Abstract
Several epidemiological studies have suggested that hepatitis C virus (HCV) infection is associated with the presence of obstructive lung disease (OLD). However, there is a strong link between HCV infection and tobacco abuse, a major risk factor for the development of OLD. In this study we analyzed clinical, laboratory and spirometric data from 1068 study participants to assess whether HCV infection, viremia, or HCV-associated end organ damage were associated with OLD. Demographics, risk behavior, serologic status for HCV and HIV, and spirometric measurements were collected from a cross-sectional analysis of the Acquired Immunodeficiency Syndrome (AIDS) Linked to the IntraVenous Experience (ALIVE) study, an observational cohort of IDUs followed in Baltimore, MD since 1988. Of 1,068 participants, 890 (83%) were HCV positive and 174 (16%) met spirometric criteria for OLD. Factors independently associated with OLD were age and BMI. HCV infection, viral load and HCV-associated end organ damage were similar in participants with and without OLD. In summary, there was no independent association between markers of HCV exposure, chronicity, viremia, or HCV-associated end-organ damage with OLD. Our findings support the strong correlation between HCV status, injection drug use, and smoking. These data suggest that HCV may not be a sole contributor to the increased prevalence of OLD described in previous studies of HCV-infected individuals.
| Abbreviations | ||
| Acquired-Immune Deficiency Syndrome (AIDS); AIDS Linked to the IntraVenous Experience (ALIVE); Body mass index (BMI); Chronic obstructive pulmonary disease (COPD); Forced Expiratory Volume in 1 second (FEV1); Forced Vital Capacity (FVC); Hepatitis C virus (HCV); Human Immunodeficiency Virus (HIV); Injection Drug Users (IDUs); kilopascals (kPa); Obstructive lung diseases (OLD); Odds Ratio (OR); Polymerase Chain Reaction (PCR); RiboNucleic Acid (RNA) | = | |
Introduction
Obstructive lung diseases (OLD), specifically asthma and chronic obstructive pulmonary disease (COPD), are prevalent conditions associated with substantial morbidity and mortality in urban, underserved areas of the United States (Citation1). Although the biological mechanisms underlying development of OLD are unresolved, latent viral infections, particularly adenovirus and HIV infection, have been proposed to increase susceptibility to development of airflow obstruction in smokers (Citation2–6).
Hepatitis C virus (HCV) infection has also been implicated as a potential viral mediator of OLD development (Citation7). From a biological standpoint, HCV infection is recognized to trigger a chronic inflammatory response and HCV virus is detectable in the lung (Citation8–10). Several epidemiological studies have suggested that HCV could be a risk factor for OLD (Citation11–13). First, HCV-infected persons suffer from a heavy burden of co-morbid diseases. Among 7,411 HCV patients in an administrative database cohort, >99% had at least one co-morbid condition, including 34% with lower respiratory disease (Citation14).
In a cross-sectional study of 187 patients from an urban hospital in Brazil, the prevalence of HCV infection was significantly higher in COPD patients than in blood donors without reported COPD (Citation13). Two small, prospective longitudinal studies suggested that HCV infection in patients with OLD was associated with an accelerated decline in lung function (Citation12, Citation15). Notably, HCV-infected patients that demonstrated virological responses to interferon therapy appeared to have a lower rate of FEV1 decline. Similar findings were observed in a small cohort of asthma patients (Citation15). In recent data presented from a large multi-site HIV cohort study, HCV seropositivity was associated with an increased prevalence of respiratory symptoms and OLD (Citation16).
However, these data require cautious interpretation because of the strong link between tobacco use and HCV infection. Worldwide, the primary risk factor for HCV acquisition is injection drug use (Citation17). Injection drug users (IDUs) have a very high prevalence of both HCV infection (around 80%) and of tobacco smoking (>70%) (Citation18). Not surprisingly, tobacco use and injection drug use are frequently observed in populations with a high burden of OLD (Citation19–22). In addition to small sample size, prior studies were limited in their ability to assess chronicity or stage of HCV-related end-organ disease and other unmeasured factors which may confound the association between HCV infection and OLD.
Since 1988, the AIDS Linked to the IntraVenous Experience (ALIVE) study has prospectively followed and obtained clinical and laboratory measurements from a cohort of IDUs in Baltimore, Maryland (Citation23). In recent years, data collected include markers of HCV exposure (HCV antibody serostatus), chronicity (HCV viral load) and HCV-associated end-organ damage (liver fibrosis assessed by transient elastography) as well as spirometry and detailed smoking behavior. In this study we analyzed clinical, laboratory, and spirometric data from 1068 ALIVE study participants to assess whether HCV infection, viremia, or related disease were associated with OLD.
