Abstract
The increasing number of treatment options for managing patients with chronic obstructive pulmonary disease (COPD) promises to improve the outcomes for COPD patients. However, determining which treatments are appropriate for individual patients has become increasingly complex. The COPD Foundation Guide for Diagnosis and Management of COPD was developed to be a practical, easy to use tool for clinicians. The Guide includes specific recommendations for diagnostic studies and treatments based on specific diagnostic criteria. This manuscript describes the rationale for the development of the Guide, the process used, the rationale for the specific recommendations and the plans for further development. The current recommendations of the COPD Foundation have been summarized in the form of Pocket Cards, which may be obtained from the Foundation at no charge (1-866-316-COPD (2673), www.copdfoundation.org).
Keywords :
Introduction
Chronic obstructive pulmonary disease (COPD) refers to a set of multifactorial, diverse diseases that share the feature of progressive airflow limitation as disease advances in addition to a variety of other important clinical features. Distinct subtypes include emphysema or airways disease, either of which may be predominant (Citation1). Spirometry, which can determine the presence of airflow limitation and measure its severity, is a central diagnostic element. However, airflow limitation is only one manifestation of COPD. Symptoms, which may be varied, exacerbations, hypoxemia and extra-pulmonary co-morbidities as well as the presence of emphysema or airways disease, can affect clinical decision making. Management of the individual COPD patient, therefore, requires an organized diagnostic and therapeutic approach and so characterization of each person's COPD requires assessments in addition to spirometry.
The COPD Foundation Pocket Guide for the Diagnosis and Management of COPD was designed to be a practical tool to assist the practicing clinician manage the diagnosis and treatment of COPD patients. The Guide was designed to aid in identifying patients for whom spirometry should be performed, how patients should be classified based on spirometry, what additional assessments should be performed and when and how these diagnostic evaluations should influence therapy.
COPD is an extremely common problem, with nearly 15 million patients diagnosed in the United States (Citation2–4). COPD is a problem frequently encountered by the clinician () (Citation4). However, COPD is often unappreciated, with at least half of patients with COPD in the United States being undiagnosed (Citation5). When diagnosed, COPD is often undertreated, with studies reporting 30–70% of diagnosed patients receiving no treatment (Citation6, 7), and treatments, when used, are often sub-optimal (Citation8). The heterogeneity of the COPD patient population and the increasing number of effective interventions further complicates disease management.
Table 1. Prevalence of COPD in a “Typical” Practice
Several efforts, including the National Heart Lung and Blood Institute's program, “Learn More, Breathe Better” (Citation9), the National Lung Health Education Program's “Test your lungs, know your numbers” (Citation10), and the COPD Foundation's program “Drive for COPD” (Citation11), have been developed to increase public and health care professional awareness of COPD and to increase detection of unrecognized disease. At the same time, a variety of resources have been developed as aids to the clinician caring for COPD patients. These materials have addressed a number of needs, but have not been as useful for the practicing clinician as might be hoped. The COPD Foundation Guide was designed to address this need. Specifically, the assists the clinician in recognizing undiagnosed COPD and in providing optimal therapy to individual patients in this heterogeneous group. To this end, the Guide has been designed to provide clear, practical recommendations for the diagnosis and management of COPD patients. It has been specifically formatted to be easy to reference and to omit information not currently relevant to clinical decision making.
The Guide is one part of the COPD Foundation program to provide support materials for clinicians caring for COPD patients. Three versions of the Guide have been prepared (). These include: (Citation1) a “skinny” two panel version with only the diagnostic and therapeutic recommendations included, (Citation2) a full six-panel version with all drugs listed using generic names, and (Citation3) a full six-panel version with all drugs listed using brand names. In addition to the Guide, a smartphone application will be developed that will include the information in the Guide together with several easily accessible tiers of additional supporting information and will allow active calculation of Spirometry Grade (SG), the modified Medical Research Council (mMRC) and COPD Assessment Test (CAT) scores.
Panel A. 2-panel version (front and back). The version includes the diagnostic summary and therapeutic table based on diagnostic features.
Panel B. 6-panel version, generic (front and back). This version adds details on the mMRC and CAT symptom scores, specific information on medications commonly used to treat COPD and information relating to smoking cessation.

Panel C. 6-panel version, brand names. This version is identical to the generic version, except that brand names are used (front and back).

Finally, the Foundation will support a discussion blog for clinicians that will provide a platform for an open interaction relating to COPD management. The entire program is supported as a public service by the COPD Foundation, which is committed to making the Guide, the smartphone applications, the blog and any other materials that may be developed available without charge.
The current manuscript describes the rationale for the development of the Guide, the process by which it was developed and plans for its ongoing support. In addition, we provide the rationale for the information included in the Guide and evidence supporting the recommendations made.
Unmet needs
Guidelines have been developed by a variety of groups to provide recommendations in a number of therapeutic areas. A formal methodology has evolved for the preparation of these documents, which is both labor intensive and expensive (Citation12–28). As a result, many organizations have prepared summary recommendations with simplified methodology and have used “consensus statements,” “position paper,” “guidance,” or other names to describe the recommendations. Several such documents are available for COPD: GOLD (a consensus statement) (Citation29, 30), the ATS/ERS Standards (a position paper) (Citation31, 32) and NICE (a guidance) (Citation33) are among the most widely referenced. A Guideline has also been prepared by a collaboration of the ACP/ACCP/ATS/ERS (Citation34). In general these documents are extensively researched and referenced, but are too long for practical use by most clinicians. Even the summary statements for the longer documents are often too long for ready reference in the setting of a busy clinical practice (Citation35) (). In addition, while strict guideline methodology produces an evidence-based set of recommendations, too often there are insufficient data to resolve important clinical questions that must be addressed daily in practice. Therefore, evidence-based guidelines may not always help the clinician who is often faced with making decisions for which such evidence is not available. The Guide was designed to provide practical recommendations for the problems that are frequently encountered in clinical practice and to do so in a format that could be readily used in the context of a busy clinical practice.
