Abstract
Whether respiratory symptoms are associated with mortality independent of lung function is unclear. The authors explored the association of the exposures i) lung function, ii) respiratory symptoms, and iii) lung function and respiratory symptoms combined, with the outcomes all-cause and cardiovascular mortality. The study included 10,491 adults who participated in the Nord-Trøndelag Health Study (HUNT) Lung Study in 1995–1997 and were followed through 2009. Cox regression was used to calculate adjusted hazard ratios (HRs) with 95% confidence intervals for all-cause and cardiovascular mortality associated with pre-bronchodilator% predicted forced expiratory volume in 1 second (ppFEV1), chronic obstructive pulmonary disease (COPD) grades, and respiratory symptoms (chronic bronchitis, wheeze, and levels of dyspnoea). Lung function was inversely associated with all-cause mortality. Compared to ppFEV1 ≥100, ppFEV1 <50 increased the HR to 6.85 (4.46–10.52) in women and 3.88 (2.60–5.79) in men. Correspondingly, compared to normal airflow, COPD grade 3 or 4 increased the HR to 6.50 (4.33–9.75) in women and 3.57 (2.60–4.91) in men. Of the respiratory symptoms, only dyspnoea when walking remained associated with all-cause mortality after controlling for lung function (HR 1.73 [1.04–2.89] in women and 1.57 [1.04–2.36] in men). Analyses of lung function and dyspnoea when walking as a combined exposure further supported this finding. Overall, associations between lung function and cardiovascular mortality were weaker, and respiratory symptoms were not associated with cardiovascular mortality. In conclusion, lung function was inversely associated with all-cause and cardiovascular mortality, and dyspnoea when walking was associated with all-cause mortality independent of lung function.
Introduction
Chronic obstructive pulmonary disease (COPD) is primarily characterised by persistent airflow limitation (Citation1). However, undiagnosed people with COPD may not seek medical advice until they experience respiratory symptoms like cough, wheeze, or dyspnoea, being unaware of possible lung function impairment (Citation1, 2). COPD may reach an advanced stage before such symptoms become prominent, and not all patients experience respiratory symptoms (Citation1). In addition, limited awareness of COPD among medical doctors and underuse of spirometry may contribute to the underdiagnosis of COPD (Citation1–3). Both lung function level and presence of respiratory symptoms are assumed to be important for the prognosis of patients with COPD (Citation1). Although the association between lung function and mortality has been thoroughly studied, the association between respiratory symptoms and mortality is less clear.
During the last three decades, several epidemiological studies have found associations between impaired lung function and increased all-cause or cardiovascular (CV) mortality (Citation4–19). Respiratory symptoms have been found to be associated with all-cause and CV mortality in some studies where lung function has not been accounted for (Citation20–23). However, whether respiratory symptoms are associated with all-cause and CV mortality independent of lung function needs to be further explored as previous literature is inconclusive (Citation11–19, Citation24).
In this large population-based study we therefore aimed to explore the association of the exposures i) lung function, ii) respiratory symptoms, and iii) lung function and respiratory symptoms combined, with the outcomes all-cause and CV mortality.
Methods
Study population
All residents in Nord-Trøndelag County in Norway aged 19 years or older were invited to participate in the second survey of the Nord-Trøndelag Health Study (HUNT2) from August 1995 to June 1997. HUNT2 has been described elsewhere (Citation25–27). Among the 65,237 participants in HUNT2 (69.5% of those invited), a 5% random sample and a symptom sample were invited to participate in the Lung Study (Citation28, 29). Briefly, the symptom sample included participants reporting attacks of wheezing or breathlessness during the last 12 months, having ever had asthma, and/or having ever used asthma medication, and who were not included in the random sample.
Among the 10,693 participants eligible for the current study, we excluded 202 participants with missing information on body mass index (BMI), systolic blood pressure (SBP), total cholesterol, diabetes mellitus (DM), and cardiovascular disease (CVD). For the analyses of CV mortality we additionally excluded 1029 participants with CVD (angina pectoris, myocardial infarction, or stroke) at baseline. The remaining 10,491 participants for the all-cause mortality analyses and 9462 participants for the CV mortality analyses were weighted to represent 51,854 and 48,355 people, respectively, from the general population of Nord-Trøndelag County. This weighting has been described in detail elsewhere (Citation28). A flow chart of inclusion and exclusion in the Lung Study is presented in .
