Abstract
Home-based exacerbation management programs have been proposed as an approach to reducing the clinical and financial burden of COPD. We demonstrate a framework to evaluate such programs in order to guide program design and performance decisions towards optimizing cost and clinical outcomes. This study models the impact of hypothetical exacerbation management programs through probabilistic Markov simulations. Patients were stratified by risk using exacerbation rates from the ECLIPSE study and expert opinion. Three scenarios were modeled, using base, worst and best case parameters to suggest potential telehealth program performance. In these scenarios, acute exacerbations could be detected early, with sensitivity and specificity ranging from 60–90%. Detected acute exacerbations could be diverted to either a sub-acute pathway (12.5–50% probability), thus entirely avoiding hospitalization, or a lower cost pathway through length-of-stay reduction (14–28% reduction). For a cohort of patients without prior hospitalization, the base case telehealth scenario results in a cumulative per-patient lifetime savings of $2.9K over ∼12 years. For a higher risk cohort of patients with a prior admission and 1 to 2 acute exacerbations per year, a cumulative $16K per patient was saved during the remaining ∼3 life-years. Acceptable prices for home-based exacerbation detection testing were highly dependent on patient risk and scenario, but ranged from $290–$1263 per month for the highest risk groups. These results suggest the economic viability of exacerbation management programs and highlight the importance of risk stratification in such programs. The presented model can further be adapted to model specific programs as trial data becomes available.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality. Its high prevalence and progressive nature place an enormous economic and social burden on patients and society. The total costs of COPD morbidity and mortality in the United States were estimated to be $23.9 billion in 1993 (Citation1), and $29.5 billion in 2010 (Citation2).
Exacerbations of COPD are acute episodes of worsening of respiratory symptoms triggered by airway and systemic inflammation, requiring treatment with antibiotics and/or systemic corticosteroids. The symptoms of exacerbations can range significantly with matching variations in the level of the required interventions. Mild to moderate exacerbations may require outpatient evaluation and possible changes in the treatment regimen. At the other end of the spectrum, exacerbations may become life threatening events, requiring emergency care, hospital admissions, and possibly intensive care unit (ICU) stays. Exacerbations requiring hospitalization are referred to as acute exacerbations (AE), to be contrasted with subacute exacerbations (SAE) that do not require hospitalization.
Acute exacerbations are expensive events and contribute >70% of COPD medical costs (Citation1, Citation3, Citation4). Moreover, exacerbation frequency is an important determinant of health-related quality of life (Citation5, 6), with many studies reporting that some patients are more prone to frequent exacerbation than others (Citation7). Controlling the frequency and/or severity of exacerbations is of paramount importance to both the quality of life of the patient and to managing healthcare costs.
One such intervention strategy is the deployment of home-based management programs designed to reduce exacerbations, particularly AE. The premise is that, before the onset of an exacerbation, there is a change in symptoms such as dyspnea, sputum characteristics, cough and a deterioration in activity level, well-being, and biomarker levels (e.g. SpO2, respiratory rate, pulmonary function, etc). An array of technologies could be deployed to monitor these signs of potential COPD deterioration. Combined with intelligent algorithms, it may then be possible to detect exacerbations early in their clinical development. If detected early enough and coupled with appropriate triage and contact with care providers, pharmacological interventions may be administered in time to prevent or reduce the extent of the exacerbation while also reducing downstream costs.
It is hypothesized that such a COPD management program could be cost-saving because of its potential to reduce hospitalizations, clinic visits, and the ancillary procedures associated with exacerbations. However such new management approaches could also be costly due to a significant commitment of human and technical resources. In one reported study, for instance, the additional cost of a self-management program for COPD exceeded the savings (Citation8). With steeply rising healthcare expenditures in the United States (Citation9) there is increased scrutiny on there is increased scrutiny on the potential financial impacts and sustainability of new interventions (Citation10), in addition to consideration of the clinical efficacy of the interventions. Therefore, it is important to investigate home-based COPD management tools from multiple perspectives, including the impact on payer healthcare budgets.
In this study, we perform an early-stage economic analysis to determine the viability and requirements of a wide range of new approaches to the care of COPD patients in the home. We present this in the context of a hypothetical COPD exacerbation management program for which the clinical data is not yet readily available. As such programs are currently under active development, scenario analysis is performed to understand the relationship between economic viability and technical and clinical performance parameters. This is done with the aim of informing researchers and R&D managers of the boundary conditions required for an exacerbation management tool to be economically acceptable to both manufacturers and payers.