Table 2. Selected recommendation documents and summaries: Length
A second limitation of most sets of recommendations is that they are developed by relatively small groups. They are often reviewed and approved by somewhat larger groups. Nevertheless, the content usually reflects the analysis of relatively few individuals. This is less of a problem where the evidence base is strong, but may be increasingly problematic when recommendations must rely on expert opinion. The Guide was developed by a similar approach. However, it is the intent of the Foundation to use the Guide as a platform to engage a much larger group in the discussion of COPD management. Although not yet an “open source-derived” document, it is hoped that the blog that will support the Guide will be a means to engage the larger clinical community in developing recommendations and identifying areas in need of additional recommendations.
Another limitation of many consensus recommendations is that their purpose, the intended audience and outcomes are seldom stated explicitly. It is widely assumed that the documents are written to inform primary care physicians, who care for most COPD patients, and that the intended result is to improve the outcomes of COPD management. This common failure to identify the target audience and intended outcomes might explain why the guidelines so often fail to meet the hopes of their authors (Citation37).
Despite widespread adoption by academic specialists, translating this consensus knowledge into improved practice patterns and outcomes has been less successful than might be hoped. The COPD Foundation Guide is a tool specifically designed to put the core recommendations for COPD diagnosis and treatment into a more accessible format. Combined with the planned web and smartphone technology, this may greatly facilitate dissemination and rapidly bring the recommendations directly into the hands of clinicians in day-to-day practice.
Process
Recognizing that currently available tools were overly large, contained much information that was valuable for understanding the disease but not immediately relevant to clinical practice, an ad hoc committee (listed as authors) was organized through the COPD Foundation with the goal of developing a practical guide. The Guide was based on the prior management card (The COPD Pocket Consultant) developed by the COPD Foundation and NewYork-Presbyterian Healthcare System. For the current version, funding from donor funds made possible the drafting of mock ups for initial critiques and revisions. These versions were reviewed by members of the COPD Foundation Medical and Scientific Advisory Council and additional selected reviewers (see acknowledgment).
Prior versions of the management card were felt to do some things well and others poorly. Its size and format were easy to use and straightforward to reference. In addition, it could be produced and distributed within the financial resources available to the COPD Foundation. Over the last half dozen years, this consultant card has been updated multiple times, and over a quarter of a million cards have been distributed to healthcare providers nationwide at no charge. On the other hand, a key diagnostic recommendation, when to use spirometry to diagnose COPD, was not clearly defined. This has remained a somewhat controversial issue. Although there is general agreement that spirometry is indicated if symptoms are present, the consensus statement from ACP, ACCP, ATS, and ERS published in 2011 strongly recommended against performing spirometry in those at risk but without specific symptoms (Citation34).
One concern with that recommendation is that symptoms, particularly early symptoms, may be ignored or incorrectly self-diagnosed. A chronic cough may be viewed as an allergic cough or a “normal smoker's cough” and self treated with over-the-counter medications. Progressive shortness of breath may be explained by “getting old, being overweight or out of shape.” In the limited time available during health care visits, these issues may never be discussed. Limiting spirometry to those complaining of symptoms may miss many of the reported 12 million with COPD but as yet undiagnosed (Citation2–4). Better delineation of which patients should have spirometry is needed and was the subject of an NHLBI workshop (Citation38) and a subsequent RFA (Citation39). Ongoing research, in which the COPD Foundation collaborates, looks to develop a new validated questionnaire linked with peak flow testing that may provide a better approach. In the interim, recognizing that comorbidities are extremely common in COPD (Citation29–33, Citation40) and that evidence suggests that COPD is an independent risk factor for a number of these conditions, the Guide suggests that spirometry should be considered in those with symptoms, and those at risk who have one or more co-morbid condition, e.g., smokers with cardiovascular disease, consistent with recommendations from a recent ATS/ERS report (Citation41).
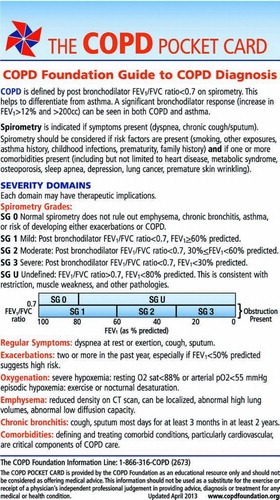
A strict definition of COPD remains controversial, although the defining feature is airflow limitation (Citation42–45). In part, this is because the “definition” has mostly been used for epidemiological rather than clinical purposes (Citation46). Reduced airflow is most easily measured as a reduction in the forced expiratory volume in one second (FEV1). With obstructive disease, the FEV1 decreases more than the forced vital capacity (FVC). As a result, the FEV1/FVC ratio has been used to define obstruction and to distinguish it from restriction, in which the FEV1 and FVC decrease in proportion. With aging, both FEV1 and FVC decline, but the FEV1 declines faster (Citation42–45).
