The main constituents of the a,-fraction obtained on paper electrophoresis in a barbital buffer are α1-lipoproteins, orosomucoid (acid glycoprotein), Schmid's (1953) and Schultze et al.'s α1-3.5 S glycoprotein (Schultze, Giillner, Heide, Schonenberger & Schwick 1955), which Schultze, Heide & Haupt (1962) recently proved to be identical with a,-antitrypsin (Citation10–12).
The variation, in heaIth and disease, of the a,-lipoproteins, or at least of lipids in the electrophoretic albumin- α1 region, has received fairly wide attention (for references see Lindgren & Nichols 1960) (Citation9). The electrophoretic α1-lipoprotein fraction migrates faster and spreads more, widely than most of the other components. Its electrophoretic behavior also varies with the duration of storage of the serum and with variations of the intermediary lipid metabolism. It is the heaviest normal al-component (about 300 mg per 100 ml) calculated as protein, but it is electrophoretically heterogenous and does not give rise to any well demarcated electrophoretic protein peak, which is evident on comparison of the paper and agar gel electrophoretic patterns after staining for lipids and proteins. The orosomucoid, which normally occurs in a concentration of about 75 mg per 100 ml (Goodman, Ramsey, Simpson & Brennan 1957) produces no demonstrable peak on paper or in agar gel electrophoresis, because it is partly masked by the slower part of the albumin fraction, and it stains only faintly with bromphenol blue (Sundblad & Wallin-Nilsson 1962) (Citation4, Citation15). The α1x component normally occurs in a concentration of about 30 mg per 100 ml (Schultze, Heide & Haupt 1962), which is not high enough to produce a distinct peak on paper electrophoresis (Citation11, Citation13).
Burtin (1960) has stated that the strongly antigenic 3.5 S a,-glycoprotein (α1Z, α1-antitrypsin) is the dominating normal α1-component. We have accepted this concept for two reasons. The precipitation maximum of the line formed by anti- α1-antitrypsin corresponds to the α1-protein peak obtained on paper and agar gel electrophoresis with different buffers. It may be deduced from data given by Jacobson (1955) on the α1- antitrypsin activity that if the molecular weight of the α1-antitrypsin is about 60,000, the mean normal concentration will be 0.18 g per 100 ml. This amount of protein is in accord with the intensity of the relatively sharply demarcated alpha-1-band obtained on paper electrophoresis. We cannot accept Burtin's statement, based on the appearance of immunoelectrophoretic patterns, that the main al-component varies little in pathological sera. On the contrary, the α1-antitrypsin varies considerably in disease (Jacobsson 1955), which is in accordance with the observed variations of the electrophoretic α1-band (Citation3).
In this article we present some patients with a new type of dysproteinemia characterized by very pronounced α1-antitrypsin deficiency. The sera were revealed as abnormal on routine inspection of the paper electrophoretic strips at the laboratory since the α1 pattern had an atypical configuration with no distinct α1-band. The numerical values of the electrophoretic α1-fractions fell within or just below the lower range of the normal variation.
Material
Selection
The sera discussed below were selected for further analysis because their electrophoretic pattern showed no distinctly demarcated band in the α1-zone despite the presence of normat or abnormally large α1-fractions.
Sera from three different cases were found during a period of six months, when approximately 1500 sera from diseased subjects were analyzed with paper electrophoresis at our laboratory. One serum had earlier been found to contain α1-fraction in subnormal concentration and was therefore reinvestigated. The 5th case was traced in the archives for paper electrophoretic strips, which Professor C.-G. Holmberg (University of Lund) kindly placed at our disposal. A number of his strips showed an appearance suggesting α1-antitrypsin deficiency. Samples from these patients will be collected for further analysis and the analysis and the result will be the subject of a later paper.
Methods
Paper electrophoretic analysis was performed with a calcium lactate containing barbital buffer according to Laurell, Laurell & Skoog (1956) (Citation8).