Study variables
All participants in HUNT2 provided information about life style factors, complaints, diseases, and demographics (Citation25, 26). Participants in the Lung Study completed an additional lung specific questionnaire and an interview, and performed flow volume spirometry (Citation29).
Lung function
Flow volume spirometry was recorded according to the 1994 American Thoracic Society recommendations (Citation30) as described elsewhere (Citation29). Pre-bronchodilator forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were obtained, and local prediction equations were used (Citation29). Lung function was classified in two ways;% predicted FEV1 (ppFEV1) was categorised as ≥100, 80–99, 50–79, and <50; pre-bronchodilator COPD was defined as FEV1/FVC <70% and airflow limitation was graded according to modified Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria as grade 1 (ppFEV1 ≥80), grade 2 (50≤ ppFEV1 <80), and grade 3 or 4 (ppFEV1 <50) (Citation1). Normal was defined as FEV1/FVC ≥70% and% predicted FVC ≥80. Participants with possible restrictive lung function impairment (FEV1/FVC ≥70% and% predicted FVC <80) were excluded from the COPD analyses as they would otherwise have been included in the “normal” category.
Respiratory symptoms
Chronic bronchitis (4.6% missing data) was measured by a question included in the main questionnaire: “Have you had a cough with phlegm for periods of at least three months during each of the last two years?” Wheeze (0.4% missing data) was measured by a question included in the interview: “During the last 12 months, have you had wheezing in your chest at any time?” Possible answers were “yes” or “no”. The lung specific questionnaire included four questions about dyspnoea during various activities from the Norwegian Respiratory Questionnaire (Citation31), and these questions were used to generate a dyspnoea scale (25.3% missing data) (Citation28), which is presented in eTable in the web appendix. This scale included dyspnoea when walking uphill, climbing stairs, walking flat, and sitting still. The dyspnoea scale was dichotomized at dyspnoea when walking when counting number of respiratory symptoms. Additionally, we created combined exposures of lung function and each of chronic bronchitis, wheeze, and dyspnoea when walking or sitting. Participants with missing data on respiratory symptoms were excluded from the analyses including respiratory symptoms. To assess potential selection bias due to missing data we compared baseline characteristics and associations with all-cause and CV mortality between participants with and without data on the dyspnoea scale.
Table 1. Statistical models for the association of lung function and respiratory symptoms with all-cause and cardiovascular mortality
Follow-up
The participants were followed from the date of attendance in HUNT2 to the date of death or until the end of follow-up, December 31 2009, whichever came first. The primary endpoints were all-cause and CV death (International Classification of Diseases 10: I00–I99). Date and cause of death were obtained from the Cause of Death Registry in Norway which is practically complete due to the unique personal identification number of all residents in Norway.
Statistical analyses
To enhance the generalizability of the results the regression analyses were weighted with an inverse probability weight (Citation32) to reflect the distribution in the general population (Citation28). Sex specific (Citation33) statistical analyses were performed with few exceptions.
Deaths per 1000 person-years of follow-up were calculated for categories of ppFEV1, COPD grades, and respiratory symptoms. Cox proportional hazard regression was used to compute adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the association of lung function and respiratory symptoms with all-cause and CV mortality.
Possible confounders included in the Cox proportional hazard models were age (<40, 40–49, …, ≥80 years), smoking (current, former, never, unknown [5.5%]), education (<10, 10–12, ≥13 years, unknown [4.9%]), BMI (<18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2), physical activity (inactive, light activity <1 hours/week, light activity 1–2 hours/week, light activity ≥3 hours/week, only vigorous activity, unknown [9.9%]), CVD (yes, no), DM (yes, no), SBP (sex specific quartiles), total cholesterol (sex specific quartiles), and when studying the association between respiratory symptoms and mortality, ppFEV1 (≥100, 80–99, 50–79, <50).
The association between lung function alone or combined with respiratory symptoms and mortality was studied in three different regression models, and the association between respiratory symptoms and mortality was studied in four models (). First, we explored the association in a simple model (Model 1) adjusting only for age at baseline. In the main model (Model 2) we also adjusted for other potential confounders identified through directed acyclic graphs (DAGs) (Citation34). In additional analyses we adjusted for lung function when studying respiratory symptoms (Model 3), and established risk factors for mortality (Model 4) that could be viewed as possible confounders or mediators although not supported by DAGs (Citation35). Women and men were combined to increase statistical power when studying the association between lung function combined with respiratory symptoms and mortality, and in additional analyses of the association between dyspnoea and CV mortality.