Methods
Study participants and design
The ALIVE cohort, as has been previously described, is composed of participants >18 years, living in inner city Baltimore, Maryland with active injection drug use at the time of enrollment (Citation23). Since 1988, participants complete health-related questionnaires and provide blood samples at visits every six months. Over follow-up, ALIVE has had >95% retention. There have been several periods of open recruitment to replenish attrition related to loss to follow-up or mortality. The ALIVE cohort is a source for study participants for specific substudies focusing on organ-specific outcomes.
All participants enrolled in the larger ALIVE cohort are asked to participate in these different substudies as they are conducted. Since 2005, as part of a liver disease sub-study, individuals underwent liver fibrosis staging by elastography (Citation24). HCV antibody and RNA testing was performed at the time of first elastography measurement. Since 2007, as part of a lung disease sub-study, individuals performed pre-bronchodilator spirometry testing at each study visit (Citation3). For this study, we performed a cross-sectional analysis nested within the on-going cohort of data from the first spirometry visit with liver scan elastography within 6 months. The study was approved by the Institutional Review Board of Johns Hopkins University (NA_00020295) and all participants provided written informed consent.
Data collection
Demographic and clinical data were collected concurrently with spirometry measurements. Active drug use and smoking status were determined by self-report through both interviewer- and computer- administered standardized questionnaires.
Routine laboratory testing at each visit included HIV serology for HIV-negative participants and, for HIV-infected participants, T-cell subsets and HIV RNA (Roche Molecular Systems, Amplicor HIV-1 Monitor test version 1.5). Antibodies to HCV were detected in serum collected at the first available visit on each subject, as previously described (Citation24).
HCV RNA testing was done on plasma collected at or near the time of the initial liver elastography measurement using the Abbott real-time PCR (Citation25). Liver fibrosis was staged by measurement of liver stiffness by transient elastography with use of a Fibroscan machine, as previously described (EchoSens, France) (Citation26, 27). Briefly, pulse-echo ultrasound acquisitions are used to measure the velocity of a shear wave propagated through the liver. Results are instantaneously received as a single, quantitative parameter of liver stiffness measurement, reported in kilopascals (kPa).
All elastography examinations were performed by certified operators trained by the manufacturer with use of a single device in the research clinic. Examinations with 8 validated measurements, a >60% success rate (the number of validated measurements divided by the total number of measurements), and limited variability (inter-quartile range of measures/median value less than 0.30) were considered to be reliable (Citation26, 27). The median values from each examination were used for analysis.
The presence of obstructive lung disease was assessed with pre-bronchodilator spirometry using a KoKo® pneumotachometer (Pulmonary Data Services Inc., Louisville, CO) in accordance with American Thoracic Society guidelines (Citation28). OLD was defined as a prebronchodilator ratio of forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) of less than 70% (Citation29).
Statistical analysis
Clinical and demographic characteristics between groups are presented as means (standard deviation) for normally distributed data and median values (interquartile range [IQR]) for non-normally distributed data or n (%). Student's t-test was used to compare continuous variables for normally distributed data and Wilcoxon–Mann–Whitney test for skewed data. For all comparisons, a p-value <0.05 was used to infer statistical significance.
To determine the associations between HCV markers and presence of OLD, logistic regression models were generated to estimate odds ratios with 95% confidence intervals. We evaluated three measures of HCV infection or disease as the exposure of interest. HCV antibody serostatus was included in models as a binary (yes/no) exposure while HCV viral load levels were incorporated grouped into quartiles. Liver fibrosis scores, measured using elastography, were categorized based on prior validation and modeling work in this cohort into three groups reflecting none/mild (<8), moderate (Citation8–12) and severe (>12) fibrosis which approximates Metavir fibrosis stages F0/F1, F2, and F3, respectively (Citation26).
All models were adjusted for covariates deemed relevant in exploratory analysis or clinical literature review. These included age, race, sex, body mass index (BMI), HIV status, current injection drug use, and current smoking status. In this cohort, HCV antiviral therapy is extremely rare and treatment responses are limited in this predominantly African American, HCV genotype-1 population. As less that 1% of HCV-infected participants had achieved a sustained virological response (Citation30), we did not incorporate HCV treatment variables into this analysis.