Agar gel electrophoresis was run with the same buffer on long glassplates (4 × 30 cm) covered with 1.3 mm thick agar layers and placed on the efficient cooling plate (+4°C) of a Pherograph instead of filter paws. The samples were run for 4 hours at 10 V potential difference per cm.
Immunoelectrophoresis was run in macroscale using the above mentioned type of agar gel electrophoresis and in microscale utilizing Heremans (1960) modification of Scheidegger's technique (Citation6).
Ouchterlony's agar diffusion plates
Lipid electrophoresis was run with a slight modification of Swahn's (1953) technique with Sudan black staining (Citation16).
Acid electrophoresis was run on filter paper with a citrate-phosphate buffer of pH 4.5. The polysaccharides were stained with Aronson's (1961) modification of the P.A.S. technique (Köiw & Grönvall 1952) (Citation1,Citation7).
Starch gel electrophoresis was run with Smithies’ vertical technique using borate buffer (1957) (Citation14).
Antisera
Rabbit-antihuman sera (Behringwerke) and horse-antihuman sera (Institute Pasteur lot no. 13460).
Trypsin inhibitors in whole serum. Dilutions of serum were incubated with trypsin, and the residual trypsin activity was otimated according to Erlanger et al. (method II) with benzoyl DL-arginine p-nitroanilide hydrochloride as substrate (Citation2).
Results
The numerical values obtained on initial analysis of five sera with an electrophoretic abnormality in the α1-zone are given in . The electrophoretic patterns obtained in cases 2 and 5 are compared with that of normal serum (). The “α1-band” is missing in the patients’ strips, but the intensity of the color in the zone between albumin and α1 is otherwise largely normal.
Figure 1. Paper electrophoretic protein pattern of sera from two patients with α1-antitrypsin deficiency and from a control. Upper: Case No. 2. Middle: Normal. Lower: Case No. 5.
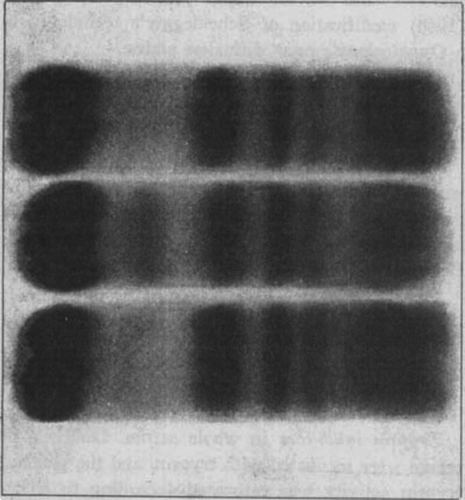
Table 1. Serum protein and lipid patterns in five patients with anti-alpha-1 antitrypsin deficiency with normal values for comparison. All quantitative values are expressed as g per 100 ml.
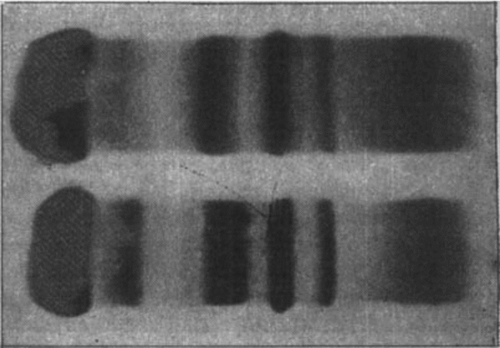
The typical, sharply demarcated α1-band was missing in the pattern obtained on agar gel electrophoresis (), while the rest of the α1-zone seemed to be normal.
Figure 2. Agar-gel electrophoresis of serum from patient 4 (above) and a healthy subject (below). Both belong to haptoglobin group 2-2.
When the papers are stained with Sudan black after conventional paper electrophoresis a faint, black zone is obtained that covers the whole α1-zone and encroaches upon the albumin zone. The α1-lipoprotein values (calculated as lipid) obtained on analysis of the atypical sera are presented in .