Trend tests across ppFEV1 levels were calculated using the sex specific median value within each ppFEV1 level as an ordinal variable in the regression model. For testing the trend across COPD grades we treated the categories as an ordinal variable.
Log-log survival curves for each covariate were inspected and formal tests of interaction with time or log time were produced to evaluate the proportional hazard assumptions. All, but a few, p-values were above 0.05. For all-cause mortality, there were weak evidence for interaction with time for COPD grade 3 or 4 among women (p = 0.049 for time and p = 0.038 for log time). However, the log-log plots showed no clear violation of the proportional hazard assumptions.
In a subsequent sensitivity analysis of the association between respiratory symptoms and all-cause mortality we excluded participants with CVD at baseline. Moreover, all analyses of CV mortality were repeated in a sample where participants with CVD at baseline were included and where CVD was instead adjusted for.
Statistical analyses were conducted using Stata, release 12.1 (StataCorp LP, College Station, Texas). All statistical tests were two-sided.
Ethics
The Norwegian Data Inspectorate licensed the research register (reference 06/00104-39/CGN), and the Regional Committee for Medical Research Ethics (reference 4.2008.59) approved the study. All participants signed informed written consents.
Results
Baseline characteristics
The 10,491 participants we had actual spirometric measurements on, 2655 from the random sample and 7836 from the symptom sample, were weighted to represent 51,854 people from the general population. and present baseline characteristics according to ppFEV1 and respiratory symptoms in women and men, respectively. In general, participants with low lung function and participants who reported respiratory symptoms tended to be older, be ever smokers, have less education, be inactive, and have more CVD and DM. BMI was lowest among participants with lowest lung function, and highest among participants reporting respiratory symptoms. Summary statistics of key variables among the symptom, random, and weighted samples are presented in eTable . That the percentage or mean of key variables were almost similar in the weighted and the random sample indicates that the weighting was successful.
Table 2. Baseline characteristics according to lung function and respiratory symptoms among women in the general population (weighteda)
Table 3. Baseline characteristics according to lung function and respiratory symptoms among men in the general population (weighteda)
Lung function and mortality
The overall all-cause () and CV () death rates per 1000 person-years were 9.70 and 2.96 in women, and 13.74 and 4.13 in men, respectively. Compared to participants with ppFEV1 ≥100 or normal airflow, women and men with ppFEV1 <80 or COPD grade 2 or higher had increased all-cause mortality (Table4). For every 10% decrease in ppFEV1 the adjusted HRs for all-cause mortality was 1.17 (95% CI 1.09–1.25) in women and 1.23 (95% CI 1.16–1.30) in men. Additional adjustments for BMI, physical activity, CVD, DM, SBP, and total cholesterol did not materially change the results (eTable ).
Table 4. Death rates and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between lung function and all-cause mortality (weighted)
Table 5. Death rates and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between lung function and cardiovascular mortality (weighted) in participants without CVD at baseline
CV mortality was increased in women with ppFEV1 <50 or COPD grade 3 or 4 and in men with ppFEV1 <80 or COPD grade 2 or higher (). For every 10% decrease in ppFEV1 the adjusted HRs for CV mortality were 1.03 (95% CI 0.91–1.16) in women and 1.24 (95% CI 1.10–1.39) in men. Additional adjustments for BMI, physical activity, DM, SBP, and total cholesterol (eTable4) or including participants with CVD at baseline in a sensitivity analysis (eTable ) did not materially change the results.
Respiratory symptoms and mortality
Chronic bronchitis, dyspnoea when walking, dyspnoea when sitting (among women only), and number of respiratory symptoms were positively associated with all-cause mortality in models not adjusted for lung function (). However, only dyspnoea when walking remained positively associated with all-cause mortality when adjusting for lung function (HR 1.73 [95% CI 1.04–2.89] in women and HR 1.57 [95% CI 1.04–2.36] in men). With additional adjustments for DM, SBP, and total cholesterol, the association between dyspnoea when walking and all-cause mortality in women attenuated (eTable ). When excluding participants with CVD at baseline in a sensitivity analysis none of the respiratory symptoms were associated with all-cause mortality when adjusting for lung function (eTable 7). Within lung function levels, participants reporting dyspnoea when walking or sitting had generally higher HRs for all-cause mortality than participants without these symptoms (). Similar trends were not seen for chronic bronchitis or wheeze within lung function levels (eFigure and 1b). Additional adjustments for DM, SBP, and total cholesterol did not materially change the results (data not shown).
