Abstract
Maintaining serum levels of alpha-1-proteinase inhibitor (A1PI) >11 μM by augmentation with plasma-derived human A1PI is currently the only specific therapy available to treat patients with the genetic deficiency of A1PI. In this study, a new, high-purity (≥90% A1PI in monomeric form), ready-to-use, liquid formulation of A1PI—GLASSIA (Kamada, Ness Ziona, Israel) was compared to PROLASTINÆ (Talecris, Research Triangle Park, NC, now Grifols), both commercially available, FDA-approved products. This multicenter, double-blind, randomized controlled trial with partial cross-over was designed to test the non-inferiority and safety of GLASSIA compared to PROLASTIN, assessing both antigenic and functional A1PI trough levels in subject serum. Non-inferiority of GLASSIA to PROLASTIN was demonstrated by remaining within the lower bounds of the confidence intervals (≤3 μM) for both antigenic and functional A1PI. The study concluded that GLASSIA, a new liquid, ready to use, formulation of A1PI, was not inferior to PROLASTIN and it was well tolerated with a safety profile comparable to PROLASTIN.
Introduction
Alpha-1-proteinase inhibitor (A1PI) inhibits neutrophil elastase (NE) and is an enzyme implicated in the pathogenesis of emphysema (Citation1, 2). NE degrades elastin as well as basement membrane and other matrix components (Citation3). A1PI binds to and inhibits NE providing protection against excess proteolytic activity, thus maintaining the proteinase-anti-proteinase balance. An imbalance between proteinase and anti-proteinase in the lungs leads to alveolar destruction that results in premature development of emphysema and a rapid decline of lung function, as measured by forced expiratory volume in 1 second (FEV1)(Citation4). More recent evidence suggests that A1PI may also help prevent the destruction of alveolar walls (Citation5). A1PI reaches the lungs through diffusion of circulating A1PI and, to a lesser extent, local production by macrophages and bronchial cells (Citation6).
A1PI deficiency, also known as alpha-1-antitrypsin deficiency (AATD), or Alpha-1, is a hereditary disorder that results in serum concentrations of A1PI below 11 μM. Low levels of A1PI may predispose individuals to early development of emphysema, which in turn is exacerbated by smoking or environmental influences such as dust and pollution (Citation5). Although A1PI deficiency is uncommon, it is not considered a rare disease. It is estimated that A1PI deficiency affects ∼70,000 to 100,000 individuals in the United States and a similar number in Europe, making A1PI deficiency one of the most common serious genetic disorders in the world (Citation7). However, due to a lack of awareness and misdiagnosis, the actual number of patients identified is much lower and is reported to be several thousand in the United States and Europe. The biology, genetics, epidemiology, clinical manifestations, and disease impact of A1PI deficiency have been well reviewed (Citation8–12).
The only currently available treatment for A1PI deficiency is augmentation with plasma-derived, human A1PI, which has been available as an FDA-approved therapy based on biochemical efficacy for over 20 years (Citation13). The goal of augmentation is to increase the levels of A1PI in the blood and lungs, and to correct the deficiency state to levels that may prevent further lung damage and slow or halt disease progression (Citation14). Wewers et al. found that disease progression increases when serum levels fall below 11 μM (Citation15). Weekly infusions of A1PI at 60 mg/kg raise serum and alveolar concentrations to predicted historical or target levels of at least 11 μM in serum (Citation3).
The rationale for the use of A1PI is described in several biochemical efficacy studies (Citation13, Citation16–18). A preponderance of evidence has supported the benefits of augmentation (Citation19) although controversy over clinical efficacy and cost-benefit continues (Citation20–22). Several guidelines and Standards of Care—American Thoracic Society/ European Respiratory Society (ATS/ERS), Canadian Thoracic Society, American College of Chest Physicians, American Association for Respiratory Care—endorse the use of A1PI for the treatment of patients with A1PI deficiency (Citation3, Citation10, Citation23). The ATS/ERS standards document recommends intravenous (IV) augmentation for individuals with AATD and emphysema (Citation3).
At the time of the current study, there were 5 FDA-approved preparations of A1PI sourced from pooled human plasma (). Non-inferiority of PROLASTIN®-C, ARALAST, ARALAST NP and Zemaira® to PROLASTIN have been previously shown (Citation24–28). However, differing product manufacturing processes may result in purity variations.
