Abstract
Background: The prevalence and characteristics of airway obstruction in older individuals varies widely with the definition used. We used a random sample of never smoking older population in Iceland to compare the prevalence and clinical profile of subjects diagnosed with Chronic Obstructive Pulmonary Disease (COPD) based on different spirometric criteria. Material and methods: The study uses data from the Age, Gene/Environment Susceptibility–Reykjavik Study, comprising survivors from the Reykjavik Study. Procedures included standardized questionnaires and pre-bronchodilator spirometry for measurement of forced expiratory volume in one second (FEV1) and forced vital capacity (FVC). Results: Total of 495 individuals (150 men and 345 women) met study criteria. Mean age 77 years (range 66-92 years) using fixed ratio (FEV1/FVC < 70%) up to 29% of the population were diagnosed with COPD Stage I. The prevalence of COPD increased with age. Only 7 among 495 (1.4%) were diagnosed with COPD using FEV1/FVC LLN and FEV1 LLN. Conclusion: Application of the GOLD criteria for diagnosis of COPD in older lifelong never smoking subjects identifies a substantial number of non-symptomatic subjects as having COPD. If airway obstruction is defined by FEV1/FVC and FEV1 being below the LLN using appropriate reference equations, only very few non-smoking older individuals fulfill the criteria for COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of disability and death in the world (Citation1) and prevalence increases with age. COPD is a disease characterized by chronic airflow limitations not fully reversible by bronchodilatation and published guidelines recommend use of spirometry to define it (Citation2). The overall prevalence of COPD is usually reported between 3% and 10%, but this varies widely depending on the diagnostic criteria (Citation3). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary has defined COPD as airflow limitation where forced expiratory volume in one second/forced vital capacity (FEV1/FVC) is < 0.7 and stages (I-IV) are defined from FEV1 as percentage of predicted value.
Stage 1 FEV1> 80%, Stage II 50-80%, Stage III 30-50% and Stage IV < 30%. These criteria are set regardless of age in attempt to simplify the diagnosis (Citation4). However, GOLD cut-off points might lead to misclassification since the FEV1/FVC ratio falls with age (Citation5) as a part of normal aging. Recent studies (Citation6, 7) in population of older individuals point towards both over-diagnosis of COPD in people over 70 years old and also underestimating young adults (Citation8, 9), if standard GOLD cut-off points are used.
In 2004, ATS and the ERS proposed the use of the lower limit of normal (LLN) instead of fixed ratio criteria (Citation10). In a recent evidence-based review comparing the use of either fixed ratio of FEV1/FVC < 0.7 and LLN it was obvious that comparative studies have rarely included a sufficient number of older non-smoking individuals, especially women (Citation11). The number of older individuals is growing worldwide and it is important to estimate the real disease burden of chronic disease such as COPD among them. COPD has been linked to many co-morbid conditions and co-morbidities such as cardiovascular diseases and diabetes mellitus (Citation12). The aim of the study was to estimate prevalence of COPD in a random sample of never-smoking older individuals in Iceland and evaluate the prevalence of co-morbid conditions in those groups defined with COPD depending on different spirometric criteria.
Materials and Methods
Study subjects
The design of Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik Study) is described in detail elsewhere (Citation13). In summary AGES-Reykjavik originates from the Reykjavik study, a cohort established in 1967 and originally comprised a random sample of 30,795 men and women (divided in 6 groups) born in 1907–1935 and living in the greater Reykjavik area in 1967 (Citation14). AGES-Reykjavik Study examinations began in 2002, and at that time 11,549 previously examined Reykjavik Study cohort members were still alive. From these persons, recruitment order was randomly assigned within the 6 Reykjavik Study groups. The AGES- Reykjavik examinations concluded in 2006 with a total sample size of 5,764 survivors of the Reykjavik Study cohort (Citation13). Spirometry was only preformed in the first two years of the study because of necessary prioritizing in time-consuming protocol.