Figure 2. Association of the combined exposure of % predicted forced expiratory volume in 1 second (ppFEV1) and reporting of dyspnoea when walking or sitting with all-cause mortality. The reference category consists of people with ppFEV1 ≥100 not reporting dyspnoea when walking or sitting. The bars represent 95% confidence intervals. Adjusted for age (<40, 40–49, …, ≥80 years), sex (woman, man), smoking (never, former, current, unknown), education (<10, 10–12, ≥13 years, unknown), body mass index (<18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2), physical activity (inactive, light activity <1 hours/week, light activity 1–2 hours/week, light activity ≥3 hours/week, only vigorous activity, unknown), and cardiovascular disease (yes, no).
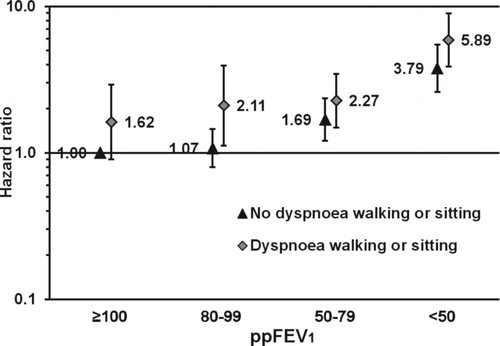
Table 6. Death rates and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between respiratory symptoms and all-cause mortality (weighted)
None of the respiratory symptoms were positively associated with CV mortality when adjusting for lung function (eTable 8). The results did not materially change with additionally adjustment for DM, SBP, and total cholesterol (eTable 9) or when including participants with CVD at baseline in a sensitivity analysis (eTable 10). Participants reporting respiratory symptoms did not have a consistent trend for increased CV mortality compared to participants not reporting respiratory symptoms within lung function levels (data not shown). However, when combining women and men, dyspnoea when walking was positively associated with CV mortality when participants with CVD at baseline were included in the model (HR 1.67 [95% CI 1.05-2.66]) (eTable 11).
There were little differences in baseline characteristics (eTable 12) and HRs for all-cause and CV mortality (eTable 13) between participants with and without data on the dyspnoea scale, indicating no substantial selection bias due to missing data.
Discussion
In this large epidemiological study from a general population in Norway we found pre-bronchodilator lung function to be strongly and inversely associated with all-cause and CV mortality. Although chronic bronchitis, dyspnoea when walking or sitting, and number of respiratory symptoms were positively associated with all-cause mortality in crude models, only dyspnoea when walking remained positively associated with all-cause mortality when controlling for lung function. None of the respiratory symptoms were associated with CV mortality independent of lung function in sex specific models.
In accordance with findings from previous epidemiological studies (Citation4–19), we found increased all-cause and CV mortality with lower lung function. However, none of these previous studies have classified participants according to both ppFEV1 and COPD grades, and few have done sex specific analyses. For the main analyses of CV mortality we excluded participants with CVD at baseline to avoid reverse causation. Thus, our results indicate that impaired lung function may be causally associated with CV mortality. Possible mechanisms that may explain our findings include systemic inflammation, impaired functional capacity, muscle dysfunction, malnutrition, oxidative stress, and comorbidities such as CVD, depression, lung cancer, and DM (Citation36).
In a population-based study from the United States, reporting at least one of cough, phlegm, wheeze, or breathlessness increased the HR for all-cause mortality compared to not reporting any symptom within pre-bronchodilator levels of COPD (Citation12). However, there was no investigation of which respiratory symptoms were responsible for the association with mortality, and the analyses were not conducted sex specific. In the current and in other studies (Citation15, Citation19), dyspnoea, but not chronic bronchitis or wheeze, has been found to be associated with all-cause mortality independent of lung function.
Interestingly, among the four levels of dyspnoea only dyspnoea when walking was associated with all-cause mortality in the current study. However, none of the respiratory symptoms remained associated with all-cause mortality independent of lung function when we excluded participants with CVD at baseline, indicating that CVD could explain some of the association between dyspnoea when walking and mortality. Nevertheless, excluding people also results in reduced power to detect an association. Future studies aiming at scrutinising the complex mechanisms involved in the sensation of dyspnoea (Citation37) may be needed to explain our findings.