Table 1. FDA-approved preparations of alpha1-proteinase inhibitor (human) at study time
This phase 2/3 study was designed to evaluate the non-inferiority of GLASSIA (Kamada, Ness Ziona, Israel) compared with PROLASTIN (Talecris Biotherapeutics, now Grifols, Research Triangle Park, NC) an FDA-approved, commercially available A1PI product. This study investigated antigenic and functional pharmacokinetics (PK) of A1PI in serum as well as product safety.
Methods
Study Drugs
GLASSIA, the test drug, (Alpha1-Proteinase Inhibitor, (Human) intravenous) is a sterile, ready-to-use, stabilizer-free, preservative-free, liquid preparation of purified 2% active A1PI in a phosphate-buffered saline solution. GLASSIA is prepared from Cohn Fraction IV of human plasma obtained from US-licensed collection centers by a modified version of the cold ethanol fractionation process. GLASSIA is then purified using specialty chromatographic methods. Not less than 90% of the A1PI in GLASSIA is of the monomeric form as measured by size-exclusion chromatography.
To further reduce the theoretical risk of viral transmission, the manufacturing process for GLASSIA includes nanofiltration through a 15-nm filter and a Solvent/Detergent treatment. Individual plasma units used for production of GLASSIA are tested for hepatitis B surface antigen (HBsAg), and for antibodies to hepatitis C virus (HCV) and human immunodeficiency virus types 1 and 2 (HIV-1/2). Each plasma unit used in the manufacturing process must be non-reactive (negative) in all tests. Furthermore, plasma used in the GLASSIA production process is also tested for parvovirus B19.
PROLASTIN, the comparator drug, is an FDA-approved commercially available lyophilized A1PI product available in the United States since 1987. PROLASTIN®-C was not available at the time of this study.
Study Design
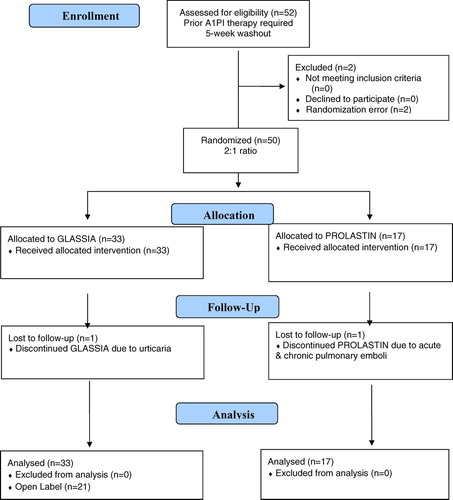
A double-blind, randomized, controlled, 2-arm, multi-center trial with a partial cross-over () (NCT00460096) was conducted at 3 sites: National Jewish Health, Denver, Colorado, the University of Texas Health Science Center at Tyler, Tyler, Texas, and the University of Florida School of Medicine, Gainesville, FL. Subjects who met inclusion and exclusion criteria (n = 50) were randomly assigned in a 2:1 ratio to receive either GLASSIA (study drug) or PROLASTIN (active comparator), respectively, for a 12-week double-blind phase with an open-label extension during Weeks 13 to 24, during which time all subjects received GLASSIA. Subjects who were on any A1PI therapy prior to the study required a washout period of 5 weeks before study initiation.
All subjects participating in the study underwent baseline testing that included: vital signs, recording of medical history, physical examination, pregnancy test for females, and blood draws for hematological and biochemical analyses, complement consumption (C3, C4), plasma A1PI level and virology. Post-bronchodilator spirometry, which included FEV1 and FVC, chest x-ray and an ECG, was also performed at baseline. GLASSIA and PROLASTIN was administered by IV infusion at a protocol-defined rate of 0.08 mL/kg per min at a dose of 60 mg/kg body weight by study staff either at the study site or at home. The infusion rate was increased or decreased at the discretion of the investigator as clinically indicated.
Prior to administration, PROLASTIN was reconstituted with sterile water for injection, supplied by the manufacturer and in accordance with the product insert instructions. GLASSIA was supplied as a liquid in ready-to-use 50-mL glass vials containing approximately 2% active A1PI. The 2 products were prepared for infusion by a specialty pharmacy in close proximity to the subject's home or at the study site pharmacy, and prepared in an opaque infusion bag in order to maintain the blind.
