AATD COPD patients demonstrated recently (Citation5).
In 2002, Stoller et al. (Citation6) reported a randomized controlled trial with 26 AATD subjects to evaluate the bioequivalence of 2 commercially available preparations of pooled human plasma AAT. Patients were studied for 24 weeks and urinary desmosine excretion was measured weekly by 2 methods, the isotope-dilution HPLC method (Citation4) and RIA (Citation7). Desmosine values showed a good correlation between the 2 methods of measurement but no significant differences occurred between values at entry and after 24 weeks of treatment.
Changes were introduced in the methods of analysis of desmosine and isodesmosine (DI) using mass spectrometry in 2003 (Citation8). This, increased specificity and sensitivity for such measurements in body fluids, which included plasma and sputum as well as urine. This method has recently been modified to include an acetylated pyridinoline internal standard, which improves accuracy and was applied in this study (Citation9).
A free, non-conjugated to a protein peptide component portion of DI in urine is measurable by mass spectrometry and is considered to be an indicator of increased elastin peptide degradation in vivo prior to excretion and its increase in urine would be consistent with an increase in elastase activity in vivo (Citation10).
In 2007 results of measurements of DI in urine, plasma and sputum in patients with COPD related to AATD and COPD patients with normal levels of AAT were published (Citation5). In both groups the levels of DI in plasma and sputum and the unconjugated free component of DI in urine were significantly elevated above control values. The patients with AATD had values of DI, which were significantly above the non-AATD patients. All the AATD patients were on augmentation therapy. Pre-augmentation therapy values were not available in this population of patients.
The availability of fluid samples for analysis of plasma, bronchoalveolar lavage fluid (BALF) and urine before starting augmentation and again after
augmentation therapy provided by the Tissue Bank of the Alpha One Foundation and University of Florida Research Program allowed such an analysis. This report compares levels of elastin degradation biomarkers before and after the administration of augmentation therapy and also compares plasma levels in cohorts of AATD patients receiving and not receiving augmentation therapy.
Methods
Body fluids for analysis
The plasma samples from patients as well as relatives of patients analyzed in this study were obtained from the Alpha-1 Foundation DNA and Tissue Bank. The Alpha-1 Foundation DNA and Tissue Bank project is sponsored by the Alpha-1 Foundation and is located at the University of Florida College of Medicine in Gainesville FL. The Alpha-1 Foundation IRB approved this protocol (659-2002).
The bronchoalveolar lavage fluid (BALF) and urine samples analyzed in this study were obtained from a previous IRB approved Lung Research Data and Tissue Bank Registry study (UF-IRB577-2002). All subjects signed an informed consent form. Alpha-1 antitrypsin phenotyping and genotyping was performed at the Alpha-1 Genetic Laboratory at the University of Florida.
There were 4 sources of body fluid samples analyzed:
Plasma samples from 47 patients receiving alpha-1 antitrypsin protein from human blood sources, commercially available, compared with 50 AATD patients not on AAT augmentation and 50 normal subjects (see );
11 patients with homozygous severe AATD who provided plasma samples before the start of IV therapy and then 12 and 24 weeks later (see ).
10 patients with homozygous AATD receiving IV replacement provided BALF at 12 weeks. The dose administered was 60 mg/Kg body weight each week in the above patient groups; and,
12 patients received a recombinantly produced AAT which was administered as an aerosol for 8 weeks (see ) (Citation11). The administered dose was 250 mg of aerosolized transgenic AAT each day. Eight patients of the 12 provided BALF and 5 of 12 patients provided urine samples at baseline and after 8 weeks of aerosol therapy.
Table 1. Patient cohorts providing plasma samples for analysis of DI
Table 2. Patients receiving AAT augmentation by intravenous infusion
Table 3. Patients receiving augmentation of AAT by aerosol administration
The following 3 methods were used to define the alpha-1 genomes: 1) Genotype by allelic discrimination using TaqMan (S&Z alleles), ABI 7500 Fast Real-time PCR System (Life Technologies, Carlsbad, CA, USA); 2) AAT Level by Immunogenic assay, Dade Behring Nephelometer BN II (Deerfield, IL, USA) and 3) PI Typing by Isoelectric Focusing, Pharmacia Biotech Multiphore II (Piscataway, NJ, USA). The method of analysis for DI in plasma, BALF and urine is as described (Citation9). The content of DI in BALF is reported as the Epithelial Lining Fluid (ELF) concentration of DI. ELF dilution factor is obtained using urea method (Citation12).