The orosomucoid content was not determined quantitatively, but it could be roughly estimated from the polysaccharide content of Winzler's M1-fraction seen as the most anodical of the colored bands after electrophoresis at pH 4.5. The appearance of the strips from two of the patients’ (Cases 1 and 5) sera are presented together with strips from a normal and a subject with increased orosomucoid fraction in .
Figure 3. Polysaccharide pattern after electrophoresis in a buffer of pH 4.5. The upper fraction is M1 i.e. mainly orosomucoid. Nr. 1. Abnormal. Normal. No. 5.
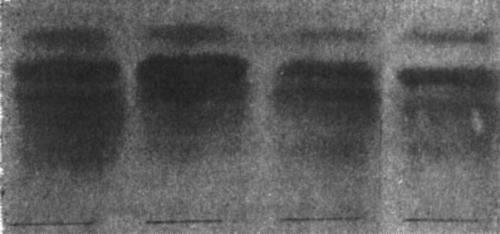
The intensity of the color of the M1-fraction varied some from case to case. In Case 1 the concentration was higher, and in Case 5 possibly somewhat lower, than in the sample from a healthy donor. A good impression of the orosomucoid content of the sera was also obtained from the results of the vertical starch gel electrophoresis, when all sera were run beside the same normal serum. In Cases 1, 2 and 3 the prealbumin fraction that appeared at the albumin frontier on staining with Amido Black was of normal intensity and normal breadth. In Case 4 the fraction was probably increased —judging from the increased spread of the fraction—and in Case 5 the fraction was definitely increased.
In addition the tryptophan-rich prealbumin seemed to occur in normal concentration in all the sera and the zone between the sharp postalbumin bands (Gc-proteins) and the albumin appeared normal, as did the albumin zone. Double postalbumin lines (Gc) of normal appearance were observed in Cases 2, 3, 4 and 5, but only the first of the two lines in Case 1. Haptoglobin groups are given in . Starch gel electrophoresis revealed no other abnormality.
The results of the immunologic studies are summarized in . Immuno-electrophoresis with Scheidegger's microtechnique utilizing rabbit-antihuman serum could not demonstrate or exclude the existence of the α1-antitrypsin precipitation bow because the critical zone was masked by the thick albumin line. An excellent survey of the three main α1-components was obtained with a horse-anti human serum lacking anti-albumin but with a lower titer against al-antitrypsin, but the α1-antitrypsin bow was now missing in four of the patients’ sera and occurred in trace amounts in the fifth. We therefore tried to prepare an antiserum specific for α1-antitrypsin. To 10 ml of the rabbit-anti human serum commercially available from Behringwerke was added in three steps (0.15 + 0.05 + 0.02) 0.22 ml serum from patient 5. After each addition the serum was placed a few hours at 4°C and the precipitate was centrifuged down. After the last addition of the patients’ serum only an insignificant turbidity appeared. The remaining specificity of the antiserum was then tested against normal sera and against the five sera with atypical α1-fraction. Some results are presented in and .
Figure 4. Immunoelectrophoretic analysis of patient sera 1 to 5. Each serum was run with a normal serum (above the slit). The “specific” anti- α1-antitrypsin serum in the slit.
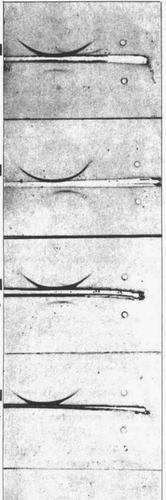
Table 2. Immunological methods used to estimate the α1-Antitrypsin
The absorbed antiserum had a remaining good titer against α1-antitrypin. It also developed a weak precipitation line with an unidentified protein in the fast α2-zone. This line was found in the few normal sera tested and in the patients’ sera except in Case 5. With this nearly specific antiserum it was evident that trace amounts of α1-antitrypsin occurred in all the patients sera and that the mobility of the protein was probably somewhat decreased. Utilizing the macro immunoelectrophoresis with unabsorbed rabbit-antihuman serum the α1-antitrypsin line is projected free from the albumin line. For the patients’ sera it became evident that the remaining α1-antitrypsin migrated more slowly than in the control serum, indicating qualitative and quantitative deviations from normal.