There were 4 primary objectives of this study: 1) demonstrate that the PK of antigenic and/or functional GLASSIA were not inferior to those of PROLASTIN as defined by trough levels of A1PI, 2) determine the efficacy of GLASSIA in maintaining antigenic and/or functional plasma levels of at least 11 μM, 3) compare A1PI trough levels (antigenic and functional) over Weeks 7 to 12 (6 infusions), and 4) demonstrate that the safety profile of GLASSIA was not inferior to PROLASTIN.
This study was performed in compliance with the Declaration of Helsinki (2000) and the International Conference on Harmonization (ICH) Guideline for Good Clinical Practice (1996). All investigative sites received institutional review board approval and all subjects signed informed consent prior to study enrollment.
Subject Population
Inclusion Criteria
Subjects 18 years of age or older with evidence of lung disease related to AATD and ìat-riskî alleles (null alleles and deficiency alleles) associated with A1PI plasma levels <11 μM were eligible for inclusion. Subjects were required to have evidence of lung disease related to A1PI deficiency identified by at least 1 of the following: a) post-bronchodilator FEV1 <80% predicted; or b) loss of lung function over a 1-year period of greater than 35 mL in FEV1; or c) evidence of pulmonary emphysema by high-resolution computed tomography. All study participants (male and female) were required to use an effective means of contraception.
Exclusion Criteria
Subjects excluded from the study met at least 1 of the following criteria: laboratory evidence of severe IgA deficiency (from medical history or by IgA testing at screening showing IgA levels less than 20% of the lower limit of normal); a history of smoking within the past 3 months or current smokers; a history of allergy to plasma proteins; participation in another experimental drug or device trial within the past 30 days; evidence of uncontrolled hypertension or pulse ≥120/min; any abnormal laboratory measurements at the screening or baseline visit that might be a safety issue; a current life-threatening malignancy; a previous organ transplant; a history of hepatitis C, hepatitis B, and/or HIV 1 or 2 infection; an acute respiratory tract infection or COPD exacerbation that required antibiotic and/or systemic steroid treatment within the previous 6 weeks; or were pregnant or nursing.
Plasma for measurement of antigenic and functional A1PI levels was drawn at baseline and weekly from Week 2 through Week 24 and analyzed at the University of Florida, Alpha-1 Research Program (Gainesville, FL).
Statistical Methods
The minimal evaluable sample size of 45 for this study was calculated using nQuery Advisor® (Version 2) assuming a non-inferiority comparison of GLASSIA to PROLASTIN. GLASSIA non-inferiority was defined as a mean antigenic and/or functional A1PI trough level ≤3 μM below that of PROLASTIN a value suggested by the FDA and used in all previous comparator studies. The comparison of the 2 treatment groups was based on the lower limit of a 2-sided 95% Confidence Interval (CI) for the difference in means (GLASSIA minus PROLASTIN). If the lower bound of this A1PI interval was ≤3 μM, then the goal of demonstrating non-inferiority was achieved.
This method is formally equivalent to a test of the null hypothesis—the difference between the average trough A1PI level in the GLASSIA group and that of the PROLASTIN group was ≤3 μM, against the alternative hypothesis that the difference was >3 μM. The CI was derived from a 2-group Wilcoxon rank-sum test. This test was inverted to provide the 95% CI used to assess non-inferiority. The 95% exact binomial lower confidence bounds were estimated for the proportion of subjects in each treatment group for whom the mean trough antigenic A1PI for Weeks 7 to12 was in excess of 11 μM.
The intent-to-treat (ITT) population included all subjects assigned to the study regardless of the treatment or amount of treatment received. Safety analysis included all subjects administered at least 1 dose of A1PI. Adverse events (AEs) were recorded from the time of signing the informed consent until study completion (Week 28). Subjects were monitored for AEs during and after each infusion. Safety was also assessed by clinically significant changes in vital signs (respiration rate, body temperature, blood pressure, and/or pulse rate), and clinically meaningful changes in laboratory assessments or radiological assessments.
The incidence of GLASSIA and PROLASTIN AEs and frequencies of potentially clinically significant findings were compared using either the chi-square or Fisher's exact tests, depending upon the size of the frequencies, and the overall rate of AEs. The potential for immunogenicity (antibody formation to A1PI) was assessed by C3 and C4 complement consumption at Baseline, Week 12 and Week 24; subject samples were analyzed at the Mayo Central Labs for Clinical Trials, Rochester, MN. The PK evaluable population included all subjects in the ITT population who received the full dose of study medication at each dose administration, and had at least 1 evaluable trough level beyond Week 6.