Somewhat unexpectedly, we found no association between dyspnoea and CV mortality independent of lung function in sex specific models. This lack of association may be due to low statistical power, misclassification of CV deaths, residual confounding, or it may reflect the reality. Additional analyses combining women and men increased the statistical power, but only the association between dyspnoea when walking and CV mortality was statistically significant when participants with CVD at baseline were included. Others have found breathlessness to be associated with CV mortality in 40–64 years old men after controlling for FEV1 (Citation18, 19). Although these studies did not exclude men with CVD at baseline, they adjusted for baseline myocardial ischemia. A Dutch study with over 40 years of follow-up found dyspnoea to be clearly associated with CV mortality also after adjusting for lung function (Citation24). However, CVD at baseline was not accounted for, the analyses were not conducted sex specific, and CV death was coded differently than in the current study (Citation24).
Strengths of the current study include the large population-based sample size which gave us the possibility to do sex specific analyses, to exclude participants with CVD at baseline, and to study lung function and respiratory symptoms as a combined exposure. In addition, our participants had a large span in age and lung function, and they were included independent of clinical diagnoses. HUNT2 had a relatively high response rate, and a non-responder study did not indicate serious selection bias (Citation38).
All-cause mortality is a robust outcome since the Cause of Death Registry in Norway is practically complete and there is minimal room for misclassification. However, the results on CV mortality must be interpreted with caution since it is possible that CV deaths may have been registered as deaths of other causes and vice versa. If this possible misclassification is non-differential it may dilute the real effect estimates (Citation35), which could explain the lack of association between respiratory symptoms and CV mortality in our data. About one quarter of the participants in the HUNT2 Lung Study did not return the lung specific questionnaire, which included the dyspnoea scale. However, it is unlikely that missing data on the dyspnoea scale have biased the results since there were no large differences in baseline characteristics and HRs for all-cause and CV mortality between participants with and without data on the dyspnoea scale. Despite thorough adjustment for possible confounding factors, there may still be residual confounding due to poorly measured or unmeasured variables. Additionally, we were not able to account for possible changes in factors such as smoking, physical activity, and BMI during follow-up. Finally, respiratory symptoms were self-reported and not validated.
In summary, pre-bronchodilator lung function was strongly and inversely associated with all-cause and CV mortality, and dyspnoea when walking was positively associated with all-cause mortality independent of lung function. Chronic bronchitis, dyspnoea when sitting, and number of respiratory symptoms were positively associated with all-cause mortality only in models where lung function was not controlled for. Respiratory symptoms were not associated with CV mortality independent of lung function. Our results suggest that objectively measured lung function is superior to subjectively reported respiratory symptoms in mortality studies.
Declaration of Interest Statement
The authors report no conflicts of interest. This study was financially supported by the Norwegian ExtraFoundation for Health and Rehabilitation through EXTRA funds (project 2007/2/0214); the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (project 46041600); and the Leif Richard Erichsen and wife Maren Hertzberg Erichsen's fund for Norwegian medical research. The HUNT2 Lung Study received funding without obligation from AstraZeneca.
The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The second survey of the Nord-Trøndelag Health Study 1995–1997 (HUNT2) was a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology), Nord-Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of COPD 2011 [updated 11 January 2012 cited 2012 21 May]. Available from: http://www.goldcopd.org.
- Fromer L. Diagnosing and treating COPD: understanding the challenges and finding solutions. Inter J Gen Med 2011; 4:729–739.
- Yawn BP, Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J Chron Obstruct Pulmon Dis 2008; 3(2):311–317.
- Gulsvik AK, Gulsvik A, Skovlund E, The association between lung function and fatal stroke in a community followed for 4 decades. J Epidemiol Community Health 2012;66(11):1030–1036.
- Mannino DM, Diaz-Guzman E, Buist S. Pre- and post-bronchodilator lung function as predictors of mortality in the Lung Health Study. Respir Res 2011; 12:136.
- Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005; 127(6):1952–1959.
- Stavem K, Aaser E, Sandvik L, Lung function, smoking and mortality in a 26-year follow-up of healthy middle-aged males. Eur Respir J 2005; 25(4):618–625.
- Schunemann HJ, Dorn J, Grant BJ, Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 2000; 118(3):656–664.
- Hole DJ, Watt GC, Davey-Smith G, Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ 1996; 313(7059):711–715.