To accomplish this, we first developed a risk stratification model that predicts the likelihood of COPD exacerbations. This model is based on results from the ECLIPSE study (Citation7), where the frequency of exacerbation was found to be related to prior history of exacerbation, or phenotype. We then created a probabilistic Markov model to capture the progressive course of COPD. Using this model, we compared the clinical progression and financial costs for patients receiving different interventions, including the hypothesized home-based management tool. This comparison was performed for patients at different risk levels. This analysis included exacerbation-associated inpatient, outpatient and pharmacy costs for the different cohorts. Scenario analysis was performed to assess clinically and economically feasible product performance-price combinations. We built both a cost-avoidance model and cost-effectiveness model (using quality-adjusted life-years, or QALY, as the effectiveness measure) to assess the cost boundary conditions under different criteria.
Methods
New approach for home-based exacerbation management
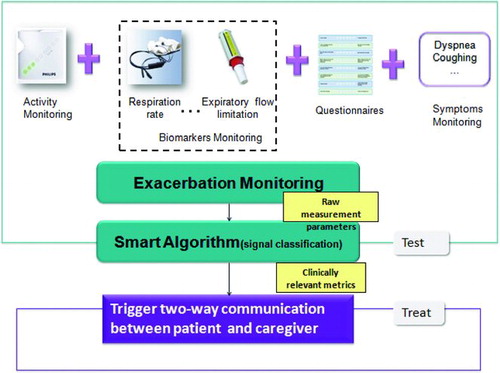
While the model used in this study is not predicated on any specific exacerbation management approach, it was developed and is presented in the context of a home-based COPD management program. Such a program can be conceptualized as having two primary components: 1) TEST: means to enable early exacerbation detection 2) TREAT: early treatment upon the detection of exacerbation. Detailed descriptions of these components can be found in and .
Table 1. Definition of the two major components of a home-based COPD exacerbation management program
Patient stratification
Home-based disease management programs have been traditionally assumed to deliver the most cost effective care to high-risk populations. The benefits of a home-based program to low-risk population remain uncertain. To study this, we stratify the COPD population into five major risk levels (). This enables an examination of the potential cost/clinical impact of a home-based management program for each risk strata. The stratification was defined based on exacerbation frequency and hospital admission rate, and was developed using data from the ECLIPSE study (Citation7), clinical trials (Citation11, 12), and expert opinions.
Table 2. Definition of patient stratification and their source of data assumptions
This patient stratification method reflects the exacerbation frequency phenotype of individual patients. In practice, this can be inferred by examining the admission and discharge record for a patient in the year preceding enrollment in the COPD management program. Level I-III consists of patients without any prior COPD AE admissions. These patients are grouped into risk levels on the basis of their observed exacerbation susceptibilities (or phenotypes) in alignment with the ECLIPSE findings. Note that one out of eight exacerbations are assumed to be AEs (Citation13). Level IV-V consists of patients with a history of one prior acute exacerbation (AE), implying at least one hospital admission. Level V is a sub-category of Level IV, including those patients recently discharged from an exacerbation-related hospitalization. These patients are particularly vulnerable to new exacerbations and subsequent readmissions. The mortality of Level IV-V patients is particularly high, with a 28% 5-year survival rate (Citation11), and a mortality of 49% within 2 years of their index admission (Citation12).
Model simulation
A probabilistic Markov model was created to simulate the progressive and recurrent nature of COPD. Markov models have previously been used to model chronic, recurrent diseases such as COPD (Citation14, 15). In this model, the evolution of disease is reflected by the transition of patients between different states, as shown in . Patients are modeled to be within a single state for a fixed unit of time (the simulation cycle time). Our model defines three states: (Citation1) stable (no exacerbation during a simulation cycle), (Citation2) unstable (one exacerbation, which may be AE or SAE) and (Citation3) death (). A 6-week cycle length was used in the model. This cycle time was chosen specifically to constrain most patients to no more than one exacerbation per cycle (Citation16), and was selected based on analysis of the time course of COPD exacerbation from onset to recovery (Citation16) and expert opinions. At the end of each 6-week simulation cycle, patients transition to other states (or stay in the same state) according to pre-defined probabilities; these probabilities are given in . Note that the annual exacerbation rates defined in have been converted to six-week exacerbation rates for modeling purposes. Hospitalization related mortality rate and all-cause COPD death probability (Citation12, Citation17) are also built into the model, as shown in .
Figure 2. Markov state diagram to model the recurrent and chronic nature of COPD. Note that only acute exacerbations (AE) leads to hospitalization.