As a result, on a population basis, the “normal range” for FEV1/FVC ratio, based on the population distribution decreases. For rigorous population studies, the lower limit of normal defined statistically on population-based sampling has been suggested as an appropriate definition for categorizing individuals as having COPD. In clinical practice, this value, which is not easy to reference and is undetermined for many populations, is difficult to use. For this reason, many consensus recommendations suggest a ratio of FEV1/FVC of 0.7 as a cutoff, with values below this level indicating the presence of COPD (Citation29, Citation31, Citation33, 34). Because this is easy to implement in clinical practice and provides information that allows clinicians to make reasonable decisions, this value and approach was adopted for the Guide.
Assessment of airflow is important. In addition to defining the presence of obstruction, the severity of airflow limitation can be gauged. As with other recommendations, the FEV1, expressed as a percentage of that predicted based on height, age and gender, is used in the Guide. Thus, those with obstruction (FEV1/FVC < 0.7) can be categorized into severity categories, and the Guide defines three spirometric grades (SG) of obstruction. These are: mild obstruction (SG 1, FEV1 ≥ 60%), moderate obstruction (SG 2, FEV1 between 30 and 60%) and severe obstruction (SG 3, FEV1 < 30%) and were designed to follow the therapeutic recommendations provided in other consensus recommendations (Citation29, Citation31, Citation33, 34). These labels and groupings were chosen as they are already in use, are straightforward to implement and provide adequate classification to support recommendations for clinical decision making.
The Guide adds two additional spirometric grades, SG 0 and SG U. Those with SG 0 have normal spirometry. The majority of SG 0 individuals will be normal. However, normal spirometry does not rule out the presence of chronic bronchitis, emphysema, or other lung disease. SG U represents those with a normal FEV1/FVC ratio but low FEV1. Classically this group has been described as having “restrictive” disease. However data from COPDGene suggests that emphysema can be seen in this group and that SG U is very common. Up to 10% of the COPDGene population has emphysema without obstruction (Citation47). Although neither SG 0 nor SG U lead to specific therapeutic options now, that may well change as we learn from ongoing studies. Including these in the spirometric grading system allows all patients to be given an SG classification.
COPD has been defined in terms of airflow, but the disease processes that cause the obstruction, which are extremely heterogeneous, can be active before the airflow limitation is present. In addition, clinical manifestations of COPD are only weakly related to airflow limitation and likely reflect other consequences of COPD as well (Citation29, Citation31, Citation33, 34). For these reasons, it was felt that a more global assessment of the COPD patient was required. In practice, decisions on what therapies should be initiated depend both on spirometry and, more commonly, on other assessments (Citation29, Citation31, Citation33, 34).
Several distinct parameters, termed “domains” in the Guide, were felt crucial to assess in addition to airflow. These include the presence of symptoms, a history of exacerbations, adequacy of oxygenation, presence of emphysema, presence of chronic bronchitis and presence of co-morbidities. These domains need to be evaluated in order to develop a comprehensive therapeutic plan, although not all are required in all COPD patients.
Recommendations
Symptoms—Should be assessed in all patients
The cardinal symptoms of COPD are dyspnea, particu–larly with exertion, cough and sputum. Spirometry should be performed when these symptoms are troubling to the patient. Often, patients may minimize symptoms. If activity has become progressively limited because it would precipitate dyspnea, spirometry should be performed. Conversely, when spirometry is assessed due to smoking and co-morbidities such as cardiovascular disease, it is important to carefully assess the presence and severity of COPD-related symptoms. Two symptom scores, the mMRC that assesses only dyspnea (Citation48), and the CAT that assesses a range of symptoms including cough, wheezing and fatigue (Citation49), are provided in the Guide. Both can be used to track the course of disease. A score of ≥10 on the CAT is regarded as a significant indicator of respiratory disease that is impacting the patient (Citation49). A score of ≥2 on the mMRC has been suggested to have similar importance, but is not well-validated (Citation29). These scores can help guide diagnostic and therapeutic decisions.
Exacerbations—Should be assessed in all patients
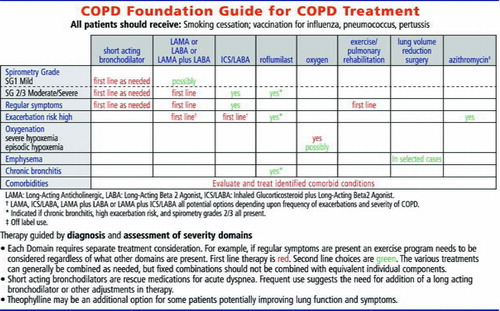
Exacerbations, particularly frequent exacerbations, defined as two or more per year, are major contributors to morbidity, mortality, and cost (Citation29, Citation31, Citation33, 34, Citation50, 51). A major advance in COPD management is the recognition that COPD exacerbations can be prevented and that individuals at risk for first or recurrent exacerbations can be identified. The strongest predictor of risk is a prior history of exacerbations followed by the severity of lung function impairment (Citation52). Moderate and severe patients by the Guide classification (SG 2 and SG 3) would include those at highest risk of exacerbation based on FEV1. Treatment of COPD patients with either of these criteria is warranted, and numerous inhaled agents including LAMA (long acting anticholinergic) (Citation53), LABA (long-acting beta 2 agonist), and LABA/ICS (inhaled glucocorticosteroid) combinations (Citation54), have been shown to decrease exacerbation rates and are approved for this indication. Data also suggest that theophylline (Citation55) and certain antibiotics (Citation56–58) may also decrease exacerbation rates, although these are not uses that are approved by the FDA. In addition, the presence of chronic bronchitis identifies a sub-group of SG 2 and SG 3 patients whose exacerbations can be prevented with roflumilast, which is approved for this use (Citation59, 60). These diagnostic features have been integrated into the therapeutic recommendations of the Guide.