Analytic method for DI
High performance liquid chromatography and tandem mass spectrometry as described (Citation9). Analyses of DI levels were done in triplicate in batches of 20 samples on subsequent days. The coefficient of variation of the method is 8%.
Statistical methods
The t-test was used to compare the effects of augmentation therapy on the large patient cohorts. The paired t-test was used to compare the changes before and after therapy in individuals. The linear regression was used to determine the relationships of age to levels of DI.
Results
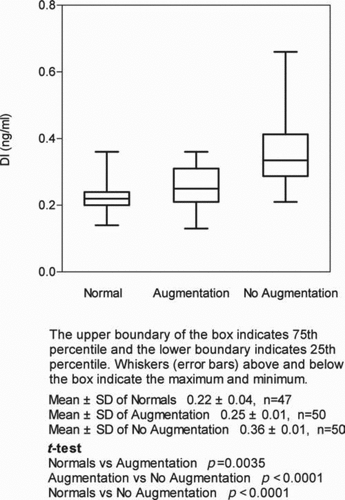
The mean levels of DI in plasma of normal individuals was 0.22 ng/ml ± 0.04SD (n = 47), which is compared with a mean of 0.25 ng/ml ± 0.01SD (n = 50) in patients receiving augmentation therapy p = 0.0035 for the difference (n = 50). Levels of DI in AATD patients not receiving augmentation (n = 50) averaged 0.36 ng/ml ± 0.01SD, p < 0.0001 for comparison of levels of DI in the AATD patients not receiving AAT therapy and the normal controls and between AATD subjects receiving and not receiving augmentation ().
Figure 1. The effect of intravenous alpha-1 antitrypsin augmentation therapy on plasma levels of desmosine and isodesmosine (DI) in alpha-1 antitrypsin deficiency.
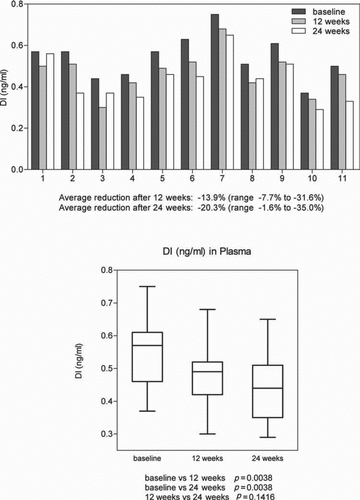
In 11 patients receiving intravenous replacement, levels of DI at baseline and at 12 and 24 weeks of therapy showed statistically significant reductions in DI in plasma at both time points; (i.e. –13.9% and –20.3%, p = 0.038)().
Figure 2. The effect of intravenous alpha-1 antitrypsin augmentation therapy on levels of desmosine and isodesmosine (DI) in plasma.
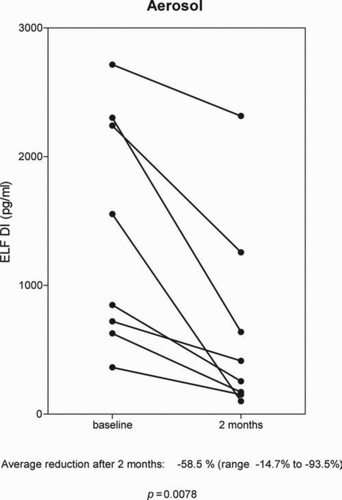
Levels of DI in BALF before and 12 weeks after receiving IV augmentation therapy were analyzed in 10 patients. Levels of DI were reduced in 8 patients and increased in 2. The overall average change was –37% (range: –12.8% to –85.9%) p = 0.0273 ().
Figure 3. The effect of Intravenous alpha-1 antitrypsin augmentation therapy on levels of desmosine and isodesmosine (DI) in bronchoalveolar lavage fluid.