Results
Subject Characteristics and Drug Administration
All 50 subjects met one or more of the inclusion criteria for evidence of lung disease related to AATD. Although the specific entry criterion met was not collected, an evaluation of the data revealed that all subjects had an FEV1 of less than 80% predicted and therefore, met at least one of these criteria. Of the 50 subjects assigned randomly to the study, 33 were administered GLASSIA and 17 were administered PROLASTIN. The 2 treatment groups had similar median days of drug exposure representing 24 total infusions per subject, 12 during the 12-week blinded phase and 12 during the 12-week open-label phase.
Most (98%) patients receiving GLASSIA, during both treatment periods, received at least 1 infusion at a rate of 0.08 mL/kg per min, which is the commonly used infusion rate by other approved A1PI products in the United States (Citation29). About half (52.0%) of the patients received at least 1 infusion at a rate higher than 0.08 mL/kg per min. About half (50.7%) of the patients received at least 1 infusion at a rate lower than 0.08 mL/kg per min. This infusion rate included approximate drug infusion time (∼40 to 45 min) and the typical 50 mL saline flush after infusion. Two subjects were withdrawn early from the study due to AEs (1 in the GLASSIA group due to possibly treatment-related urticaria; the other in the PROLASTIN group due to acute and chronic non-treatment-related pulmonary emboli). Forty-eight subjects completed the entire study through Week 28.
Subject demographics were similar among the groups (). The majority of subjects were phenotype ZZ—GLASSIA, 28 (84.8%); PROLASTIN, 15 (88.2%) (). There were no clinically significant differences in baseline characteristics between the groups. Mean FEV1 values and% predicted FEV1 were similar between Baseline, Week 12 and Week 24 in both treatment groups. A similar result was found for the FVC values. There were no meaningful differences in prior medications with the most commonly used being Advair®, albuterol, oxygen, Spiriva® and multivitamins.
Table 2. Patient demographics
Table 3. Baseline characteristics
Non-Inferiority as Defined by Trough Levels of Antigenic and Functional A1PI
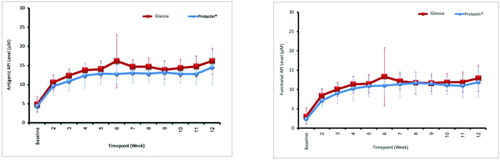
The proportion of subjects with mean trough antigenic A1PI levels exceeding 11 μM during Weeks 7 to 12 was 100% for subjects in the GLASSIA group and 75% for subjects in the PROLASTIN group (81.3% reported by FDA when calculated for the Summary Basis for Regulatory Action, June 14, 2010). Non-inferiority of GLASSIA compared with PROLASTIN was demonstrated by the lower bounds of the confidence intervals being ≤ 3 μM for both antigenic and functional A1PI (). The median antigenic A1PI values for Weeks 7 to 12 were: GLASSIA 14.5 μM (range 11.6 to 18.5 μM) and PROLASTIN 12.6 μM (range 10.4 to 19.2 μM) (). The median functional A1PI values for Weeks 7 to 12 were: GLASSIA 11.8 μM (range 8.2 to 16.9 μM) and PROLASTIN 11.2 μM (range 7.7 to 18.0 μM).
Table 4. Non-inferiority; Trough circulating levels of antigenic and functional A1PI (average of weeks 7 to 12)
Safety
Adverse Events
Out of 50 subjects, 49 experienced at least 1 AE with the majority being mild or moderate in severity and not related to study drug. Four subjects experienced serious AEs (SAEs) during the course of the study, all categorized as unrelated to study drug. Three occurred following dosing (GLASSIA: endoscopic retrograde cholangiopancreatography and COPD exacerbation, PROLASTIN: pulmonary emboli) and 1 prior to dosing (GLASSIA: pneumothorax). There were no deaths. Two subjects were withdrawn early; 1 due to urticaria (GLASSIA: categorized as possibly treatment-related) and 1 due to pulmonary emboli (PROLASTIN: categorized as unrelated to study drug). The most commonly reported AEs occurring during both phases in ≥10% of subjects were respiratory (cough, COPD exacerbation, upper respiratory tract infection, nasopharyngitis), and there were no clinically significant differences in AEs between treatment groups ().