Using Ouchterlony's agar gel diffusion plates, a series of dilutions of normal sera and of the patients’ sera were compared with regard to the intesity and the location of the precipitation line induced by the “specific” antiserum. To get a rough idea of the concentration of the residual α1-antitrypsin in the ‘patients’ sera they were compared with normals. The dilutions resulting in comparable precipitation lines are given in together with the secondary calculated concentration.
Discussion
The electrophoretic α-fractions increase relatively regularly in diseases with associated inflammatory reactions. The α2 increase is secondary to an increased haptoglobin synthesis. Of the dominant α1-components, the α1-lipoproteins (H.D.L. 2 and 3) tend to decrease but the orosomucoid increases in concentration roughly in proportion to the haptoglobins. This rise cannot, however, be ascribed to the increase of the α1-fraction seen on paper electrophoresis, since the orosomucoid absorbs only one tenth of the bromphenol blue taken up by egg albumin (Sundblad & Wallin-Nilsson 1962) (Citation15). Even a substantial increase would cause little change in the pattern on the electrophoretic strip.
The distinct band normally seen in the slow α1-region on paper electrophoresis corresponds to a glycoprotein recently identified as α1-antitrypsin. The variation in concentration of this enzyme inhibitor in some diseases was described by Jacobsson (1955) who found it to vary like the orosomucoid (Citation3). The α1-antitrypsin is seen as a sharper demarcated band on agar-gel than on paper electrophoresis (). Subnormal concentration of α1-antitrypsin has to be suspected if no band sharpening (increased color intensity) can be traced in the slow α1-zone. As stressed by Jacobsson, the α1-antitrypsin concentration is low in the nephrotic syndrome (Citation3). Judging from the appearance of the paper electrophoretic strips it must often occur in low concentration in advanced liver cirrhosis. But in both of these conditions the α1-antitrypsin band can be seen with the naked eye on the electrophoretic strip even if the α1-fraction is subnormal.
In sera from the five patients described in this paper no α1-band was demonstrable on paper electrophoresis, for which reason the sera were analysed further. The immunoelectrophoretic studies revealed that the sera contained only trace amounts of α1-antitrypsin (a few percent of the normal). It appeared as if the residual α1-antitrypsin molecules had slightly decreased electrophoretic mobility in agar gel, which may indicate a structural abnormality.
Detailed studies on the concentration measured as antitrypsin after fractionation will be reported in a future paper.
No protein line was missing in the pattern obtained on starch gel electrophoresis of the sera. This is in conformity with the finding that the α1-antitrypsin migrates within the albumin zone.
The clinical material is too small to warrant any definite conclusions concerning possible connections between the α1-antitrypsin deficiency and the patients’ clinical pictures. It is, however, striking that three of the patients (Citation1, 2 and Citation3) had widespread pulmonary lesions (Cases 1 and 2 with cystic degeneration) and that the sister of the patient in Case 1 had the same lung disease and obviously the same plasma protein deficiency. Case 4 is interesting since the slight malabsorption syndrome may be secondary e.g. to defective pancreatic function. The nature of the disease in Case 5 is still obscure, but nothing indicates either pulmonary, pancreatic or intestinal dysfunction. The age of the patients and the case histories also indicate that the deficiency is well consistent with a normal life, though the α1-antitrypsin deficiency may decrease the resistance of the patients in some way.
Family studies are in progress and the preliminary results suggest that the defect may be inherited. It is possible that the three patients with pulmonary disease have some inborn error of metabolism and that the others have some type of acquired α1-antitrypsin deficiency.
