The use of computed tomography (CT) scanning to assess emphysema has exploded since the mid-1980s. Since the definition of emphysema, permanent enlargement of airspaces and destruction of alveolar walls (Citation1) is a structural definition and pathologic examination of tissue is limited to post-mortem or resected specimens, CT has been become very popular because it allows investigators and clinicians to obtain useful, relatively non-invasive structural information about the lung (Citation2–4). The exquisite anatomic information that CT provides has resulted in a proliferation of CT devices throughout the world such that virtually every hospital and research organization in the developed world now has easy access to a CT scanner. The end result is that almost all investigators have access to high quality images of the lung for clinical and research purposes. Another important advantage of CT is that, unlike clinical or physiological measurements of airflow limitation, it can differentiate between the contributions of emphysema and airways disease—the two underlying phenotypes in patients with chronic obstructive pulmonary disease (COPD).
Although routine clinical assessment of emphysema is typically performed using visual grading, the inter- and intra-observer variability that is associated with subjective visual scoring can be very high (Citation5, 6). Importantly, since CT images are densitometry maps of the lung, attention quickly turned to computer aided assessments of CT densitometry (). Early studies demonstrated that CT lung density measurements were abnormally low in patients with pulmonary emphysema (Citation7) and that these low attenuation regions corresponded to regions of emphysematous destruction using both visual (Citation8) and pathological scoring (Citation9, 10).
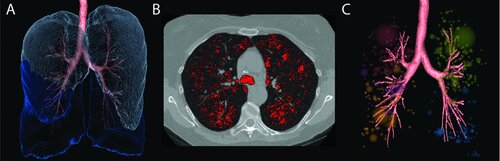
Figure 1. Quantitative CT measurements of emphysema. A three-dimensional rendering of the lung registered to the three dimensional reconstruction of the airway tree (A), two-dimensional axial CT image with the CT densitometry values below −950 HU highlighted in red (B), and the three-dimensional low attenuation cluster analysis of emphysema with colored regions represented as connected regions of the lung with CT densitometry values below −950 HU registered to the three-dimensional reconstruction of the airway tree (C) for a COPD subject. Images A and C were generated using Apollo image analysis software (VIDA Diagnostics, Coralville, IA) and Image B was generated using MATLAB R2007b (The Mathworks, Natick, MA).
Lung density masks, first introduced by Müller and colleagues, showed that CT densitometry values below a given threshold value (originally defined as −910 Hounsfield Units (HU)) correlated with quantitative pathology (Citation11). Over the subsequent years this threshold analysis has been redefined using modern CT scanners and techniques resulting in the redefinition of the threshold density for thin slice high resolution CT scans to be −950 HU (Citation3) and for multi-slice CT scans to be −960 HU (Citation12). However, the −960 HU threshold for multi-slice CT scans has not gained traction in the literature.
Another well-established densitometry approach uses fixed percentiles of the frequency distribution of Hounsfield units. Gould and colleagues (Citation13) showed that the lowest 5th percentile of x-ray attenuation values correlated with distal airspace size taken from the same lateral regions of resected lung∕lobe in patients that underwent resection for a peripheral lung tumor. More recently, a study by Dirksen and colleagues (Citation14) in patients with severe alpha-1 antitrypsin deficiency concluded that the decline in the density of any percentile in the 10th to 30th range (corresponding to densities ranging from −950 to −890 HU) may provide a sensitive measure of emphysema progression. This has led to the promotion of the lowest 15th percentile as a measurement of emphysema and emphysema progression.
Finally, there have been other techniques introduced to quantify emphysema that take into account not just how many low attenuating voxels are present, but how these low attenuation regions are clustered together to create a metric that measures the size and spatial distribution of emphysema on the CT scan (Citation15). It is thought that since the radiologist considers several factors in the assessment of emphysema, including the type, distribution, and the potential cause of the low attenuation (i.e. small airways disease, gas trapping or image noise), a quantitative approach that also includes an assessment of emphysematous hole, or cluster, size may improve the detection and quantification of emphysema (Citation16, 17). There are data that suggest that a low attenuation cluster analysis does correlate better with radiologist scoring (Citation16) suggesting that there is more to emphysema analysis than just finding low attenuation regions, and it is how these regions are spatially located that may provide further information. Analyses that take into account these different features of low attenuating regions are often called “texture” measures and because these analyses are performed by computers they are very reproducible and have even been shown to be more sensitive at detecting early disease than radiologists (Citation18).