Adequacy of oxygenation—Should be assessed in all patients with FEV1 < 60% predicted
For individuals with hypoxemia at rest (pO2 < 55 mmHg), supplemental oxygenation prolongs life (Citation61, 62). It is now standard of care to provide supplemental oxygenation to these individuals. The Guide recommends oxygen supplementation for either pO2 < 55 mmHg or percutaneous oxygen saturation < 88%. These are roughly equivalent values, but the oxygen saturation is somewhat more variable. Some published recommendations do not include percutaneous saturation, although this is accepted by payors and Medicare in the United States (Citation63). Recognizing that percutaneous oximetry is readily performed in clinical practices and requirement for an arterial blood gas would likely lead to under-diagnosis, under-treatment and preventable mortality, the Guide accepts either percutaneous oximetry as a convenient assessment that can be supplemented by arterial blood gas assessment when needed.
In contrast to the clear data regarding mortality for oxygen therapy in individuals hypoxemic at rest, there are several important topics related to oxygen therapy that are controversial. These include whether treatment is indicated for individuals with episodic hypoxemia that may occur with exercise (Citation64). The Guide makes no recommendations on these topics. It is common practice to treat such individuals. An ongoing study (NCT00692198) addressing this question should help clarify the issue. We expect to include some discussion in the smartphone application and hope there will be a lively discussion when the blog is activated.
Presence of emphysema—Should be assessed in all patients with FEV1 < 30% predicted or with very severe dyspnea
There are several ways to determine if emphysema is present. A low diffusion capacity that is not explained by reduced lung volume or anemia in a patient with obstruction is strongly suggestive, but may not be very sensitive. Computed tomography (CT), in contrast, provides a quantitative assessment of emphysema severity, determines emphysema location and can distinguish several distinct subtypes of emphysema (Citation65, 66). These assessments are not theoretical. CT scan is required to identify individuals who are appropriate for volume reduction surgery (Citation67), which can improve functional status, quality of life and reduce mortality in selected individuals with severe disease (Citation68). This treatment is drastically under-utilized, however. Because of the importance of recognizing individuals who may be candidates for this treatment option, the Guide recommends assessing whether emphysema is present in selected cases.
Presence of chronic bronchitis—Should be assessed in all patients
As noted above, individuals with chronic bronchitis have increased risk of COPD exacerbations (Citation52). In addition, they represent a subset of COPD patients whose exacerbations are responsive to treatment with roflumilast (Citation59). For this reason, it is important to identify whether chronic bronchitis is present. The most commonly used definition of chronic bronchitis, symptoms of cough and sputum for most days for three months in two successive years, was initially proposed as a “tentative” definition until something more definitive could be developed (Citation69). It remains in use as nothing “more definitive” has been proposed. However, the assessment of chronic bronchitis is inherently a clinical impression, with the key feature of persistent cough with or without sputum.
Presence of co-morbidities—Should be assessed in all patients
It is now recognized that many extra-pulmonary conditions are associated with COPD () (Citation29–33, Citation40). These are present in the COPD patient with a higher frequency than would be expected based on chance alone. Not all COPD patients are affected with the co-morbidities, but many have multiple co-morbidities (Citation70, 71). As may be expected, the presence of these co-morbidities adversely affects prognosis and may be the dominant problems patients face.
Table 3. Co-morbidities and COPD
Because the treatment of co-morbid conditions in the COPD patient population is generally the same as treatment in the broader population, there was considerable discussion on whether to include this in “COPD diagnosis.” However, the key issue most commonly faced by clinicians is whether to pursue diagnostic studies to evaluate the presence of these co-morbidities.
As the presence of COPD should increase the “index of suspicion,” it was felt that inclusion in the Guide was warranted. It is hoped that more aggressive diagnosis will lead to reduced morbidity from these associated conditions, which are often unrecognized but are frequently treatable. The smartphone application will include additional information on this topic together with appropriate links. It is also hoped that there will be lively discussion on the blog.
The Guide recommends testing all patients for alpha 1 anti-trypsin deficiency. The “classic” presentation of alpha 1 anti-trypsin deficiency is basilar emphysema in a young patient with modest smoking history. However, patients with alpha 1 deficiency may present at any age and with any pattern of emphysema. Many are misdiagnosed as asthma for many years. As replacement therapy may slow disease progression, a high index of suspicion for alpha 1 anti-trypsin deficiency is warranted (Citation72).
The Guide also includes some general treatment recommendations. Vaccination for influenza, pneumococcal pneumonia and pertussis was felt to be warranted based on available data and clinical practice. In addition, smoking cessation is always warranted (Citation29, Citation31, Citation33, 34). Although this is true not only for COPD patients but for all smokers, there is an incorrect but frequent attitude that COPD patients may be “hard core” and refractory to intervention. Specific recommendations for use of nicotine replacement therapy, buproprion and varenicline, all of which are approved for use to aid with cessation and have been documented to have efficacy in COPD patients (Citation73–75), were provided.