The condition does not seem to be so very rare since as many as three cases were traced on examination of 1,500 consecutive electrophoretic strips.
Summary
The fairly well outlined band normally occurring in the slow α1-zone on paper electrophoretic strips is produced by the glycoprotein α1-antitrypsin.
Case reports of five patients with markedly reduced concentration of α1-antitrypsin in the serum are presented together with the results of the serum analysis. Immunological findings indicate that the concentration of the α1- antitrypsin in their sera was only a few percent of normal.
Some connection between degenerative pulmonary disease and α1-antitrypsin deficiency is suggested.
The primary cause may be an inborn error.
Case Reports
Case 1
E.K. Male, born 1928. None of the patient's relatives were known to have pubnary diseases. The patient was admitted in 1958 for acute pneumonia. Since that year he has had recurrences of bronchitis. In September 1961 he had an aattc attack with respiratory distress, fever and severe cough. Chest X-ray showed left-sided pneumothorax and diffuse emphysema. In addition both lungs showed signs of vesicular emphysema. Body section roentgenography showed small to orange-sized cysts on both sides. The Roentgen appearance of the bronchial tree was normal and no contrast filling of the cysts was obtained. At thoracotomy in December 1961 cystic parts of the left lung were excised and severe emphysema with multiple cysts was noted.
Histological examination of a biopsy specimen of the lung showed severe emphysema with bronchiectasis and fibrosis.
Case 2
O.H. Male, born 1925. A sister and a brother of the patient have bronchial asthma and emphysema. The patient was admitted to hospital in 1944 because of pneumonia. Chest-roentgenography of the chest in 1954 and 1957 showed no signs of a pathological condition. In recent years the patient has been troubled by increasing breathlessness on exertion. He was admitted to hospital in December 1961 because of right-sided bronchopneumonia with fever (40°C) and leukocytosis (9,600-5,600 per cu.mm.). The bronchopneumonia promptly responded to penicillin and terramycin, and the body temperature returned to normal within two days. Chest-roentgenography showed typical emphysema with obliteration of the sinus, flattening of the diaphragmatic arch and poor mobility of the diaphragm. The patient was not examined bronchographically. The residual volume of the lungs was increased to 6 litres. The patient's sister also has pronounced emphysema, and electrophoretic analysis of the serum revealed that the α1-band was missing.
Case 3
L.U. Male, born 1919. None of the patient's relatives were known to have asthma or other pulmonary diseases. The patient was admitted in 1945 and 1955 because of pneumonia. He has since had recurrent respiratory tract infections of bronchitic type, particularly during the winter months. During the last 6 months he has had increasing breathlessness on exertion. He had an acute attack of bronchitis in June 1961. Chest roentgenography at that time showed signs of bronchiectasis and emphysema in both lungs. Bronchography revealed generally widened bronchi on both sides as in congenital bronchiectasis. Spirometry showed moderately reduced lung function. The electrocardiographic recording showed no abnormalities. Differential count showed 7 percent eosinophils.
Case 4
A.L. Female, born 1887. None of her relatives are known to have pulmonary diseases. At 46 years rheumatoid arthritis appeared at the same time as menopause. In 1956 she was treated for 6 months with Deltacortril. She has since been taking Butazolidin continually.
In 1957 she was admitted to the Surgical Department because of acute cholecystitis. Spontaneous fractures of the 6th and 11th thoracic vertebrae were noted, and later that year also of the 7th thoracic vertebra. Roentgenographic signs of general osteoporosis were also observed, and the patient was treated for some time with Ultranol.
Since 1956 the patient has had hypertension with a diastolic pressure of about 100 and systolic pressure of about 200 mm Hg. A roentgenologic enlargement of the heart was noted in 1956. Repeated roentgenography has failed to reveal any changes of the lung parenchyma, apart from a transient sign of congestion in association with slight incompensation in 1956.