Obviously there are many factors that affect the CT density of the lung including the level of inspiration during breath-hold scanning, the radiation dose (Citation19), slice thickness (Citation19) and the choice of reconstruction algorithm (Citation20), which has led to many recommendations on standardization for both serial investigations and multi-center studies (Citation21). Although it seems that there are many issues with the quantification of emphysema on CT scans, the research community has become very sensitive to the above variables and are now carefully controlling for them in clinical studies. The Radiological Society of North America, recognizing that quantitative image biomarkers must be standardized, has instituted a working group within their Quantitative Imaging Biomarkers Alliance (QIBA) to define the standard operating procedures and best practices for quantitative imaging of COPD and asthma (Citation22).
Although it is important for imaging measurements to be highly reproducible and significantly correlated with pathology and physiological parameters, a potential imaging biomarker must also be significantly related to clinically meaningful outcomes in COPD, including mortality, disease progression, exacerbations and treatment response. In this regard, CT measures of the extent of emphysema have been shown to be an independent predictor of all-cause mortality in a large community-based cohort of subjects with and without COPD (Citation23).
Other studies evaluating COPD subjects over time have demonstrated that emphysema severity evaluated using CT was independently associated with FEV1 decline (Citation24–26) and several circulating blood biomarkers of inflammation were ide ntified that correlated significantly with CT measurements of emphysema progression (Citation26).
Exacerbations of COPD are also being increasingly recognized as an important burden both to the patients as well as society. In the United States alone, in 2000, COPD was responsible for 8 million physician office and hospital outpatient visits, 1.5 million emergency department visits and 726,000 hospitalizations (Citation27). Moreover, a large number of patients that receive hospital-based care for COPD exacerbations are readmitted within a short period of time (Citation28) and therefore there is increased motivation to identify those patients that are more susceptible to exacerbations. Furthermore, exacerbations have also been shown to be correlated with a more rapid rate of emphysema decline in COPD subjects (Citation29). However, it has been shown that COPD disease severity determined by spirometry-based criteria may not always be adequate for identifying patients at risk for exacerbations (Citation30) and therefore there has been growing interest in phenotyping patients based on the underlying contributions of airways disease and emphysema.
Han and colleagues (Citation31) showed that the frequency of COPD exacerbations was related to both emphysema and airways disease, and that for subjects with greater emphysema severity (defined as the percentage of all lung voxels with CT attenuation less than −950 HU) there was a 1.18-fold increase in exacerbation frequency for each 5% increase in emphysema.
Another important area where CT measurements have been used is for identifying patients or subgroups of patients who may demonstrate improvements following lung volume reduction surgery (LVRS) or bronchial valve treatment for severe emphysema or pharmacologic intervention. The multi-center, randomized controlled clinical trial performed by the National Emphysema Treatment Trial (NETT) Research Group followed patients with severe emphysema that underwent LVRS (Citation32) and showed that patients identified as having predominantly upper-lobe emphysema, determined using a visual scoring system, and a low maximal workload after rehabilitation had a lower risk of morality than patients that were not randomized to the surgery group. Furthermore, this group was also more likely to have an improvement in exercise capacity and an improvement in symptoms following surgery. CT measurements of emphysematous hole size obtained before LVRS noted that large holes located in the upper lobes were significantly correlated with maximal cardiopulmonary exercise performance and physiologic measures before and 3 months after LVRS (Citation33).