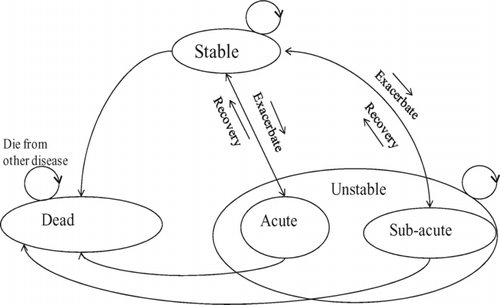
Table 3. Probability Estimate Per Markov Cycle (6 weeks)
Note that previously published Markov models (Citation18, 19) define states using GOLD stages or disease severity. In contrast, the states in our model are selected to focus explicitly on exacerbation rate. This was done because the hypothesized interventions specifically affect exacerbation rates. Moreover, exacerbation events are associated with well-defined costs, allowing the costs of disease progression to be tallied as the simulation evolves.
We analyzed the Markov model via cohort analysis and Monte Carlo simulation. In the cohort analysis, the patient population was considered as a whole. As the Markov model evolves, the proportion of patients in different states changes according to the transition probabilities and cost and utilities are accrued as described in the following section.
Cost and utilities
As the simulations evolved, costs were calculated for each group from the perspective of an American public insurer (e.g. Medicare). Indirect costs such as lost productivity and informal care costs were not evaluated in this study. Our models synthesized exacerbation-associated inpatient, outpatient and pharmacy costs based on the existing literature (see ). Because of the wide variations in reported costs in different countries, the analysis presented here focused on North American published costs. All costs are expressed in 2011 US dollars ($). Health outcomes are expressed as life-years (LY) and quality-adjusted life-years (QALY). QALY is a measure of health outcomes addressing both quantity and quality of life. It assumes that a year of life in perfect health is worth 1 QALY (1 LY × 1 utility value = 1QALY) and that a year of life in a state of less than this perfect health (e.g. utility = 0.5) is worth 0.5 QALY (1 LY × 0.5 utility value = 0.5 QALY). QALYs are therefore expressed in terms of “years lived in perfect health”.
Table 4. Cost and utility parameters used in the Markov model
A number of potential QALY discount rates were considered; for example, 3.5% has been recommended by the UK National Institute for Health and Clinical Excellence (NICE) (Citation28) while 3% has been proposed as a value for the United States (Citation29). To maintain a conservative estimate, we choose the slightly higher discount rate of 3.5% for this study.
Utility values per disease state were based on published data from an empirical study of COPD patients (Citation22). The model assumed a reduction in utility during any cycle in which a patient experiences an exacerbation. During cycles in which patients experienced a sub-acute exacerbation, the utility was reduced by 15% (Citation24). If the patient experienced an acute exacerbation during one of the six-week cycles, the utility for that cycles was reduced by 50% (Citation27).
Effect of proposed home-based exacerbation management program
The foregoing discussion describes the costs for a population of patients undergoing usual care. We additionally model the hypothetical effect of a proposed home-based exacerbation management program. This program is defined for modeling purposes by the six parameters given in and summarized here.
Table 5. Using 6 parameters and 3 scenarios to define the technology efficacy and cost
Technical parameters
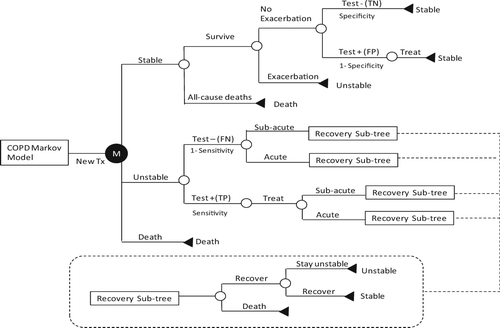
This includes (Citation1) the sensitivity of the home exacerbation detection method (SEN) and (Citation2) the specificity of the home exacerbation detection (SPE). Sensitivity refers to the probability of a positive test in a patient with an exacerbation. Specificity refers to the probability of a negative test in a patient without an exacerbation. These can be written as SEN = TP/ (TP + FN), SPE = TN/(TN + FP), where TP is true positive (the test correctly identifies an exacerbation), FN is false -negative (early detection does not occur), TN is true negative (no exacerbation and the test is negative) and FP is false positive (the test is positive but no exacerbation is occurring). A TP will accrue the clinical and financial benefits of early detection, as described by the clinical and cost parameters, below. In contrast, a FP will cause unneeded treatment and thus incur unnecessary cost while a FN will not generate any clinical or financial benefits but will still incur the recurring monitoring costs (see ).
Figure 3. A simplified scheme to demonstrate the Markov model of the treatment branch. Note that “Test” refers to the home exacerbation detection test and “Treat” refers to the early exacerbation treatment upon the detection of the exacerbation. False Positive (FP) will cause unneeded treatment, thus incur unnecessary cost; False Negative (FN), or missed diagnosis, will omit patients from the early treatment, thus will not reduce admissions but still incur monitoring cost.