In addition, the freely available tobacco quit line, (1-800-QUITNOW), which has demonstrated efficacy (Citation76), was specifically mentioned. Additional information and links relating to smoking cessation will be provided in the App. While tobacco smoking is not the only risk factor for COPD (Citation1), no specific recommendations related to other risk factors were felt to be justified at this time. Exercise is recommended as first line therapy for all patients with symptoms and pulmonary rehabilitation in patients with SG 2/3 disease (Citation77, 78). Unfortunately, pulmonary rehabilitation is not widely available at the present time and may not be adequately covered by payors. Nevertheless, its benefits for symptomatic COPD patients are supported by the highest levels of evidence (Citation77, 78). Rehabilitation may also decrease hospitalizations and improve disease-related health status (quality of life).
Goals and next steps
The Guide was created to be a practical and easily used tool that can aid clinicians with the diagnosis and management of COPD patients. As such, it is hoped that COPD diagnosis will improve and become more accurate: unrecognized COPD patients need a proper diagnosis, and misdiagnosed COPD patients need their diagnosis corrected. Diagnosis is only part of the difficulty clinicians face in COPD management. Properly selecting a therapeutic regimen appropriate for individual patients is becoming more difficult. COPD treatment options are increasing, and novel treatments, many of which are in development, are desperately needed. However, COPD is also extremely heterogeneous, and most treatments are appropriate for subsegments of the COPD population. It is hoped the Guide will provide clinicians with a practical and easily used tool for selecting treatments appropriate for individual patients.
The Guide can have additional uses. The diagnostic and therapeutic recommendations of the Guide represent “best practice” as recognized by the COPD Foundation. As such, the Guide has the potential to be used as a benchmark to gauge COPD management. The unambiguous and easy-to-apply recommendations included in the Guide could also serve as clear performance measures and be used as a basis for quality of care assessments.
The Guide was designed to be short and extremely practical. However, dissemination and implementation of any new physician tool such as the Guide requires careful planning (Citation79). The COPD Foundation Guide to COPD Diagnosis and Management has the potential to allow the end user to feel more confident and competent in the up-to-date management of COPD and may also allow the physician to be more efficient in managing patients. Development of a smartphone version will facilitate the use of the Guide by all interested health professionals. In addition, an electronic format is greatly preferred by many physicians.
For these reasons, the COPD Foundation will develop a smartphone application. It will include all of the information that is in the print version of the COPD Pocket Consultant Guide with additional information for using the diagnostic categories and for implementing the therapeutic recommendations together with expanded descriptions of the severity domains. Hyperlinks will be provided to external resources.
Expanded topics will include: smoking cessation, oxygen therapy, management of co-morbid conditions, pulmonary rehabilitation, and management of exacerbations. It will also allow physicians to record patients’ COPD Assessment Test (CAT) or mMRC results in real-time along with spirometry values and exacerbation history to assist in determining appropriate therapy based on the Therapy Chart. The software will also be able to flag patients for whom assessment of oxygenation or CT scan would be appropriate. The full medications list will contain brief details of medications, including a hyperlink to the FDA website for additional drug information. Hyperlinks will be provided to consensus guidelines for management of COPD associated co-morbidities.
Development of an electronic version of the Guide, however, creates new and important opportunities in eHealth. It is the intention of the COPD Foundation to develop a dynamic website that will facilitate eHealth interactions. To this end, a patient smartphone application will also be developed with active links to the physician resource. Interested patients would be able to register to receive features such as incentive reminders, updates or practice “tips and tricks”. In a recent study using a BlackBerry smartphone as a daily COPD symptom diary, over 99% of daily symptoms diaries were recorded from a cohort of 100 COPD patients over a 3-year period, some of whom admitted to have never turned on a computer before (Citation80). The development of the internet-based tools, which have the potential to greatly advance the management of COPD patients, is a major commitment of the COPD Foundation.
The management of COPD has improved substantially over the last 10 years. It is hoped that even greater improvements will emerge in the years ahead. This is likely to include the development of new treatments and refined ways of utilizing current treatments. Because COPD patients have varied social and economic challenges as well as a complex and varied pattern of other medical problems, management of COPD patients requires many skills best described as the “art of medicine.”
Preparation of consensus statements and guidelines is most commonly done by experts. Individuals who are primarily clinicians are much less likely to be contributors. It is hoped that the planned blog: the COPD Foundation Management Discussion Group, will help address this. The blog is being designed to serve as a platform for the open discussion of COPD management. It will be organized around the recommendations made in the Guide. It is hoped that the discussions in the Blog will help with subsequent revisions to the Guide. Similarly, discussions in the blog will help determine which content areas in the App need revision.
It is the commitment of the COPD Foundation that the Guide and supporting activities be freely available to all clinicians. It is hoped that these resources will help clinicians provide optimal care to COPD patients.
Acknowledgments
We happily acknowledge the careful review and comments made by the following individuals in the preparation of the Guide and of this manuscript: Leigh Anderson, Joseph Auxier, Kristina Bailey, Graham Barr, Richard Casaburi, Bartolomeo Celli, Mark Ginsburg, MeiLan Han, Craig Hersh, Patricia Jellen, Salman Kahn, Ravi Kalhan, Jerry Krishnan, Oleena Lineberry, Fernando Martinez, Eduardo Mireles-Cabodevila, Ankit Nahata, Steve Nelson, Imre Noth, Jill Ohar, Amol Patil, Robert Sandhaus, Neil Schacter, Frank Sclurba, Xavier Soler, James Stoller, Austin Thompson, Gerry Turino, Robert Wise and Natalie Yip.
Declaration of Interest Statement
The COPD Foundation has received no financial support for the production or publication of this manuscript. Mockups of the Pocket Card were supported by a donation to New York-Presbyterian Healthcare System.