Since the NETT study, minimally invasive techniques for lung volume reduction have been proposed, such as the insertion of one-way values. These studies have used CT to assess lung volume changes longitudinally following valve implantation and have demonstrated significant reductions in the volume of the treated lobe, although there were no changes in global measurements such as FEV1 (Citation34). Moreover, the data also showed that CT volume reduction in the treated lobe was significantly correlated with clinically meaningful improvements in symptom scores. The large randomized, prospective, multicenter Endobronchial Valve for Emphysema Palliation Trial (VENT) also evaluated the efficacy of endobronchial valves and demonstrated that greater heterogeneity of emphysema between lobes and intact interlobar fissures on CT were related to clinically important functional and physiological changes following therapy (Citation35).
Finally, studies using CT measurements have been shown to be more sensitive than FEV1 for detecting disease progression (Citation36), and as a result, new trials have adopted CT as a primary outcome measure for evaluating the therapeutic effect of augmentation therapy in subjects with apha-1 antitrypsin deficiency (Citation37, 38).
In summary, CT provides a way to non-invasively measure the extent of emphysema within the lungs of living subjects. Importantly, these measurements have been demonstrated to correlate significantly with mortality, to be sensitive to disease progression over short periods of time, have shown potential for evaluating and predicting COPD phenotypes at risk for exacerbations and have been shown to identify suitable candidates for surgical interventions and treatment. Even though there is still no clear biomarker for the progression of emphysema, and CT studies indicate that a large sample size may still be needed to identify the natural progression of the disease, the inclusion of imaging biomarkers to quantify the extent of emphysema may be useful for patient stratification or surrogate endpoints in new drug and interventional trials. It is thought that the use of CT to quantify emphysema will allow for more targeted therapies and potentially lead to better outcomes in patients with emphysema.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Snider GL, Kleinerman JL, Thurlbeck WM, Bengali ZH. The definition of emphysema - report of a national-heart-lung-and-blood-institute, Division of Lung-Diseases Workshop. Amer Rev Respir Dis 1985; 132(1):182–185.
- Gevenois PA, De Vuyst P., De Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1996 Jul; 154(1):187–192.
- Gevenois PA, De M, V, De VP, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995 Aug; 152(2):653–657.
- Turner MO, Mayo JR, Muller NL, Schulzer M, FitzGerald JM. The value of thoracic computed tomography scans in clinical diagnosis: a prospective study. Can Respir J 2006 Sep; 13(6):311–316.
- Barr RG, Berkowitz EA, Bigazzi F, Bode F, Bon J, Bowler RP, A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD 2012 Apr; 9(2):151–159.
- Bankier AA, De Maertelaer V, Keyzer C, Gevenois PA. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 1999 Jun; 211(3):851–858.
- Wegener OH, Koeppe P, Oeser H. Measurement of lung density by computed tomography. J Comput Assist Tomogr 1978 Jul; 2(3):263–273.
- Coddington R, Mera SL, Goddard PR, Bradfield JW. Pathological evaluation of computed tomography images of lungs. J Clin Pathol 1982 May; 35(5):536–540.
- Hruban RH, Meziane MA, Zerhouni EA, Khouri NF, Fishman EK, Wheeler PS, High resolution computed tomography of inflation-fixed lungs. Pathologic-radiologic correlation of centrilobular emphysema. Am Rev Respir Dis 1987 Oct; 136(4):935–940.
- Kuwano K, Matsuba K, Ikeda T, Murakami J, Araki A, Nishitani H, The diagnosis of mild emphysema. Correlation of computed tomography and pathology scores. Am Rev Respir Dis 1990 Jan; 141(1):169–178.
- Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988 Oct; 94(4):782–787.
- Madani A, Zanen J, De M, V, Gevenois PA. Pulmonary emphysema: objective quantification at multi-detector row CT—comparison with macroscopic and microscopic morphometry. Radiology 2006 Mar; 238(3):1036–1043.
- Gould GA, Macnee W, McLean A, Warren PM, Redpath A, Best JJ, CT measurements of lung density in life can quantitate distal airspace enlargement—an essential defining feature of human emphysema. Am Rev Respir Dis 1988 Feb; 137(2):380–392.