In April 1962 the patient was re-admitted because of severe pain in the chest. The pain was ascribed to the multiple vertebral fractures and no evidence for coronary incompetence could be produced. Roentgen examination now also showed compression fractures of the 2nd lumbar and 4th lumbar vertebrae since 1957.
On further investigation of the osteoporosis the fecal fat was found to be as much as 26 g per 24 hours (normal values 6–7 g) when the patient was on a normal diet.
The patient left hospital before it was possible to examine her for a malabsorption syndrome.
Case 5
I.B. Female, born 1932. None of the Relatives were known to have pulmonary diseases. The patient had been admitted to hospital for obscure abdominal pain in 1939, for acute nephritis in 1944, and for chronic appendicitis and mesenteric lymphadenitis in 1944. The woman complained of intermittent diffuse joint pain without swelling or stiffness. The E.S.R. varied between 40–100 mm/hour. She was readmitted in October 1960. No objective joint changes were then noted. Chest X-ray showed no abnormalities. The E.S.R. was still increased (30–40 mm/hour), The patient had no anemia. The urinary sediment and protein content were normal. The only explanation that could be found for the increased E.S.R. was rightsided sinusitis. Apart from periodic fatigue the patient has felt well since she left hospital. Further investigation is planned.
Acknowledgment
Our thanks are due to Miss Nancy Skoog for technical assistance.
References
- Aronson, T.: A study of the periodic acid-Schiff method for staining of protein-bound serum polysaccharides. Acta Soc. Med. upsalien. 66, 181, 1961.
- Erlanger, B., Kokowsky, N. & Cohen, W.: The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. 95, 271, 1961.
- Jacobsson, K.: Studies on the trypsin and plasmin inhibitors in human blood serum. Scand. J. Clin. Lab. Invest. Suppl. 14, 1955.
- Goodman, M., Ramsey, D., Simpson, W., Brennan, M.: The use of chicken antiserum for the rapid determination of plasma protein components. J. Lab. Clin. Med. 50, 758, 1957.
- Grabar, P. & Burtin, P.: Les protéines du plasma humain normal. L'analyse immunoelectrophoresis. Masson, Paris, 1960.
- Heremans, J. F. Les globulines sérique du système gamma. editions Arscia S.A., Bruxelles, 1960.
- Köiw, E. & Grönvall, A.: Staining of proteinbound carbohydrate after electrophoresis of serum on filter paper. Scand. J. Clin. Lab. Invest. 4, 244, 1952.
- Laurell, C.-B., Laurell, S. & Skoog, N.: Buffer composition in paper electrophoresis. Clin. Chem. 2, 99, 1956.
- Lindgren, F., Nichols, A.: Structure and function of human serum lipoproteins. The Plasma Proteins. II. Academic Press, New York, 1960.
- Schmid, K.: Preparation and properties of serum and plasma proteins. XXIX. Separation from human plasma of polysaccharides, peptides, and proteins of low molecular weight. Crystallization of an acid glycoprotein. J. Amer. Chem. Soc. 75, 60, 1953.
- Schultze, H. E., Göllner, I., Heide, K., Schönenberger, M., Schwick, G.: Zur Kentnis des α-Globulin des menschlichen Normalserums. Z. Naturforschung 10 b, 463, 1955.
- Heide, K., Haupt, H.: α1-antitrypsin aus Humanserum. Klin. Wschr. 40, 427, 1962.
- Heide, K. & Haupt, H.: Ueber ein noch nicht beschriebenes α1-glycoprotein des menschlichen Serums. Naturwissenschaften 49, 133, 1962.
- Smithies, O.: An improved procedure for starchgel electrophoresis : Further variations in the serum proteins of normal individuals. Biochem. J. 71, 585, 1959.
- Sundblad, L., Wallin-Nilsson, M. : Electrophoretic determination of orosomucoid. Scand. J. Clin. Lab. Invest. 14, 72, 1962.
- Swahn, B.: Studies on blood lipids. Scand. J. Clin. Lab. Invest. 5, Suppl. 9, 1953.