SR has consulted or been a member of an advisory board for Able Associates, Adelphi Research, Almirall/Prescott, APT Pharma/Britnall, Aradigm, AstraZeneca, Boehringer Ingelheim, Chiesi, CommonHealth, Consult Complete, COPDForum, DataMonitor, Decision Resources, Defined Health, Dey, Dunn Group, Eaton Associates, Equinox, Gerson, GlaxoSmithKline, Infomed, KOL Connection, M. Pankove, MedaCorp, MDRx Financial, Mpex, Novartis, Nycomed, Oriel Therapeutics, Otsuka, Pennside Partners, Pfizer (Varenicline), Pharma Ventures, Pharmaxis, Price Waterhouse, Propagate, Pulmatrix, Reckner Associates, Recruiting Resources, Roche, Schlesinger Medical, Scimed, Sudler and Hennessey, TargeGen, Theravance, UBC, Uptake Medical, VantagePoint Mgmt. He has lectured for American Thoracic Society, AstraZeneca, Boehringer Ingelheim, California Allergy Society, Creative Educational Concept, France Foundation, Information TV, Network for Continuing Ed, Novartis, Pfizer, SOMA. He has received industry-sponsored grants from AstraZeneca, Biomarck, Centocor, Mpex, Nabi, Novartis, Otsuka.
BT has served as a consultant for Boehringer Ingelheim and on advisory boards for GlaxoSmithKline, Novartis, Forest Pharmaceuticals.
JC has no financial disclosure to report.
BY has consulted for and received honoaria form Boehringer Ingelheim, Merck, Novartis and Forest Pharmaceuticals.
AM has received honoraria for CME and attending advisory board meetings for pharmaceutical companies including AstraZeneca, Boehringer Ingelheim, GlaxoSmithKlein, Merck, Novartis, Pfizer and Takeda.
SC has no financial disclosure to report.
JW has no financial disclosure to report.
DM has received honoraria/consulting fees and served on speaker bureaus for GlaxoSmithKline plc, Novartis Pharmaceuticals, Pfizer Inc., Boehringer-Ingelheim, AstraZeneca PLC, Forest Laboratories Inc., Merck, and Creative Educational Concepts. Furthermore, he has received royalties from Up-to-Date.
References
- Shapiro SD, Reilly JJJ, Rennard SI. Chronic bronchitis and emphysema. In: RJ Mason VCB, TR Martin, TE King Jr., DE Schraufnagel, JF Murray, JA Nadel, editors. Textbook of Respiratory Medicine, 5th ed. Philadelphia: Saunders, 2010; 919–967.
- Centers for disease control and prevention. Atlanta, Ga. National health interview survey. www.cdc.gov/nchs/nhis.htm
- Centers for Disease Control and Prevention. Atlanta, Ga. Behavioral risk factor surveillance system: survey data and documentation. http://www.cdc.gov/brfss/technical-infodata.htm
- Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. Chronic obstructive pulmonary disease surveillance-united states, 1999–2011. Chest 2013; in press.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ 2002; 51:1–16.
- Make B, Dutro MP, Paulose-Ram R, Marton JP, Mapel DW. Undertreatment of copd: A retrospective analysis of us managed care and medicare patients. Int J Chron Obstruct Pulmon Dis 2012; 7:1–9.
- Takahashi T, Ichinose M, Inoue H, Shirato K, Hattori T, Takishima T. Underdiagnosis and undertreatment of copd in primary care settings. Respirology 2003; 8:504–508.
- Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The national emphysema treatment trial (NETT): Part I: Lessons learned about emphysema. Am J Respir Crit Care Med 2011; 184:763–770.
- National Heart, Lung and Blood Institute, Bethesda MD. COPD: Learn more breathe better. 2013. www.nhlbi.nih.gov/health/public/lung/copd/index.htm
- Petty TL, Doherty DE. The National Lung Health Education Program: Roots, mission, future directions. Respir Care 2004; 49:678–683.
- COPDFoundation. Drive4copd. 2013.
- Fretheim A, Schunemann HJ, Oxman AD. Improving the use of research evidence in guideline development: 15. Disseminating and implementing guidelines. Health Res Pol Syst/BioMed Cent 2006;4:27.
- Fretheim A, Schunemann HJ, Oxman AD. Improving the use of research evidence in guideline development: 5. Group processes. Health Res Pol Syst/BioMed Centr 2006; 4:17.
- Fretheim A, Schunemann HJ, Oxman AD. Improving the use of research evidence in guideline development: 3. Group composition and consultation process. Health Res Pol Syst/BioMed Centr 2006; 4:15.
- Oxman AD, Fretheim A, Schunemann HJ. Improving the use of research evidence in guideline development: Introduction. Health Res Pol Syst/BioMed Central 2006; 4:12.
- Oxman AD, Schunemann HJ, Fretheim A. Improving the use of research evidence in guideline development: 16. Evaluation. Health Res Pol Syst/BioMed Cent 2006; 4:28.
- Oxman AD, Schunemann HJ, Fretheim A. Improving the use of research evidence in guideline development: 14. Reporting guidelines. Health Res Pol Syst/BioMed Cent 2006; 4:26.
- Oxman AD, Schunemann HJ, Fretheim A. Improving the use of research evidence in guideline development: 12. Incorporating considerations of equity. Health Res Pol Syst/BioMed Cent 2006; 4:24.
- Oxman AD, Schunemann HJ, Fretheim A. Improving the use of research evidence in guideline development: 8. Synthesis and presentation of evidence. Health Res Pol Syst/ BioMed Central 2006; 4:20.