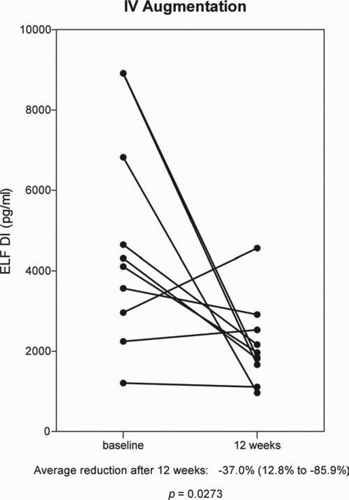
In 12 patients, comparison of before and after aerosol augmentation therapy measurements of DI in plasma showed a mean decrease of 6.5% and a range of percent change from 22.3% to –50.8% from base line. This change in concentration of DI in 12 patients was not statistically significant (p = 0.1675) . The reduction in DI levels in 9 of the 12 patients is statistically significant, an average decrease of 12.1% (range: –0.2% to –50.8%) p = 0.0142. The mean increase in DI in 3 patients was 4.3%, which is not statistically significant.
Figure 4. The effect of aerosol alpha-1 antitrypsin augmentation therapy on levels of desmosine and isodesmosine (DI) in plasma.
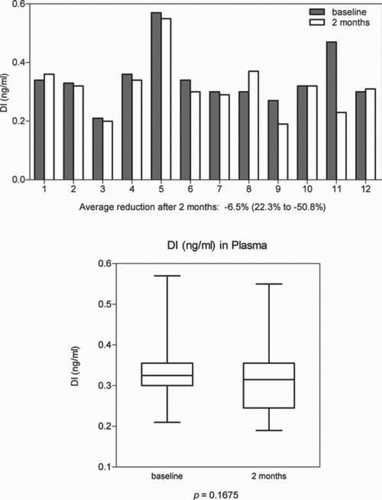
In 8 patients receiving AAT by aerosol administration, DI levels in BALF were all reduced. Mean reduction was –58.5% (range: –14.7% to –93.5%) p = 0.0078 (). Analysis of DI in BALF was also done using the total protein content of BALF as a correction factor and the results were similar to that shown for epithelial lining fluid volumes determined by the urea concentration.
Figure 5. The effect of aerosol alpha-1 antitrypsin augmentation therapy on levels of desmosine and isodesmosine (DI) in bronchoalveolar lavage fluid.
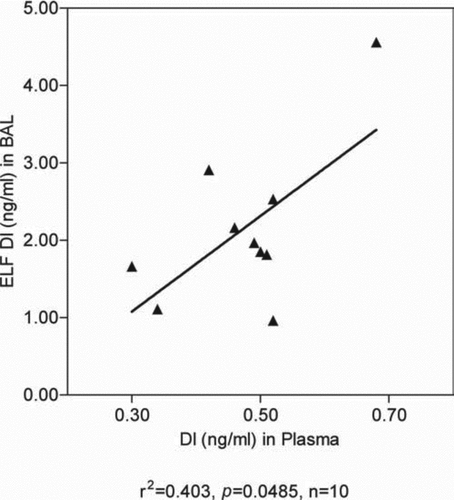
In 10 patients who were receiving intravenous augmentation it was possible to compare levels of DI in bronchoalveolar lavage fluid (BALF) and plasma in the same patients at the same time. There is a positive and significant correlation between the two ().
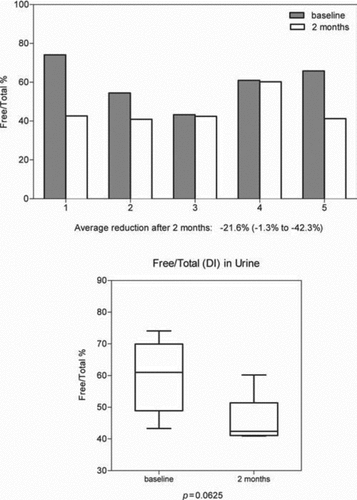
In 5 subjects’ urine samples were obtained before and after administration of aerosol augmentation therapy. In 4 of the 5 subjects there was a reduction in the free component of DI excretion with a range of 0.1–13.0%. One subject showed an increase in excretion of 75.3%. Total excretion of DI was increased post-therapy in 3 subjects and decreased in 2. The percent of free DI over total DI excretion was reduced in all 5 subjects with an average reduction of 21.6% and a range of –1.33% to –42.36%. This result was slightly below statistical significance (p = 0.0625) ().