The AGES-Reykjavik Study is approved by the Icelandic National Bioethics Committee (VSN: 00-063), the Icelandic Data Protection Authority and the Institutional Review Board serving the National Institution on Aging (NIA)
Data selection
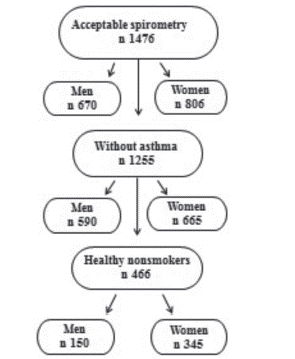
Selected parts from the AGES-Reykjavik study questionnaire,were used. These included demographic data, smoking history, symptoms and spirometry results (FEV1 and FVC). Inclusion criteria for acceptable spirometry was having at least two spirometry attempts, not more than 300 ml difference between the attempts and to be able to blow out for at least six seconds. Those with history of asthma were excluded because only pre-bronchodilator measurements were used for the study. Total of 1255 participants (590 men and 665 women) had acceptable spirometry results and no prior history of asthma. Participants with lifetime smoking history (smoked regularly and smoked at least 100 cigarettes or 20 cigars in lifetime) were excluded from the study. In the final group of 495 participants, 150 men and 345 women were included to the study ().
Spirometry
Spirometry was performed with a Vitalograph Gold Standard Plus (Vitalograph Ltd., Buckingham, UK) at each survey (Citation15). Spirometry was done in a sitting position. The procedure was explained in details before starting. Three attempts were made for each individual. The Vitalograph was regularly calibrated with 1L syringe. Only pre-bronchodilatation measurements were performed.
Spirometric definition of COPD
The following definitions for COPD were used in our analysis:
GOLD criteria for staging COPD, FEV1/FVC < 0.7 (fixed ratio) and FEV1 predicted was used for further staging the disease (FEV1 ≥ 80% predicted; stage I, ≥50% and < 80%; stage II, ≥ 30% and < 50%; stage III < 30%; stage IV) (Citation4). LLN is age-corrected and is defined statistically by the lower fifth percentile of a reference population and can be calculated by subtracting 1.64 times the standard deviation from the mean, i.e. the expected value (Citation10).
Predicted and percent predicted values were calculated for FEV1, FVC, FEV1/FVC ratio and LLN for FEV1, FVC and FEV1/FVC ratio using predicted equations for Caucasian adult males and females derived from the Third US National Health and Nutrition Examination Survey (NHANES –III) (Citation16). In this study we compare fixed ratio (FEV1/FVC < 0.7) to FEV1/FVC LLN with FEV1 < 80% (FEV1 < 80%) predicted and FEV1/FVC LLN with FEV1 LLN (FEV1 LLN).
Data analysis
All statistical calculation was performed using Stata version 10.1 (Stata Corporation; College Station, TX). Continuous response variables were analysed on the original scale except the FEV1/FVC ratio, which was logit transformed. To test the assumption of normality the residuals from regression analyses, by sex with age adjustment, were inspected using normal quantile plots and deemed adequately normally distributed. MacNemar's symmetry test was used to test for marginal homogeneity between the prevalence estimates from the different spirometric criteria.
Results
Main characteristics of the studied sample
Total of 495 individuals (150 men and 345 women) met the study criteria (). Sample characteristics are presented in . The age of participants ranged from 66–92 years (Mean (±SD) 76.9 ± 5.7). Mean (±SD) body max index for men was 26.4 kg/m−2 (± 3.4) and 26.7 (± 4.6) for women. The mean (±SD) FEV1/FVC ratio was 74% (± 6.0) the same for men and women, while mean (± SD) FEV1 for males and females was 2.85 L (± 5.7) and 1.88 L (± 0.4), respectively, p = 0.00001. Cardiovascular disease and obesity were the most common co-morbidities. A total of 21 (4%) of the subjects reported chronic phlegm most mornings more than 3 months a year.
Table 1. Demography, co-morbidity and spirometric data
Prevalence of COPD using different spirometry criteria
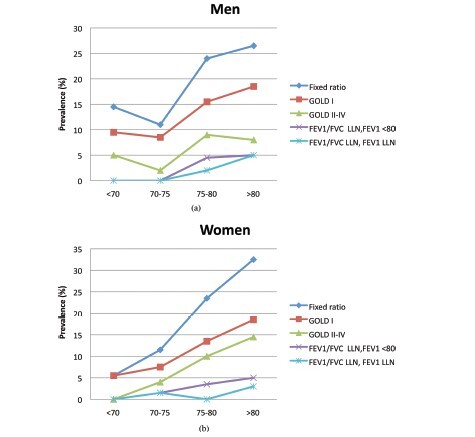
As shown in the prevalence of COPD increases proportional with age using all the studied criteria and is as high as 26% among men and 32% among women in the > 80 years age group. Use of the fixed ratio criteria (GOLD stage I and higher) produced prevalence estimates that were significantly greater than for FEV1/FVC LLN withFEV1<80% (FEV1<80%) predicted and FEV1/FVC LLN with FEV1 LLN (FEV1 LLN) in age groups over 70 years (p < 0.03–0.00001). The fixed ratio estimates were up to 18 percentage points higher than those for GOLD stages II-IV (). The LLN criterion (FEV1LLN and FEV1 < 80%) were up to 9,5 percentage points lower than estimates for GOLD stages II-IV. Finally, use of FEV1LLN in place of FEV1<80% further reduced estimates down to 3.5% of the population.