- Lange P, Nyboe J, Appleyard M, Spirometric findings and mortality in never-smokers. J Clin Epidemiol 1990; 43(9):867–873.
- Baughman P, Marott JL, Lange P, Health outcomes associated with lung function decline and respiratory symptoms and disease in a community cohort. COPD 2011; 8(2):103–113.
- Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med 2006; 100(1):115–122.
- Ekberg-Aronsson M, Pehrsson K, Nilsson JA, Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res 2005; 6:98.
- Mannino DM, Buist AS, Petty TL, Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003; 58(5):388–393.
- Knuiman MW, James AL, Divitini ML, Lung function, respiratory symptoms, and mortality: results from the Busselton Health Study. Ann Epidemiol 1999; 9(5):297–306.
- Lange P, Nyboe J, Appleyard M, Relation of ventilatory impairment and of chronic mucus hypersecretion to mortality from obstructive lung disease and from all causes. Thorax 1990; 45(8):579–585.
- Vollmer WM, McCamant LE, Johnson LR, Respiratory symptoms, lung function, and mortality in a screening center cohort. Am J Epidemiol 1989; 129(6):1157–1169.
- Ebi-Kryston KL, Hawthorne VM, Rose G, Breathlessness, chronic bronchitis and reduced pulmonary function as predictors of cardiovascular disease mortality among men in England, Scotland and the United States. Int J Epidemiol.1989; 18(1):84–88.
- Ebi-Kryston KL. Respiratory symptoms and pulmonary function as predictors of 10-year mortality from respiratory disease, cardiovascular disease, and all causes in the Whitehall Study. J Clin Epidemiol 1988; 41(3):251–260.
- Frostad A, Soyseth V, Haldorsen T, Respiratory symptoms and long-term cardiovascular mortality. Respir Med 2007; 101(11):2289–2296.
- Frostad A, Soyseth V, Andersen A, Respiratory symptoms as predictors of all-cause mortality in an urban community: a 30-year follow-up. J Intern Med 2006; 259(5):520–529.
- Hewitt J, Smeeth L, Bulpitt CJ, Respiratory symptoms in older people and their association with mortality. Thorax 2005; 60(4):331–334.
- Carpenter L, Beral V, Strachan D, Respiratory symptoms as predictors of 27 year mortality in a representative sample of British adults. BMJ 1989; 299(6695):357–361.
- Figarska SM, Boezen HM, Vonk JM. Dyspnea severity, changes in dyspnea status and mortality in the general population: the Vlagtwedde/Vlaardingen study. Eur J Epidemiol 2012; 27(11):867–876.
- Helseundersøkelsen i Nord-Trøndelag 2012 [updated May 11 2012; cited 2012 May 21]. Available from: http://www.ntnu.edu/hunt.
- Krokstad S, Langhammer A, Hveem K, Cohort Profile: The HUNT Study, Norway. Int J Epidemiol [published online ahead of print 9 August 2012]. (doi:10.1093/ije/dys095).
- Holmen J, Midthjell K, Krüger Ø, The Nord-Trøndelag Health Study 1995–97 (HUNT 2): Objectives, contents, methods and participation. Norsk Epidemiolog 2003; 13(1):19–32.
- Leivseth L, Nilsen TI, Mai XM, Lung function and anxiety in association with dyspnoea: The HUNT study. Respir Med 2012; 106(8):1148–1157.
- Langhammer A, Johnsen R, Gulsvik A, Forced spirometry reference values for Norwegian adults: the Bronchial Obstruction in Nord-Trøndelag Study. Eur Respir J 2001; 18(5):770–779.
- Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152(3):1107–1136.
- Brogger JC, Bakke PS, Gulsvik A. Comparison of respiratory symptoms questionnaires. Int J Tuberc Lung Dis 2000; 4(1):83–90.
- Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res [published online ahead of print January 10, 2011]. (doi:10.1177/0962280210395740).
- Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax 1999; 54(12):1119–1138.
- Hernán MA, Robins JM. Causal Inference 2012 [updated 2012 Nov 25 cited 2012 Des 11]. Available from: http://www.hsph.harvard.edu/faculty/miguel-hernan/files/hernanrobins_v1.10.19.pdf.
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009; 33(5):1165–1185.
- Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med 1999; 159(1):321–340.
- Langhammer A, Johnsen R, Holmen J, Cigarette smoking gives more respiratory symptoms among women than among men. The Nord-Trøndelag Health Study (HUNT). J Epidemiol Commun Health 2000; 54(12):917–922.