Table 5. Adverse events occurring in ≥ 10% of patients
During Treatment Period 1 (the blinded phase), the most common AEs ≥5% and considered related to study drug were headache—3 (9%) subjects in the GLASSIA group and 1 (6%) subject in the PROLASTIN group—and hypertension—1 subject in each group; 3% and 6%, respectively. In Treatment Period 2 (open-label phase), there were 6 subjects with possibly related AEs (1 subject each with urticaria, influenza-like illness, decreased platelet count, joint swelling, dizziness and rash, and lethargy. Infusion-related events were similar in both groups and managed by rate reductions.
Clinical and Laboratory Evaluations
There were no clinically meaningful changes in mean hematology values over time in either treatment group during either treatment period, and shifts in hematology parameters from baseline were not clinically meaningful.
Immunogenicity Reactions
Complement consumption values remained relatively stable throughout the study period and there were no statistically significant differences in mean C3 or C4 consumption between treatment groups.
Discussion
This randomized, controlled, multicenter study compared the biochemical efficacy and safety of GLASSIA, a new liquid, ready-to-use, stabilizer-free, preservative-free form of A1PI, with that of PROLASTIN in patients with A1PI deficiency. Non-inferiority of GLASSIA compared with PROLASTIN was established based on antigenic and functional trough A1PI serum levels and similar safety profiles. This study also demonstrated that weekly infusions of GLASSIA administered at 60 mg/kg were sufficient to maintain the serum A1PI (trough) level at greater than 11 μM, a target level generally accepted in A1PI augmentation.
Several clinical studies have demonstrated non-inferiority when comparing the effectiveness of various A1PI products with each other. The non-inferiority of ARALAST (Baxter Healthcare Corporation), compared with PROLASTIN was previously demonstrated by measuring the ratio of mean trough A1PI serum levels of ARALAST (n = 14)/PROLASTIN (n = 14), which was 0.905 (CI exceeding criterion of 0.80; p = 0.026) at Weeks 8 to 11 with a slope exceeding -0.01 over Weeks 11 to 23 (Citation26). In a 10-week multicenter, randomized (2:1) controlled study, Stocks et al. demonstrated the non-inferiority of Zemaira (CSL-Behring) compared with PROLASTIN, showing that steady-state trough serum A1PI levels achieved by weekly infusion were similar (Citation28). Mean trough serum antigenic A1PI levels of the 2 groups (Zemaira, n = 30; PROLASTIN, n = 14) were within 3 μM. Stocks, et al demonstrated the non-inferiority of PROLASTIN-C (Talecris Biotherapeutics, now Grifols), a new concentrated form of alpha1-proteinase inhibitor (human), compared with PROLASTIN in an 8-week multicenter, randomized, double-blind cross-over study measuring the mean plasma A1PI concentration versus time (Citation25).
The mean AUC0-7 days was 155.9 mg*h/mL for PROLASTIN-C (n = 23) and 152.4 mg*h/mL for PROLASTIN (n = 22) creating nearly superimposable concentration versus time curves. All A1PI (human) non-inferiority studies to date have demonstrated that the tested products were able to maintain weekly trough serum antigenic A1PI levels above the predicted historical or target levels of 11 μM. Consistent with these previously conducted non-inferiority studies of A1PI, this study demonstrated the non-inferiority of GLASSIA compared with PROLASTIN.
A majority of patients studied were of phenotype ZZ, the most commonly identified severely deficient phenotype of A1PI deficiency with the potential to develop both liver disease and emphysema.
Although the effectiveness of A1PI has not been evaluated with a well-powered, prospective, randomized, placebo-controlled study, several observational and case control studies have demonstrated improved survival or slowed rate of emphysema progression in this population (Citation16). Some members of the international medical community appreciate the long-term benefits that A1PI augmentation may offer while others question the clinical efficacy and advisability of augmentation therapy (Citation13, Citation19). Some theoretical and biochemical evidence support the use of A1PI in the treatment of A1PI deficiency.
A meta-analysis of 5 long-term (greater than 1 year) controlled studies with a total of 1509 patients concluded that A1PI augmentation slowed the progressive decline in FEV1 seen in patients with A1PI deficiency (Citation16). Longitudinal data from the Alpha One International Registry demonstrated that patients receiving A1PI augmentation have a lower mortality rate compared with patients never treated, as well as a slower FEV1 decline (Citation30). Physician recommendations and patient educational material stress that augmentation therapy is not a cure and cannot restore lost lung function. Further clinical evidence in placebo-controlled trials is needed to confirm the clinical benefits of A1PI therapy.