Results
Participant characteristics
Of 1,068 participants included in this analysis, 890 (83%) were HCV seropositive and 174 (16%) met criteria for spirometric obstruction. The mean age of participants was 48.1 years, approximately one-third were female, and 942 (88%) were African-American (). A total of 918 (86%) were current smokers with a median 20 pack-years smoked (IQR 12-33). A total of 438 (41%) reported active injection drug use in the previous 6 months. The smoking intensity was similar comparing HCV seropositive persons to HCV-uninfected persons (21 vs. 20 pack-years; p = 0.26). Approximately 30% of participants were infected with HIV with a mean CD4 cell count of 358 and 46% had complete virologic suppression.
Table 1. Baseline demographics of study cohort
When stratifying by OLD status, participants with OLD had a lower FEV1 (2.30 vs. 2.93L, p < 0.01) and a lower FEV1% predicted with 73.1% predicted compared to 92.1% predicted (p < 0.01). Participants with OLD were older (49.4 vs. 47.8 years, p = 0.016) and had a lower BMI (24.5 vs. 26.3 kg/m2, p < 0.01; ). The prevalence of HIV did not differ by OLD status (30% for those with and without OLD). Similarly, the prevalence of current injection drug use also did not differ by OLD status (41% for both OLD present and absent groups).
In univariate analysis, age and BMI were associated with a differential odds of OLD. For each 10-year increase in age, the odds of OLD increased 29% (OR 1.29; 95% CI 1.05–1.60; p = 0.016). Higher BMI was associated with a reduced odds of OLD (OR 0.92 per kg/m2; 95% CI 0.89–0.96; p < 0.001). Gender, race, current smoking status and pack-years smoked (modeled continuously) were not significantly associated with OLD. While there was no association between smoking and presence of OLD, in univariate analysis each 10 pack-years smoked was associated with 29 ml lower FEV1 (95% CI 1 ml to 56 ml lower; p = 0.04). Modeling pack-years smoking using a high-intensity threshold (>40 pack-years compared with ≤4 0 pack-years) was associated with a 61% increase in the odds of COPD (OR 1.61; 95% CI 1.05–2.46; p = 0.026). In multivariate modeling, age, BMI and African-American race were associated with OLD (see Tables –4). Other covariates including female gender, HIV status, current injection drug use or active tobacco were not associated with OLD.
Table 2. Adjusted association between HCV serostatus and obstructive lung disease
Association between HCV markers, HCV-related disease and OLD
Multivariate logistic regression was used to determine if there were independent associations between HCV exposure (HCV antibody serostatus), chronicity or viremia (HCV viral load) or HCV-associated end organ damage (liver fibrosis score) and the presence of OLD after accounting for relevant confounders. After adjusting for age, race, sex, BMI, HIV serostatus, current injection drug use and current smoking, we observed no association between HCV antibody status and OLD (OR 0.82; 95% CI 0.51–1.31; p = 0.42) (). Moreover, in a separate model, higher HCV viral load was not associated with the presence of OLD (OR 0.89 per log10 increase in HCV RNA level; 95% CI 0.75–1.07; p = 0.21). Similarly, there was no association between quartiles of HCV viral load and OLD (). Compared to HCV seronegative individuals, having HCV viral load in the highest quartile was not associated with OLD (OR 0.75; 95% CI 0.42–1.36; p = 0.14).
Table 3. Adjusted association between HCV viral load and obstructive lung disease
There was no association between severity of HCV-related end-organ damage as measured by liver fibrosis score and the presence of OLD. The mean fibrosis score in OLD participants was 8.54 kPa compared to 8.98 among those without OLD (p = 0.54). Compared to participants having fibrosis scores indicating none to minimal fibrosis as the referent group, neither those participants with moderate fibrosis or with severe fibrosis/cirrhosis displayed a higher likelihood of OLD [OR 0.92 (95% CI 0.58–1.46) and 1.24 (95% CI 0.75–2.04), respectively] (). presents the adjusted odds of OLD for HCV serostatus, viral load levels, and fibrosis stage. As can be seen graphically, the likelihood of having OLD was similar in patients irrespective of HCV infection status, level of viremia or fibrosis stage.
Figure 1. Odds ratios of markers of HCV infection and the presence of OLD. Width of line represents 95% CI.