WEB APPENDIX
Lung Function and Respiratory Symptoms in Association with Mortality: The HUNT Study
L. Leivseth, T.I.L. Nilsen, X.M. Mai, R. Johnsen, and A. Langhammer
eTable 1. The dyspnoea scale used in the Nord-Trøndelag Health Study 1995–1997 Lung Study
eTable 2. Respiratory symptoms, lung function, medical diagnoses, and other characteristics of participants in the symptom, random, and weighted sample
eTable 3. Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between lung function and all-cause mortality (weighted)
eTable 4. Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between lung function and cardiovascular mortality (weighted) in participants without CVD at baseline
eTable 5. Sensitivity analyses –participants with CVD at baseline included: Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between lung function and cardiovascular mortality (weighted)
eTable 6. Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between respiratory symptoms and all-cause mortality (weighted)
eTable 7. Sensitivity analyses –participants with CVD at baseline excluded: Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between respiratory symptoms and all-cause mortality (weighted)
eTable 8. Death rates and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between respiratory symptoms and cardiovascular mortality (weighted) in participants without CVD at baseline
eTable 9. Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between respiratory symptoms and cardiovascular mortality (weighted) in participants without CVD at baseline
eTable 10. Sensitivity analyses –participants with CVD at baseline included: Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between respiratory symptoms and cardiovascular mortality (weighted)
eTable 11. Additional analyses –women and men combined: Death rates and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between dyspnoea and cardiovascular mortality (weighted)
eTable 12. Respiratory symptoms, lung function, medical diagnoses, and other characteristics of participants with and without data on the dyspnoea scale
eTable 13. Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations of having missing data on the dyspnoea scale with all-cause and cardiovascular mortality (weighted)
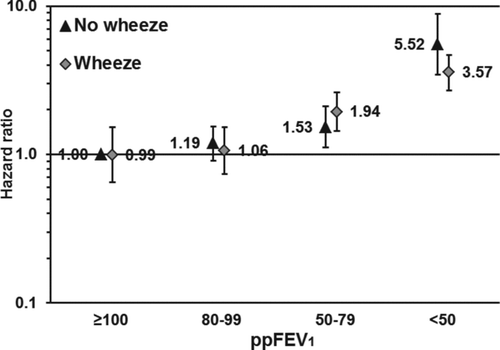
Figure 1a. Association of the combined exposure of% predicted forced expiratory volume in 1 second (ppFEV1) and reporting of chronic bronchitis with all-cause mortality. The reference category consists of participants with ppFEV1 ≥ 100 not reporting chronic bronchitis. The bars represent 95% confidence intervals. Adjusted for age (<40, 40–49, . . . , ≥ 80 years), sex (woman, man), smoking (never, former, current, unknown), education (<10, 10–12, ≥13 years, unknown), body mass index (<18.5, 18.5–24.9, 25.0–29.9, ≥ 30.0 kg/m2), physical activity (inactive, light activity <1 hours/week, light activity 1–2 hours/week, light activity ≥3 hours/week, only vigorous activity, unknown), and cardiovascular disease (yes, no).
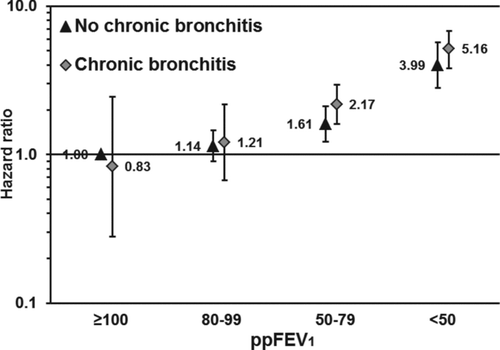
Figure 1b. Association of the combined exposure of% predicted forced expiratory volume in 1 second (ppFEV1) and reporting of wheeze with all-cause mortality. The reference category consists of participants with ppFEV1≥ 100 not reporting wheeze. The bars represent 95% confidence intervals. Adjusted for age (<40, 40–49, . . . , ≥80 years), sex (woman, man), smoking (never, former, current, unknown), education (<10, 10–12, ≥13 years, unknown), body mass index (<18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2), physical activity (inactive, light activity <1 hours/week, light activity 1–2 hours/week, light activity ≥ 3 hours/week, only vigorous activity, unknown), and cardiovascular disease (yes, no).