Figure 7. The effect of aerosol alpha-1 antitrypsin augmentation therapy on urinary desmosine and isodesmosine (DI): Ratios of free DI to total DI.
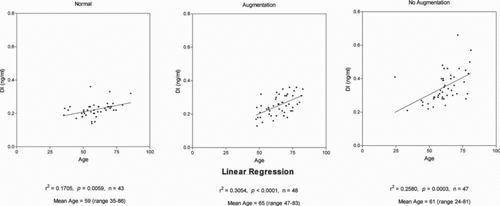
As shown in , there is a statistically significant positive correlation of plasma levels of DI with advancing age in normal subjects as well as in patients with AATD receiving and not receiving augmentation therapy.
Discussion
This study demonstrates statistically significant decreases in levels of DI in plasma in AATD patients on long term intravenously administrated AAT replacement. The measurments of DI in BALF where 8 of 10 patients demonstrated statistically significant reductions in levels suggests that I.V. augmentation therapy is reducing elastin degradation specifically in the lung, a desired result of therapy.
The reductions in plasma levels of DI in populations of patients with AATD receiving and not receiving augmentation therapy, as well as specific individuals before and after receiving AAT therapy is consistent with a decrease of elastin degradation systemically and a systemic anti-inflammatory effect.
The significant reductions of DI in BALF in patients receiving aerosol administration of AAT is an indication that nebulized AAT is reducing the activity of neutrophil elastase in the lung per se and suggests that this route of administration may be effective therapy in AATD. The reductions in plasma levels of DI in this limited number of patients was not as consistent with aerosol administration as the reductions of DI in plasma of patients receiving IV administration.
The more inconsistent reduction in DI levels in plasma with aerosol administration may be related to the lower weekly total dose of AAT being administered by aerosol, compared with the intravenous dose. A 70-kg body weight subject received a 43% lower weekly dose by aerosol than IV. The positive correlation of DI levels in plasma and BALF sampled at the same time of 12 weeks of therapy () is consistent with the levels of DI in lung positively contributing to levels in plasma.
The reduction in the percent of free DI in urine suggests less elastase degradation of elastin fragments in vivo prior to excretion, which would also be consistent with an anti-inflammatory effect of AAT augmentation (Citation10). This result, obtained in only 5 patients with available specimens, was slightly below statistical significance (p = 0.0625).
It is noteworthy that the 50 patients receiving augmentation therapy had plasma levels still above the normal range of DI. This result raises the prospect that higher doses of augmentation therapy may achieve even more effective reductions in elastase activity in AATD individuals.
Analysis of levels of DI in 43 control subjects without lung disease or alpha-1 antitrypsin deficiency (AATD) allowed us to relate plasma levels of DI to age. There was a positive correlation of increase in plasma DI levels with advancing age in non-AATD normals, as shown in (r2 = 0.1705 and p = 0.0059). These data included smokers as well as non-smoking normals. As shown, there are positive correlations of plasma DI level with age in the AATD cohorts receiving and not receiving augmentation.
The information from patients in these cohorts obtained from a questionnaire on their history of smoking was considered not reliable enough to enter into this analysis. The patient questionnaire allowed individuals with a history of having smoked 20-pack-years of cigarettes in their lifetimes or less to be categorized as non-smokers. When patients started and stopped smoking could not be determined and there was no information to rule out second hand smoke exposure. Accordingly, we analyzed each cohort with respect to age without separating tobacco smoke-exposed and those not exposed.
The increase in plasma levels of DI related to age in all three cohorts of subjects in this study is evidence that age is associated with increased degradation of body elastin. The exact mechanisms causing this effect are unclear. However, these data in human subjects are consistent with such evidence in senescence-accelerated mice (Citation13) and in studies of human aortic elastin showing reduced aorta elasticity with aging and a progressive reduction of the cross-links of aortic elastin in the aging subject (Citation14). Increased oxidation of elastin with age may play a role since it has been shown in vitro that oxidized elastin is subject to increased degradation by elastases (Citation15,16). Positive correlations of plasma levels of DI with age have recently been shown in 2 recent studies also using Mass spectrometric analytical methods (Citation17,18).