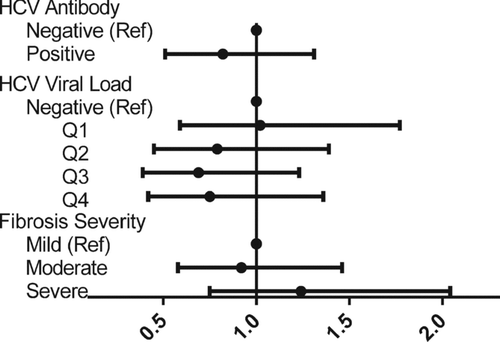
Table 4. Adjusted association between Fibroscan score and obstructive lung disease
Restrictive physiology (defined as FEV1/FVC ≥ 0.70 and FVC < 80% predicted) was present in 97 (9.08%) of the cohort. To determine if the lack of association between Hepatitis C markers and OLD was being attenuated by the presence of restrictive lung disease in the comparator population, a sensitivity analysis was performed excluding individuals with spirometric restriction.
There was no change in the observed lack of associations seen in the primary analysis. We also determined the association between FEV1 modeled continuously and HCV markers. In univariate analysis, there was no association between hepatitis C serostatus positivity or hepatitis C viral load level and absolute FEV1 (p = 0.38 and 0.32, respectively). In univariate analysis, more severe fibroscan score was associated with lower absolute FEV1. Compared to mild fibrosis, moderate fibrosis was associated with a 168 ml lower absolute FEV1 (p = 0.01), yet severe fibrosis was associated with 199 ml lower absolute FEV1 (p = 0.01). However, this association was attenuated when adjusting for age [moderate fibrosis 122 ml lower FEV1 (p = 0.05) and severe fibrosis 118 ml lower FEV1 (p = 0.10)]. Modeling smoking with current smoking status or high intensity (>40 pack-years) in multivariate models did not change the primary results.
Discussion
In this study of more than 1,000 current and former IDUs with a >80% prevalence of HCV infection and pervasive tobacco exposure, we found no evidence that HCV infection was independently associated with the presence of OLD. This study represents, to our knowledge, the largest evaluation of the association between HCV infection and OLD. Specifically, we found no association between the presence or chronicity of HCV infection, the degree of viremia, or the severity of HCV-related liver disease and OLD. These findings provide strong evidence that prior reports of an association between HCV and OLD may be have been confounded by the strong relationship between HCV infection as a marker of heavy tobacco exposure.
Previous reports demonstrating an association between HCV infection and OLD have suffered from two primary limitations. First, most studies have had small sample sizes ranging from 68 to 187 (Citation11, Citation13). More importantly, prior reports have often included HCV-infected participants drawn from very different study populations compared to the HCV-negative populations. Not surprisingly, there was evidence for differential tobacco use between the comparison groups (Citation11, Citation13). In contrast, the ALIVE cohort includes IDUs with and without HCV infection followed concurrently in the same setting by the same protocol.
Although other studies investigated the association between HCV antibody serostatus and COPD, we evaluated multiple markers of HCV exposure, chronicity of infection, and disease. From a biological standpoint, it seems reasonable that if HCV is directly contributing to OLD, then increasing levels of the virus or increasing severity of related end-organ damage would also be associated with increased likelihood or severity of obstruction. However, again, we found no association between HCV viral load or liver fibrosis stage and OLD. Although we observed an association between liver fibrosis and FEV1modeled continuously, this association was attenuated when adjusting for age. We feel that these findings demonstrate that age confounds the fibrosis-absolute FEV1 association, as a certain degree of time is required to develop fibrosis, and in that same time, FEV1 may decline.
Importantly, the analysis of this cohort reveals a negative association between African-American race and OLD during multivariate analysis despite a lack of an association during univariate analysis. The effect of race on OLD is controversial in the literature with some reports suggesting that African-American race is associated with OLD, while others indicate a protective effect (Citation19, Citation31). However, the ALIVE cohort is largely comprised of African-Americans (86%) and thus the true effect of race on the presence of OLD is difficult to ascertain from this cohort.
Smoking is a well-established risk factor for OLD yet in this study there was not a statistically significant association between pack-years smoked and OLD in this study. However, we did observe lower FEV1 in those with more pack-years smoked as well as an association between intense smoking history and OLD. The ALIVE cohort is composed of volunteers with a relatively homogenous tobacco exposure and intensity of smoking which makes it difficult to ascertain the effect of smoking on OLD given the inadequate number of nonsmokers or mild intensity smokers. However this homogeneity of tobacco exposure allows us to remove the effect of smoking in the HCV-OLD pathway as a potential confounder.