- Dirksen A, Friis M, Olesen KP, Skovgaard LT, Sorensen K. Progress of emphysema in severe alpha 1-antitrypsin deficiency as assessed by annual CT. Acta Radiol 1997 Sep; 38(5):826–832.
- Mishima M, Hirai T, Itoh H, Nakano Y, Sakai H, Muro S, Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 1999 Aug 3; 96(16):8829–8834.
- Gietema HA, Muller NL, Fauerbach PV, Sharma S, Edwards LD, Camp PG, Quantifying the extent of emphysema: factors associated with radiologists’ estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol 2011 Jun; 18(6):661–671.
- Coxson HO, Whittall KP, Nakano Y, Rogers RM, Sciurba FC, Keenan RJ, Selection of patients for lung volume reduction surgery using a power law analysis of the computed tomographic scan. Thorax 2003 Jun; 58(6):510–514.
- Uppaluri R, Hoffman EA, Sonka M, Hartley PG, Hunninghake GW, McLennan G. Computer recognition of regional lung disease patterns. Am J Respir Crit Care Med 1999 Aug; 160(2):648–654.
- Madani A, De M, V, Zanen J, Gevenois PA. Pulmonary emphysema: radiation dose and section thickness at multidetector CT quantification–comparison with macroscopic and microscopic morphometry. Radiology 2007 Apr; 243(1):250–257.
- Boedeker KL, McNitt-Gray MF, Rogers SR, Truong DA, Brown MS, Gjertson DW, Emphysema: effect of reconstruction algorithm on CT imaging measures. Radiology 2004 Jul; 232(1):295–301.
- Coxson HO. Quantitative chest tomography in COPD research: chairman's summary. Proc Am Thorac Soc 2008 Dec 15; 5(9):874–877.
- Quantitative Imaging Biomarkers Alliance. Radiological Society of North America. 2013. 13-5-2013. http://www.rsna.org/QIBA_.aspx
- Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med 2013 Mar 15; 187(6):602–608.
- Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011 Sep 29; 365(13):1184–1192.
- Nishimura M, Makita H, Nagai K, Konno S, Nasuhara Y, Hasegawa M, Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012 Jan 1; 185(1):44–52.
- Coxson HO, Dirksen A, Edwards LD, Yates JC, Agusti A, Bakke P, The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med 2013 Apr; 1(2):129–136.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. MMWR Surveill Summ 2002 Aug 2; 51(6):1–16.
- Dalal AA, Shah M, D'Souza AO, Rane P. Costs of COPD exacerbations in the emergency department and inpatient setting. Respir Med 2011 Mar; 105(3):454–460.
- Tanabe N, Muro S, Hirai T, Oguma T, Terada K, Marumo S, Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011 Jun 15; 183(12):1653–1659.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010 Sep 16; 363(12):1128–1138.
- Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology 2011 Oct; 261(1):274–282.
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003 May 22; 348(21):2059–2073.
- Rogers RM, Coxson HO, Sciurba FC, Keenan RJ, Whittall KP, Hogg JC. Preoperative severity of emphysema predictive of improvement after lung volume reduction surgery: use of CT morphometry. Chest 2000 Nov; 118(5):1240–1247.
- Coxson HO, Nasute Fauerbach PV, Storness-Bliss C, Muller NL, Cogswell S, Dillard DH, Computed tomography assessment of lung volume changes after bronchial valve treatment. Eur Respir J 2008 Dec; 32(6):1443–1450.
- Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010 Sep 23; 363(13):1233–1244.
- Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchison DC, Ulrik CS, A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med 1999 Nov; 160(5 Pt 1):1468–1472.
- Dirksen A, Piitulainen E, Parr DG, Deng C, Wencker M, Shaker SB, Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J 2009 Jun; 33(6):1345–1353.
- Stockley RA, Parr DG, Piitulainen E, Stolk J, Stoel BC, Dirksen A. Therapeutic efficacy of alpha-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res 2010; 11:136.