The mean value of plasma DI in the normal subjects in this study (0.22 ng/ml ± 0.04SD) is slightly higher than the mean normal value of plasma DI in a previous publication from this laboratory (Citation5) in 13 subjects (8 male, 5 female) (0.19 ng/ml ± 0.01SD). This difference may be related to the strict exclusion of smokers or second-hand smoke exposed in the prior study.
A previous study (Citation19) demonstrated an anti-inflammatory effect of AAT augmentation therapy based on reduced levels of leukotriene B-5, interleukin-8 and neutrophil elastase in sputum, after weeks of supplementation therapy with IV AAT. This result suggests that in addition to a direct inhibition of neutrophil elastase, augmentation therapy may reduce elastin degradation by reducing elastase production by neutrophils and macrophages through an additional antiinflammatory effect.
The positive results reported in the present study with measurements in plasma and BALF indicates that these body fluids can reflect changes in systemic elastin degradation as well as organ systems. The use of HPLC MS/MS technology has made measurements in these body fluids other than urine more sensitive and precise and deserves further application as biomarkers to evaluate potential therapies in COPD.
The plasma, BALF and urine for analysis in this study were stored in the deep frozen state (–20°C) for several years and there can be concern that such prolonged storage, even in the frozen state, could affect the content of DI. We believe that there is ample evidence that this is not the case:
The levels of DI in the normal subjects and in the AATD cohorts were in the same range as had been obtained from prior studies from this laboratory on freshly sampled plasma from similar cohorts (Citation5,Citation8,Citation9);
We have performed repeated analyses of frozen samples of plasma and urine stored in our laboratory for over 6 years and the quantitation of DI by HPLC/MS/MS is unchanged;
the purpose of this study was to determine differences in the DI levels before and after administration of augmentation therapy so before and after samples are exposed to the same storage conditions; and,
the chemical bonding of DI is stable and thus far no chemical mechanisms have demonstrated molecular degradation of DI in body fluids. Acid Hydrolysis of samples has been shown not to degrade DI (Citation9).
The plan and results of this study raise the consideration that AATD patients not receiving augmentation therapy may have higher DI levels in plasma because of a differing severity of disease. However, the mean FEV1 level in patients receiving augmentation therapy was 41.3%, S.D. = 22.1, yet the mean FEV1 in those not those not receiving augmentation was 72.7%, S.D. = 29.3. Prior studies demonstrate that DI levels in urine and plasma are higher with increasing severity of disease as indicated by the FEV1, which is counter to this premise (Citation17,18,Citation20).
A possible mechanism for lowering plasma levels of DI is increased renal clearance of DI. However, there is no evidence that augmentation therapy alters renal function from the preliminary pharmacodynamic studies of AAT products and physiologically the small amount of protein infused in a relatively small volume of fluid once per week seems unlikely to induce the reduction in DI observed in plasma and also unlikely in BALF.
The positive correlation of age and plasma levels of DI in these patient cohorts does not have an effect on the concluding results obtained in this study. In that regard, the patients receiving augmentation therapy had a higher mean age than the patients not on augmentation and yet their mean levels of DI were statistically significantly lower.
This study has limitations. The study of levels of DI before and after the start of augmentation therapy would have been stronger by having an increased number of patients and being a prospective rather than a retrospective analysis. However, we were dependent on what plasma and BALF samples were available from the University of Florida Tissue Bank. Prior studies from our laboratory indicate that DI are stable molecules in frozen fluids and the frozen state for years. The availability of BALF is limited by the invasiveness of the procedure. Ideally, one would have desired more than one before and one during therapy measurements from BALF and plasma to show consistency of levels in the two states but such samples were not available. Hopefully, such studies can be carried out in the future.
Acknowledgments
This work was supported by funds from the James P. Mara Center for Lung Disease (New York, NY, USA), the Flight Attendants Medical Research Institute (Miami, FL, USA), the Charles A. Mastronardi Foundation (Wilmington DE (USA), the Ned Doyle Foundation (New York) and the Alpha One Foundation (Miami) and also by funds from Ethel Kennedy, John Kennedy and Judith Sulzberger (New York).