Our study is primarily limited by the cross-sectional nature of the analysis. However, while correlations in cross-sectional studies cannot be inferred as causation, our finding of no effect were less susceptible to this limitation; only rarely will the reverse be true and prospective studies reveal an association that was null in cross-sectional analysis. In fact, there have been small prospective studies that suggested that chronic HCV infection was associated with an accelerated decline in the lung function of COPD patients (Citation12). On-going follow-up in the ALIVE cohort will allow further longitudinal evaluation of HCV markers on lung function decline over time.
Although this analysis may not be generalizeable to general populations, the characteristics of this study cohort, including injection drug use and heavy tobacco dependence, are consistent with known risk factors for acquisition of hepatitis C infection. Thus, these findings are generalizeable to populations at increased risk for hepatitis C infection. Because post-bronchodilator spirometry was not collected for these participants, the distinction between reversible and irreversible airflow obstruction is not possible. This limits the ability of our analysis to determine the association between HCV and asthma versus chronic obstructive pulmonary disease.
In summary, we found no direct association between markers of HCV exposure, chronicity, viremia, or HCV-associated end-organ damage with OLD. Our findings document the strong correlation between HCV status, injection drug use, and smoking, and emphasize the need for rigorous attention to these relationships in selecting comparison groups for epidemiological studies of HCV and OLD. In total, these data suggest that HCV may not be a sole contributor to the increased prevalence of OLD. As a result there is a substantial need for smoking cessation to be incorporated into risk reduction among the injection drug user community.
Declaration of Interest Statement
This work was supported by the National Institutes of Health [Grants R01-DA-04334, R01-DA-12568, R01-HL-90483, and R01-DA-16078) and GDK was supported by the American Cancer Society (MRSG-07-284-01-CCE).
The authors report no conflict of interest. Dr. Fischer assumes responsibility for the content of the manuscript including data and analysis. WF contributed to study design, analysis plan, interpretation and writing and editing of the report. BD contributed to study design, analysis plan, interpretation and editing of the report, and was responsible for data analysis. CM contributed to study design, analysis plan, interpretation and editing of the report. DT contributed to data collection, interpretation of results, and editing of the report. RB contributed to study design, analysis, interpretation and editing of the report. SM contributed to study design, interpretation and editing of the report. RW contributed to study design, analysis plan, interpretation and editing of the report. GK contributed to study design, analysis plan, interpretation and writing and editing of the report. A portion of these data was previously presented in abstract form at The American Thoracic Society International Meeting 2012.
References
- Snyder LD, Eisner MD. Obstructive lung disease among the urban homeless. Chest 2004; 125(5):1719–1725. Epub 2004/05/12.
- Diaz PT, King ER, Wewers MD, Gadek JE, Neal D, Drake J, HIV infection increases susceptibility to smoking-induced emphysema. Chest 2000; 117(5 Suppl 1):285S. Epub 2000/06/10.
- Drummond MB, Kirk GD, Astemborski J, Marshall MM, Mehta SH, McDyer JF, Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax 2012; 67(4):309–314. Epub 2011/11/18.
- Hayashi S, Hogg JC. Adenovirus infections and lung disease. Curr Opin Pharmacol 2007; 7(3):237–243. Epub 2007/04/21.
- Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 2008; 118(8):2771–2784. Epub 2008/07/26.
- Meshi B, Vitalis TZ, Ionescu D, Elliott WM, Liu C, Wang XD, Emphysematous lung destruction by cigarette smoke. The effects of latent adenoviral infection on the lung inflammatory response. Am J Respir Cell Mol Biol 2002; 26(1):52–57. Epub 2001/12/26.
- Moorman J, Saad M, Kosseifi S, Krishnaswamy G. Hepatitis C virus and the lung: implications for therapy. Chest. 2005; 128(4):2882–2892. Epub 2005/10/21.
- Idilman R, Cetinkaya H, Savas I, Aslan N, Sak SD, Bastemir M, Bronchoalveolar lavage fluid analysis in individuals with chronic hepatitis C. J Med Virol. 2002; 66(1):34–39. Epub 2001/12/19.