More than 2 decades of experience with A1PI products in several thousand patients in the United States suggests that this therapy is safe and generally well tolerated. Adverse reactions to A1PI augmentation are rare (0.03 events per patient-month) and are generally mild (Citation31). AEs vary by product, patient characteristics, and infusion rate. Sporadic cases of anaphylaxis have been reported (Citation10).
A similar, low incidence of AEs was reported in this study. The safety profile of GLASSIA was similar to that of the active comparator, PROLASTIN. There were no clinically meaningful differences in AEs between treatment groups or between treatment periods. The majority of AEs were of mild or moderate intensity. GLASSIA was well tolerated and demonstrated an acceptable safety profile in this patient population.
Conclusion
This study demonstrated the biochemical non-inferiority of GLASSIA compared with PROLASTIN based on antigenic and functional trough A1PI levels. All of the AATD patients who received GLASSIA were able to maintain median trough antigenic A1PI levels > 11 μM. GLASSIA was well tolerated in this patient population with a safety profile that was both acceptable and similar to PROLASTIN.
Declaration of Interest Statement
Dr. Sandhaus has received grants from the Alpha-1 Foundation and the National Institutes of Health. He has been an investigator, consultant, and/or advisor to Kamada Ltd., CSL Behring, AstraZeneca, Grifols (formerly Talecris Biotherapeutics), Pfizer, Intrexon and Johnson & Johnson. Dr. Stocks has received grants from the National Institutes of Health and has been an investigator/consultant/speaker for Grifols (formerly Talecris Biotherapeutics), CSL Behring, Baxter Healthcare Corporation, and Kamada Ltd. Dr. Rouhani has no conflicts. Dr. Brantly has received grants from the Alpha-1 Foundation and the National Institutes of Health. Dr. Brantly is currently an investigator for Kamada Ltd. and Grifols (formerly Talecris Biologics). Pnina Strauss, is the Senior Director Clinical Development at Kamada, Ltd, Israel.
This study was sponsored by Kamada Ltd, Israel. Baxter Healthcare Corporation provided funding for editorial support. The authors did not receive compensation related to the development of this manuscript. Authors are fully responsible for all content and editorial decisions and meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. Writing and editorial assistance was provided by Barbara Rinehart, MS of ReSearch Pharmaceutical Services, Inc., which was contracted by Baxter Healthcare Corporation.
Acknowledgments
The authors thank Neil Inhaber, MD, and Maureen Finnerty, RN, MSN, for helpful discussion and critical review of this manuscript.
References
- Eriksson S. Alpha1-antitrypsin deficiency: lessons learned from the bedside to the gene and back again. Chest 1989; 95:181–189.
- Tobin MJ, Cook PJ, Hutchinson DC. Alpha 1 antitrypsin deficiency: the clinical and physiological features of pulmonary emphysema in patients homozygous for Pi type Z. A survey by the British Thoracic Association. Br J Dis Chest 1983; 77(1):14–27.
- American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003; 168:818–900.
- Cole RB, Nevin NC, Blundell G, Relation of alpha-1-antitrypsin phenotype to the performance of pulmonary function tests and to the prevalence of respiratory illness in a working population. Thorax 1976; 31(2):149–157.
- Petrache I, Fijalkowska I, Zhen L, A novel antiapoptotic role for alpha-1antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med 2006; 173:1222–1228.
- Sandhaus RA. Elastase may play a central role in the neutrophil migration through connective tissue. In: Taylor JC, Mittman C, eds. Pulmonary emphysema and proteolysis. Orlando, FL: Academic Press, 1997:227–233.
- de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest 2002; 122(5): 1818–1829.
- World Health Organization. Alpha-1-antitrypsin deficiency. Memorandum from a WHO meeting. Bull WHO 1997; 75:397–415.
- Dickens JA, Lomas DA. Why has it been so difficult to prove the efficacy of alpha-1-antitrypsin replacement therapy? Insights from the study of disease pathogenesis. Drug Des Devel Ther 2011; 5:391–405.
- Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet 2005; 365(9478):2225–2236.