Figure 2. (a)Prevalence of COPD in nonsmoking elderly men using GOLD criteria, FEV1/FVC LLN and FEV1< 80% predicted and FEV1/FVC LLN and FEV1 LLN. (b). Prevalence of COPD in nonsmoking elderly women using GOLD criteria, FEV1/FVC LLN and FEV1< 80% predicted and FEV1/FVC LLN and FEV1 LLN.
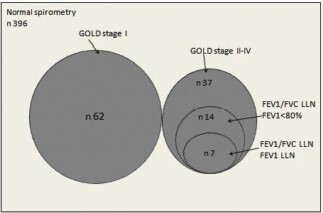
shows the relationship between COPD diagnosed using different criteria. None of those with GOLD stage I would be diagnosed with COPD using LLN criteria. Of subjects having GOLD stages II-IV, 38% would be diagnosed with COPD using FEV1/FVC LLN with FEV1<80% and 19% when using FEV1/FVC LLN with FEV1 LLN. Only 7 among the 495 (1.4%) were diagnosed with COPD using FEV1/FVC LLN with FEV1 LLN.
As shown in Tables and mean age was similar using LLN criteria and fixed ratio. Mean FEV1 was 1.15 L ± 0.39 using LLN criteria vs. 1,82 L ± 0,56 when the fixed ratio criteria as was mean FEV1/FVC 56 ± 7 vs. 65 ± 5, respectively (). Symptoms like chronic phlegm was reported by 6% when using fixed ratio criteria but none using LLN criteria. Cardiovascular disease and obesity were the most common co-morbitities. Comparing the different criteria prevalence of cardiovascular disease was similar, but obesity was less common when using the fixed ratio (FEV1/FVC < 80%) criteria than LLN criteria (see ). When looking at prescribed inhalation drugs for COPD (beta agonists and steroids) only 2% of the individuals using the fixed ratio had been prescribed the drugs and up to 14% when using the LLN criteria.
Table 2. Demographic and spirometric data, compared for different criteria
Table 3. Symptoms and co-morbidity for different criteria
Discussion
This study clearly demonstrates that applying GOLD guidelines for diagnosis of COPD among healthy, never-smoking older adults does classify a substantial number of them as having COPD. This is more prevalent with increasing age.
Comparision with previous studies is sometimes difficult as these study populations are younger than ours, not truly epidemiological and have investigated relatively few elderly women. In the current study using fixed ratio (FEV1/FVC < 0.7) up to 32% of older women were diagnosed with COPD and up to 26% of older men, with this diagnosis increasing with age. When using LLN equations the prevalence of COPD decreases to 1.4%. This is similar to previous studies. Hardie et al. (Citation7) studied 71 healthy, older never-smokers who were able to perform an acceptable spirometry. Approximately 35% of those healthy, older never-smokers had an FEV1/FVC% < 0.7 and would be classified as having at least COPD GOLD stage I. This percentage increased with age and, in those aged over 80 years, 50% would be classified as having COPD.
A study by Swanney et al. found that prevalence of airway obstruction in healthy never-smokers aged over 60 years varied for each guideline used and the GOLD guidelines caused false positive rates of up to 60% when applied to entire population (Citation17). A study by Hansen et al. on the NHANES III study population showed that approximately 20% of older adults with observed FEV1/FVC% above the NHANES-III fifth percentile had FEV1/FVC% ratios < 0.7 (Citation18). A study by Szanto et al. on the prevalence of airway obstruction in the elderly by using a cross-sectional spirometric study of nine age cohortsbetween the ages of 60 and 93 years reveiled that using the lower limit of normal, LLN yielded the lowest prevalence of 10.1%, but according to GOLD criteria (FEV1/FVC <0.7) the prevalence of obstruction was 22.5% regardless of which one of three different spirometric reference values were used (Citation19).