Declaration of Interest Statement
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wewers MD, Casalaro MA, Sellers SE, Replacement therapy for alpha-1 antitrypsin deficiency associated with emphysema. NEJM 1987; 316(17):1055–1062.
- Stone PJ, Morris LA 3rd, Franzblau C, Snider GL. Preliminary evidence that augmentation therapy diminishes degradation of cross-linked elastin in alpha-1-antitrypsin-deficient humans. Respiration 1995, 62:76–79.
- Gottlieb DJ, Luisetti M, Stone PJ, Short-term supplementation therapy does not affect elastin degradation in severe alpha-antitrypsin deficiency. The American-Italian AATD Study Group. AJRCCM 2000; 162:2069–2072.
- Stone PJ, Bryan-Rhafdi J, Lucey EC, Measurement of urinary desmosine by isotope dilution and high performance liquid chromatography. Correlation between elastase-induced air-space enlargement in the hamster and elevation of urinary desmosine. Am Rev Respir Dis 1991; 144:2844–2890.
- Ma S, Lin YY, Turino GM. Measurement of desmosine and isodesmosine by mass spectrometry in COPD. Chest 2007; 131:1363–1371.
- Stoller JK, Rouhani F, Brantly M, Biochemical efficacy and safety of a new pooled human plasma alpha-1 antitrypsin, respitin. Chest 2002; 122:66–74.
- King GS, Mohan VS, Starcher BC. Radioimmunoassay for desmosine. Connect Tissue Res 1980; 7:263–267.
- Ma S, Lieberman S, Turino GM, Lin YY. The detection and quantitation of free desmosine and isodesmosine in human urine and their peptide-bound forms in sputum: Proc Natl Acad Sci USA 2003; 100:12941–12943.
- Ma S, Turino GM, Lin YY. Quantitation of desmosine and isodesmosine in urine, plasma, and sputum by LC-MS/MS as biomarkers for elastin degradation. J Chromatogr B 2011; 879:1893–1898.
- Rodriguez JR, Seals JE, Radin A, Lin JS, Mandl I, Turino GM. Neutrophil lysosomal elastase activity in normal subjects and in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1979; 119:409–417.
- Spencer LT, Humphries JE, Brantly ML (for the Transgenic Human Allpha1-Antitrypsin Study Group). Antibody response to aerosolized transgenic human alpha1 antitrypsin. NEJM 2005; 352:19.
- Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal R: Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 1986; 60(2):532–538.
- Atanasova M, Konova E, Georgieva M, Age-related changes of anti-elastin antibodies in senescence-accelerated mice. Gerontology 2010; 56:310–318.
- Watanaabe M, Sawai T, Nagura H, Age-related alteration of cross-linking amino acids of elastin in human aorta. Tohoku J. Exp Med 1996; 180:115–130.
- Cantor JO, Shteyngart B, Cerreta JM, Synergistic effect of hydrogen peroxide and elastase on elastin injury in vitro. Exper Biol Med 2006; 231(1):107–111.
- Umeda H, Aikawa M, Libby P. Liberation of desmosine and isodesmosine as amino acids from insoluble elastin by elastolytic proteases. Biochem Biophys Res Commun 2011; 411;281–286.
- Lindberg CA, Engstrom G, Gerhardsson de Verdier M, Nihlen U, Anderson M, Forsman-Semb K, Svartengren M. Total desmosine in plasma and urine correlate with lung function. ERJ 2012; 29:839–845.
- Huang JT-J, Chaudhuri R, Albarbarawi O, Barton A, Grievson C, Rauchhaus P, Weir CW, Messow M, Stevens N, McSharry C, Feuerstein G, Mukhopadhyay S, Brady J, Palmer CAN, Miller D, Thomson NC. Clinical validity of plasma and urinary desmosine as biomarkers for chronic obstructive pulmonary disease. Thorax 2012; 67:502–508.
- Stockley RA, Bayley DL, Unsal I, Dowson LJ. The effect of augmentation therapy on bronchial inflammation in alpha-1 antitrypsin deficiency. AJRCCM 2002; 165:1494–1498.
- Fregonese L, Ferrari F, Fumagalli F, Long-term variability of desmosine/isodesmosine as biomarker in alplha-1 antitrypsin deficience related COPD. COPD 2011; 8:329–333.