- Stolk J, Seersholm N, Kalsheker N. Alpha-1-antitrypsin deficiency: current perspective on research, diagnosis, and management. Int J COPD 2006; 1(2):151–160.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009 Jun 25; 360(26):2749–2757.
- Sandhaus RA. Augmentation therapy in alpha-1 antitrypsin deficiency. COPD J 2009 Jun; 6(3):147–148.
- Petrache I, Hajjar J, Campos M. Safety and efficacy of alpha-1-antitrypsin augmentation therapy in the treatment of patients with alpha-1-antitrypsin deficiency. Biologics 2009; 3:193–204.
- Wewers MD, Casolaro MA, Sellers SE, Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med 1987; 316(17):1055–1062.
- Chapman KR, Stockley RA, Dawkins C, Wilkes MM, Navickis RJ. Augmentation therapy for alpha1 antitrypsin deficiency: a meta-analysis. COPD J 2009 Jun; 6(3):177–184.
- Stockley RA, Parr DG, Piitulainen E, Stolk J, Stoel BC, Dirksen A. Therapeutic efficacy of alpha-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res 2010 Oct 5; 11:136.
- Tonelli AR, Rouhani F, Li N, Schreck P, Brantly ML. Alpha-1-antitrypsin augmentation therapy in deficient individuals enrolled in the Alpha-1 Foundation DNA and Tissue Bank. Int J Chron Obstruct Pulmon Dis 2009; 4:443–452.
- Sandhaus RA. Alpha-1 antitrypsin deficiency: whom to test, whom to treat? Semin Respir Crit Care Med 2010 Jun; 31(3):343–347.
- Gøtzsche PC, Johansen HK. Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease. Cochrane Database Syst Rev 2010a Jul 7; (7): CD007851.
- Gøtzsche PC, Johansen HK. Intravenous alpha-1 antitrypsin augmentation therapy: systematic review. Dan Med Bull 2010b Sep; 57(9):A4175.
- Russi EW. Alpha-1 antitrypsin: now available, but do we need it? Swiss Med Wkly 2008; 138(13–14):191–196.
- Abboud RT, Ford GT, Chapman KR; Standards Committee of the Canadian Thoracic Society. Alpha1-antitrypsin deficiency: a position statement of the Canadian Thoracic Society. Can Respir J 2001; 8(2):81–88.
- ARALAST NP Prescribing Information. Baxter Healthcare Corporation. Westlakes Village, CA, 2010. http://www.baxter.com/downloads/healthcare_professionals/products/Aralast_NP_PI.pdf
- Stocks JM, Brantly ML, Wang-Smith L, Campos MA, Chapman KR, Kueppers F, Pharmacokinetic comparability of ROLASTIN®-C to PROLASTIN® in alpha1-antitrypsin deficiency: a randomized study. BMC Clin Pharmacol 2010; 10:13.
- Stoller JK, Rouhani F, Brantly M, Biochemical efficacy and safety of a new pooled human plasma alpha(1)-antitrypsin, Respitin. Chest 2002; 122:66–74.
- Louie SG, Sclar DA, Gill MA. ARALAST: a new alpha1-protease inhibitor for treatment of alpha-antitrypsin deficiency. Ann Pharmacother 2005; 39(11):1861–1869.
- Stocks JM, Brantly M, Pollock D, Barker A, Kueppers F, Strange C, Donohue JF, Sandhaus R. Multi-center study: the biochemical efficacy, safety and tolerability of a new alpha1-proteinase inhibitor, Zemaira. COPD J 2006; 3:17–23.
- Healthcare Providers. Augmentation Therapy. http://www.alpha-1foundation.org/healthcare/?c = 03a-Augmentation-Therapy. Accessed 3/15/2012.
- Luisetti M, Miravitlles M, Stockley RA. α-1-antitrypsin deficiency: a report from the 2nd meeting of the Alpha One International Registry, Rapallo (Genoa, Italy), 2001. Eur Respir J 2002; 20:1050–1056.
- Stoller JK, Fallat R, Schluchter MD, O'Brien RG, Connor JT, Gross N, O'Neil K, Sandhaus R, Crystal RG. Augmentation therapy with alpha1-antitrypsin: patterns of use and adverse events. Chest 2003 May; 123(5):1425–1434.
- Stoller JK, Aboussouan LS. alpha1-Antitrypsin deficiency. 5: intravenous augmentation therapy: current understanding. Thorax 2004 Aug; 59(8):708–712.