A study from Korea published in 2009 showed that the difference of prevalence between LLN and fixed ratio of FEV1/FVC was even higher among subjects with age ≥ 65, 14.9% and 31.1%, respectively (Citation20). The authors of a study on over 10.000 individuals from the BOLD study recruited from 14 sites concluded that the use of the FEV1/FVC, LLN criterion instead of the FEV1/FVC < 0.7 should minimise known age biases and better reflect clinically significant irreversible airflow limitation (Citation21). The recent American Thoracic Society and European Respiratory Society Joint Statement (Citation22) recommends using the LLN of the FEV1/FVC in place of the fixed-ratio criterion to diagnose airflow obstruction
It is well established that older individuals often have one or more chronic conditions (Citation23, 24). Co-morbid conditions are common among COPD patients. One study found that the median number of co-morbidities was nine (Citation25). Prior studies have shown that hypertension, cardiovascular disease and diabetes are highly prevalent co-morbidity for older COPD patients (Citation26). In our study, co-morbid conditions were common, of those cardiovascular disease and obesity were most commonly found. When comparing individuals diagnosed with COPD using fixed ratio of < 0.7 compared with LLN criteria, obesity was less common when using the LLN criteria. The role of weight needs to be taken into consideration as weight loss often is associated with severe COPD, (Citation27) and weight gain contributes to lower FEV1 and FVC) (Citation28). Medically treated hypertension was similar between the different criteria.
The strength of the study is the well defined population of both males and females. They represent a sample of older individuals from the Reykjavik metropolitan area who have all lived within a 30-km radius and been exposed to rather low amount of air pollution (Citation29).
The weaknesses of the study are that spirometries were all done without giving bronchodilating medications and a difference of 300 ml was accepted between measurements. GOLD defines COPD based on post-bronchodilator spirometry tests because COPD is thought to be a disease characterized by irreversible airway obstruction. It is possible that some of the subjects in the present study with low FEV1 would have an increase in FEV1 and FEV1/FVC% after inhalation of a bronchodilating drug.
We excluded those with history of asthma in order to minimize this from occurring but we did not exclude subjects with chronic conditions such as hypertension. The prevalence of COPD based on GOLD criteria and LLN has been compared based on the use of post-bronchodilator values or not in a community based population sample of 2235 subjects from Norway, finding a lower difference in prevalence with increasing age (Citation30). The number of older individuals in this study is however low as pointed out in a recent paper focusing on what defines abnormal lung function in older adults with COPD (Citation31).
It is difficult to estimate the practical and economic consequences of over-diagnosing COPD among the elderly. It is unlikely that individuals without symptoms will be tested and treated and we see that very few in this group are regular users of inhaled drugs. Diagnosing someone with COPD as a part of a preoperative evaluation might lead to wrong decision about operability and even unnecessary investigations.
Based on our results and results from previous studies, it seems that application of the GOLD criteria for diagnosis of COPD may lead to over diagnosis of COPD in non-smoking older individuals. Using LLN criteria in the older individuals will lead to fewer false positive results and could possibly decrease the costs of further tests and treatments.
Declaration of Interest Statement
The Ages Reykjavik study has been funded by NIH contract N01-AG-1-2100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and Althingi (the Icelandic Parliament).
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2008 [cited; 2012] Available from: www.goldcopd.org
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, Van WC, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555.
- Halbert RJ, Isonaka S, George D, Interpreting COPD prevalence estimates: What is the true burden of disease? Chest 2003; May 123(5):1684–1692.
- Pauwels RA, Buist AS, Caverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management ant prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care 2001; 163:1256–1273.
- Enright PL, Kronmal RA, Higgins M, Schenker M, Haponik EF. Spirometry reference values for women and men 65 to 85 years of age: Cardiovascular health study. Am Rev Respir Dis 1993; 147:125–133.
- Schermer TRJ, Smeele IJM, Thoonen BPA, Lucas AEM, Grootens JG, van Boxem TJ, Heijdra YF, van Weel C. Current clinical guideline definitions of airflow obstruction and COPD overdiagnosis in primary care. Eur Respir J 2008; 32:945–952.
- Hardie JA, Buist AS, Vollmer WM, Ellingsen O, Bakke PS, Mørkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J 2002; 20:1117–1122.
- Hnizdo E, Glindmeyer HW, Petsonk EL, Enright P, Buist AS. Case definitions for chronic obstructive pulmonary disease. COPD 2006; 3(2):95–100.
- Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Antó JM, Künzli N, Janson C, Sunyer J, Jarvis D, Svanes C, Gislason T, Heinrich J, Schouten JP, Wjst M, Burney P, de Marco R. Underestimation of airflow obstruction among young adults using FEV1/FVC< 70% as a fixed cut-off: A longitudinal evaluation of clinical and functional outcomes. Thorax 2008; 63(12):1031–1032.
- Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, Calverley PMA, Chavannes N, Dillard T, Fahy B, Fein A, Heffner J, Lareua S, Meek P, Martinez F, McNicholas W, Muris J, Austegard E, Pauwels R, Rennard S, Rossi A, Siafakas N, Tiep B, Vestbo J, Wouters E, ZuWallack R. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946.
- Hosesein FAAM, Zanen P, Lammers JJW. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: An evidence-based review. Respir Med 2011; 105:907–915.
- Feary JR, Rodriques LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major co-morbidities in subjects with COPD abd incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax 2010; 65(11):956–962.
- Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia E, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/environment susceptibility–Reykjavik Study: Multidisciplinary applied phenomics. Am J Epidemiol 2007; 165:1076–1087.
- Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Prevalence of coronary heart disease in Icelandic Men 1968–1986. The Reykjavik study. Euro Heart J 1993; 14:584–591.
- Chinn S, Gislason T, Aspelund T, Gudnason V. Optimum expression of adult lung function based on all-cause mortality: Results from the Reykjavik study. Respir Med 2007; 101(3):601–609.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Epidemiol 1999; 159:179–187.
- Swanney M, Ruppel G, Enright P, Pedersen O, Crapo R, Miller M, Jensen R, Falaschetti E, Schouten J, Hankinson J, Stocks J, Quanjer P. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax 2008; 63(12):1046–1051.
- Hansen JG, Pedersen L, Overvad K, Omland Ø, Jensen HK, Sørensen HT. The prevalence of Chronic Obstructive Pulmonary Disease among Danes aged 45–84 years: Population-based Study. COPD 2008; 5:347–352.
- Szanto O, Montnemery P, Elmstahl S. Prevalence of airway obstruction in the elderly: Results from a cross-sectional spirometric study of nine age cohorts between the ages of 60 and 93 years. Prim Care Respir J 2010; 19(3):231–236.
- Jing J, Huang T, Cui W, Xu F, Shen H. Should FEV1/FEV6 Replace FEV1/FVC ratio to detect airway obstruction? A metaanalysis. Chest 2009; 135(4):991–998.
- Vollmer WM, Gislason Þ, Burney P, Enright PL, Gulsvik A, Kocabas A, Buist AS for the BOLD Collaborative Research Group. Comparison of spirometry criteria for the diagnosis of COPD: Results from the BOLD study. Eur Respir J 2009; 34:588–597.
- Pellegrino R, Brusasco V, Viegi G, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Definition of COPD: Based on evidence or opinion? Eur Respir J 2008; 31:681–682.
- Schneider K, O'Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States’ Medicare population. Health Qual Life Outcomes 2009; 7:82.
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 2002: 162: 2269–2276.
- Barr RG, Celli BR, Mannino DM, Co-morbidities, patient knowledge, and disease management in a national sample of patients with chronic obstructive pulmonary disease. Am J Med 2009; 122:348–355.
- Holguin F, Folch E, Redd SC, Mannino DM. Co-morbidity and mortality in COPD-related hospitalizations in the United States, 1979–2001. Chest 2005; 128:2005–2011.
- Balasubramanian V, Varkey B. Chronic obstructive pulmonary disease: effects beyond the lungs. Curr Opin Pulm Med 2006; 12:106–112.
- Wannamethee SG, Shaper AG, Whincup PH. Body fat distribution, body composition and respiratory function in elderly men. Am J Clin Nutr 2005; 82(5):996–1003.
- Hazenkamp-von Arx ME, Götschi Fellmann T, Oglesby L, Ackerman-Liebrich U, Gislason T, Heinrich J, Jarvis D, Luczynska C, Mansanera AJ, Modig L, Norbäck D, Pfeifer A, Poll A, Ponzio M, Soon A, Vermeire P, Künzli N. European study centers of ECRHS II: Method and first winter results. J Air Waste Manag Assoc 2003; 53(5):617–628.
- Johannessen A, Omenaas ER, Bakke PS, Gulsvik A. Implications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: A community study. Thorax 2005; 60:842–847.
- Bhatt NY, Wood KL. What defines abnormal lung function in older adults with chronic obstructive pulmonary disease? Drugs Aging 2008; 25(9):717–728.