- Oxman AD, Schunemann HJ, Fretheim A. Improving the use of research evidence in guideline development: 7. Deciding what evidence to include. Health Res Pol Syst/BioMed Central 2006; 4:19.
- Oxman AD, Schunemann HJ, Fretheim A. Improving the use of research evidence in guideline development: 2. Priority setting. Health Res Pol Syst/BioMed Cent 2006; 4:14.
- Schunemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 13. Applicability, transferability and adaptation. Health Res Pol Syst/BioMed Cent 2006; 4:25.
- Schunemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 10. Integrating values and consumer involvement. Health Res Pol Systems/BioMed Cent 2006; 4:22.
- Schunemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 9. Grading evidence and recommendations. Health Res Pol Syst/BioMed Cent 2006; 4:21.
- Schunemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 1. Guidelines for guidelines. Health Res Pol Syst/BioMed Central 2006; 4:13.
- Schunemann HJ, Oxman AD, Fretheim A. Improving the use of research evidence in guideline development: 6. Determining which outcomes are important. Health Res Pol Syst/BioMed Centr 2006; 4:18.
- Boyd EA, Bero LA. Improving the use of research evidence in guideline development: 4. Managing conflicts of interests. Health Res Pol Syst/BioMed Centr 2006; 4:16.
- Edejer TT. Improving the use of research evidence in guideline development: 11. Incorporating considerations of cost-effectiveness, affordability and resource implications. Health Res Pol Syst/BioMed Centr 2006; 4:23.
- http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.
- http://www.ers-education.org/pages/default.aspx?id=2005.
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with copd: A summary of the ats/ers position paper. Eur Respir J 2004;23:932-946.
- http://www.nice.org.uk/nicemedia/live/13029/49425/49425.pdf.
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schunemann H, Wedzicha W, MacDonald R, Shekelle P. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155:179–191.
- Wilson KC, Irwin RS, File TM, Jr., Schunemann HJ, Guyatt GH, Rabe KF. Reporting and publishing guidelines: Article 12 in integrating and coordinating efforts in copd guideline development. An official ATS/ERS workshop report. Proc Am Thor Soc 2012; 9:293–297.
- http://www.nice.org.uk/nicemedia/live/13029/49399/49399.pdf.
- McIvor RA, Chapman KR. The coming of age of asthma guidelines. Lancet 2008; 372:1021–1022.
- National Heart, Lung and Blood Institute, Rockville, MD. NHLBI workshop: A case-finding strategy for moderate-to-severe COPD in the United States. 2008. http://www.nhlbi.nih.gov/meetings/workshops/case-finding-exesum.htm
- National Heart, Lung and Blood Institute, Rockville, MD. NHLBI. RFA-hl-12-011: Development and testing of a case finding methodology in COPD. 2011. http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-12-011.html
- Fabbri LM, Luppi F, Beghe B, Rabe KF. Complex chronic comorbidities of copd. Eur Respir J 2008; 31:204–212.
- Fabbri LM, Boyd C, Boschetto P, Rabe KF, Buist AS, Yawn B, Leff B, Kent DM, Schunemann HJ. How to integrate multiple comorbidities in guideline development: Article 10 in integrating and coordinating efforts in copd guideline development. An official ATS/ERS workshop report. Proc Am Thorac Soc 2012; 9:274–281.
- Enright PL, Ruppel GL. Don't use the flawed fixed ratio to diagnosis copd. Respir Care 2009; 54:1500; author reply 1500.
- Guder G, Brenner S, Angermann CE, Ertl G, Held M, Sachs AP, Lammers JW, Zanen P, Hoes AW, Stork S, Rutten FH. GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study. Respir Res 2012; 13:13.
- Mannino DM, Diaz-Guzman E. Interpreting lung function data using 80% predicted and fixed thresholds identifies patients at increased risk of mortality. Chest 2012; 141:73–80.
- Garcia-Rio F, Soriano JB, Miravitlles M, Munoz L, Duran-Tauleria E, Sanchez G, Sobradillo V, Ancochea J. Overdiagnosing subjects with copd using the 0.7 fixed ratio: Correlation with a poor health-related quality of life. Chest 2011; 139:1072–1080.
- Rennard SI, Agusti A, Vestbo J. What is COPD anyway? Amer J Resp Crit Care Med (in press).
- Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, Crapo JD, Silverman EK. Clinical and radiographic predictors of GOLD-unclassified smokers in the copdgene study. Am J Respir Crit Care Med 2011; 184:57–63.
- Ferris BG. Epidemiology standardization project. Am Rev Respir Dis 1979; 118:1S–120S.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the copd assessment test. Eur Respir J 2009; 34:648–654.
- Sin DD, Golmohammadi K, Jacobs P. Cost-effectiveness of inhaled corticosteroids for chronic obstructive pulmonary disease according to disease severity. Amer J Med 2004; 116:325–331.
- Anzueto A. Impact of exacerbations on COPD. Euro Respir Rev: J Euro Respir Soc 2010; 19:113–118.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–1554.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- ZuWallach RL, Mahler DA, Reilly D, Church N, Emmett A, Rickard K, Knobil K. Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest 2001; 119:1661–1670.
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr., Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, Marchetti N, Martinez FJ, Madinger NE, McEvoy C, Niewoehner DE, Porsasz J, Price CS, Reilly J, Scanlon PD, Sciurba FC, Scharf SM, Washko GR, Woodruff PG, Anthonisen NR. Azithromycin for prevention of exacerbations of copd. N Engl J Med 2011; 365:689–698.
- Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med 2008; 178:1139–1147.
- Cogo R, Ramponi A, Scivoletto G, Rippoli R. Prophylaxis for acute exacerbations of chronic bronchitis using an antibacterial sublingual vaccine obtained through mechanical lysis: A clinical and pharmacoeconomic study. Acta Bio-medica: Atenei Parmensis 2003; 74:81–87.
- Rennard SI, Calverley PM, Goehring UM, Bredenbroker D, Martinez FJ. Reduction of exacerbations by the pde4 inhibitor roflumilast—The importance of defining different subsets of patients with COPD. Respir Res 2011; 12:18.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet 2009; 374:685–694.
- Stuart-Harris C, Bishop JM, Clark TJH, Dornhorst AC, Cotes JE, Flenley DC, Howard P, Oldham PD. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981; 1:681–686.
- Group NOTT. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Nocturnal oxygen therapy trial group. Ann Intern Med 1980; 93:391–398.
- Centers for Medicare and Medicaid Services, Baltimore, MD. National coverage determination (NCD) for home use of oxygen (240.2). 1993. http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=169&ncdver=1&NCAId=169&bc=AAAAAAAACAAAAA%3D%3D&
- Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: Recommendations for future research: An NHLBI workshop report. Am J Respir Crit Care Med 2006; 174:373–378.
- Barr RG, Berkowitz EA, Bigazzi F, Bode F, Bon J, Bowler RP, Chiles C, Crapo JD, Criner GJ, Curtis JL, Dass C, Dirksen A, Dransfield MT, Edula G, Erikkson L, Friedlander A, Galperin-Aizenberg M, Gefter WB, Gierada DS, Grenier PA, Goldin J, Han MK, Hanania NA, Hansel NN, Jacobson FL, Kauczor HU, Kinnula VL, Lipson DA, Lynch DA, MacNee W, Make BJ, Mamary AJ, Mann H, Marchetti N, Mascalchi M, McLennan G, Murphy JR, Naidich D, Nath H, Newell JD, Jr., Pistolesi M, Regan EA, Reilly JJ, Sandhaus R, Schroeder JD, Sciurba F, Shaker S, Sharafkhaneh A, Silverman EK, Steiner RM, Strange C, Sverzellati N, Tashjian JH, van Beek EJ, Washington L, Washko GR, Westney G, Wood SA, Woodruff PG. A combined pulmonary-radiology workshop for visual evaluation of COPD: Study design, chest ct findings and concordance with quantitative evaluation. COPD 2012; 9:151–159.
- Galban CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, Galban S, Rehemtulla A, Kazerooni EA, Martinez FJ, Ross BD. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nature Med 2012; 18:1711–1715.
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The national emphysema treatment trial (nett) part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011; 184:881–893.
- Society AT. Chronic bronchitis, asthma and pulmonary emphysema: A statement by the committee on diagnostic standards for nontuberculous respiratory diseases. Am Rev Respir Dis 1962; 85:762–768.
- Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the united states, 1979 to 2001. Chest 2005; 128:2005–2011.
- Barr RG, Celli BR, Mannino DM, Petty T, Rennard SI, Sciurba FC, Stoller JK, Thomashow BM, Turino GM. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Amer J Med 2009; 122:348–355.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Enright PL, Kanner RE, O'Hara P, Owens GR, Scanlon PD, Tashkin DP, Wise RA. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of fev1. JAMA 1994; 272:1497–1505.
- Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, Gonzales D, Dozier G, Patel MK, Jamerson B. Smoking cessation in patients with chronic obstructive pulmonary disease: A double-blind, placebo-controlled, randomised trial. Lancet 2001; 357:1571–1575.
- Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in patients with mild to moderate copd: A randomized controlled trial. Chest 2011; 139:591–599.
- U.S. Department of Health and Human Services. Rockville, MD. Clinical practice guideline treating tobacco use and dependence: 2008 update. 2008. http://hphc.hrsa.gov/buckets/treatingtobacco.pdf
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, Zuwallack R, Herrerias C. Pulmonary rehabilitation: Joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest 2007; 131:4S–42S.
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, Garvey C, Goldstein R, Gosselink R, Lareau S, MacIntyre N, Maltais F, Morgan M, O'Donnell D, Prefault C, Reardon J, Rochester C, Schols A, Singh S, Troosters T. American Thoracic Society/European Respiratory Society Statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173:1390–1413.
- Boulet LP, Becker A, Bowie D, Hernandez P, McIvor A, Rouleau M, Bourbeau J, Graham ID, Logan J, Legare F, Ward TF, Cowie RL, Drouin D, Harris SB, Tamblyn R, Ernst P, Tan WC, Partridge MR, Godard P, Herrerias CT, Wilson JW, Stirling L, Rozitis EB, Garvey N, Lougheed D, Labrecque M, Rea R, Holroyde MC, Fagnan D, Dorval E, Pogany L, Kaplan A, Cicutto L, Allen ML, Moraca S, FitzGerald JM, Borduas F. Implementing practice guidelines: A workshop on guidelines dissemination and implementation with a focus on asthma and COPD. Canadian respiratory journal: journal of the Canadian Thoracic Society 2006; 13 Suppl A:5–47.
- Johnston NW, Lambert K, Hussack P, Gerhardsson de Verdier M, Higenbottam T, Lewis J, Newbold P, Jenkins M, Norman GR, Coyle PV, McIvor RA. Detection of copd exacerbations and compliance with patient reported daily symptom diaries using a blackberry-based information system. Chest 2013, in press.