- Kubo K, Yamaguchi S, Fujimoto K, Hanaoka M, Hayasaka M, Honda T, Bronchoalveolar lavage fluid findings in patients with chronic hepatitis C virus infection. Thorax 1996; 51(3):312–314. Epub 1996/03/01.
- Yamaguchi S, Kubo K, Fujimoto K, Honda T, Sekiguchi M, Sodeyama T. Analysis of bronchoalveolar lavage fluid in patients with chronic hepatitis C before and after treatment with interferon alpha. Thorax 1997; 52(1):33–37. Epub 1997/01/01.
- Erol Sea. Hepatitis C infection and COPD. Hepatitis Month 2009; 9(1):39–44.
- Kanazawa H, Hirata K, Yoshikawa J. Accelerated decline of lung function in COPD patients with chronic hepatitis C virus infection: a preliminary study based on small numbers of patients. Chest 2003; 123(2):596–599. Epub 2003/02/11.
- Silva DR, Stifft J, Cheinquer H, Knorst MM. Prevalence of hepatitis C virus infection in patients with COPD. Epidemiol Infect 2010; 138(2):167–173. Epub 2009/07/01.
- Louie KS, St Laurent S, Forssen UM, Mundy LM, Pimenta JM. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infect Dis 2012; 12:86. Epub 2012/04/13.
- Kanazawa H, Yoshikawa J. Accelerated decline in lung function and impaired reversibility with salbutamol in asthmatic patients with chronic hepatitis C virus infection: a 6-year follow-up study. Am J Med 2004; 116(11):749–752. Epub 2004/05/18.
- Gingo MG, MP; Lucht, L; Kessinger, C; Rissler, B; Weinman, R; Slivka, WA, McMahon, D; Sciurba, FC; Morris, A. Pulmonary function in human immunodeficiency virus infected individuals [abstract]. Am J Respir Crit Care Med 2010; 181(A5201).
- Meffre C, Le Strat Y, Delarocque-Astagneau E, Dubois F, Antona D, Lemasson JM, Prevalence of hepatitis B and hepatitis C virus infections in France in 2004: social factors are important predictors after adjusting for known risk factors. J Med Virol 2010; 82(4):546–555. Epub 2010/02/19.
- Esteban JI, Esteban R, Viladomiu L, Lopez-Talavera JC, Gonzalez A, Hernandez JM, Hepatitis C virus antibodies among risk groups in Spain. Lancet 1989; 2(8658):294–297. Epub 1989/08/05.
- Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 182(5):693–718. Epub 2010/08/31.
- Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet 2011; 378(9795):991–6. Epub 2011/09/13.
- Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med 2004; 170(4):408–413. Epub 2004/05/01.
- Yamini D, Basseri B, Chee GM, Arakelyan A, Enayati P, Tran TT, Tobacco and other factors have a negative impact on quality of life in hepatitis C patients. J Viral Hepat 2011; 18(10):714–720. Epub 2010/08/21.
- Vlahov D, Anthony JC, Munoz A, Margolick J, Nelson KE, Celentano DD, The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr 1991; 109:75–100. Epub 1991/01/01.
- Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000; 284(4):450–456. Epub 2000/07/25.
- Mehta SH, Astemborski J, Kirk GD, Strathdee SA, Nelson KE, Vlahov D, Changes in blood-borne infection risk among injection drug users. J Infect Dis 2011; 203(5):587–594. Epub 2011/02/02.
- Kirk GD, Astemborski J, Mehta SH, Spoler C, Fisher C, Allen D, Assessment of liver fibrosis by transient elastography in persons with hepatitis C virus infection or HIV-hepatitis C virus coinfection. Clin Infect Dis 2009; 48(7):963–972. Epub 2009/02/25.
- Mehta SR, Kosakovsky Pond SL, Young JA, Richman D, Little S, Smith DM. Associations between phylogenetic clustering and HLA profile among HIV-infected individuals in San Diego, California. J Infect Dis 2012; 205(10):1529–1533. Epub 2012/03/27.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Standardisation of spirometry. Eur Respir J 2005; 26(2):319–338. Epub 2005/08/02.
- From the Global Strategy for the Diagnosis Management and Prevention of COPD Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. Available from: http://www.goldcopd.org/ [database on the Internet].
- Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, Limited uptake of hepatitis C treatment among injection drug users. J Commun Health 2008; 33(3):126–133. Epub 2008/01/01.
- Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006; 130(5):1326–1333. Epub 2006/11/14.