Clinical parameters
A proposed benefit of early detection and intervention of exacerbations is that it may prevent severe exacerbations, thus reducing the ratio of AE to SAE (with the overall exacerbation frequency stays unchanging). This is captured as the acute exacerbation reduction ratio (ERR). A second potential benefit is a reduction of the severity of AE if the exacerbation is detected early. This is reflected as a shortened length-of-stay (LOS) and captured as the LOS reduction ratio (LRR).
Economic parameters
The recurring cost of any home-based exacerbation intervention program (which is beyond the cost of usual care) consists of: (Citation1) the cost of the monitoring system (Cost of Early Detection, or CED) and (Citation2) the cost of early treatment when the monitoring system gives a positive result (Cost of Early Treatment, or CET). The cost savings of early detection are approximated by reducing the cost of AE by the LRR while also shifting the overall balance of costs from the more costly AE to SAE (measured by ERR).
We consider three scenarios for the performance of the proposed home-based COPD management program. Each scenario is defined by a different combination of these six parameters, representing a base-case, a best case, and a worst case performance scenario. The parameters are detailed in .
In addition to simulating patient cohorts with the parameters given in , one-way sensitivity analysis was performed with respect to the cost of the early exacerbation detection tests (CED). This tests a range of values to determine its effect on the overall cost. To further understand the joint uncertainty and patient-to-patient variability in the efficacy of the telehealth program, a second order Monte Carlo simulation was used in which key parameters were randomly drawn from pre-defined probabilistic distributions. Therefore, Monte Carlo simulations have the advantage of capturing the uncertainty of the parameters, providing not only expected values, but also the associated variability and confidence interval.
Results
Cohort analysis
Two hypothetical cohorts were created based on the different proportion of risk levels. The first cohort is made of 42% Level III (frequent exacerbators) and 58% Level II (infrequent exacerbators) patients. Level I patients are excluded as they rarely exacerbate. This cohort resembles the clinical cohort reported in the ECLIPSE study. The second cohort is composed of Level IV-V patients, those patients with at least one prior index AE (with hospital admission). This cohort represents the end-stage patient population: their condition deteriorates rapidly and they have frequent hospital admissions. Cohort 1 was simulated for a 20 year period while Cohort 2 patients were followed for 10 years. The accumulated cost and utility for these two cohorts was tracked over the simulation period. The simulations were run assuming usual care as well as the three scenarios for the home management program ().
Results for each scenario and cohort are given in . Using the base-case scenario as an example, the home based program was shown to save cohort 1 a cumulative $2.9K per patient during the remaining years (∼12 years) of the patient life. In contrast, in cohort 2, a cumulative $16K per patient was saved during the remaining years (∼3 years) of the patient's life. In this base-case scenario, the cost of home-management in both cohorts is entirely offset through reduced hospital utilization, with additional cost savings as reported in .
Table 6. Simulated impact of home-based exacerbation management
Sensitivity Analysis on the Cost of Home Monitoring Early Detection (CED)
The base case analysis assumes that patients at different risk levels consume the same amount of services (CED = $100 per 6 weeks). However, it is reasonable to argue that high-risk patients would benefit from more active monitoring and supervision than low-risk patients, resulting in a higher CED for those patients. A sensitivity analysis was performed to identify the maximum CED for the two patient cohorts and the five patient risk levels. In the context of a cost-avoidance model, we -estimated the long-term cost savings to payers for a range of CED values. The break-even point was found as the CED for which the total cumulative recurring costs for the COPD home management program equaled the total cost saving through hospitalization and LOS reduction (cost savings, or ΔCost = 0).
The results for this analysis are given in and . The analysis demonstrates a significant difference between the thresholds for the two patient cohorts. As expected, the maximum monthly service fee is lowest for the low-risk groups and increases as the patient risk and service utilization rises.
Figure 4. The service pyramid indicates the intensity of services and cost boundary of each risk level patient to be cost-saving to the payers. The table on the right shows the maximum recurring cost (per month) for home-based exacerbation management. If the service cost exceeds this number, payers would incur added costs rather than savings from the exacerbation management program.
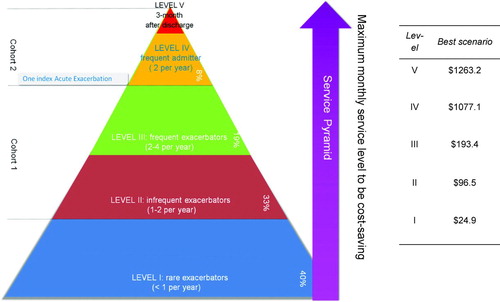
Table 7. Break-even service costs for different patient risk groups
A cost-effectiveness analysis was also performed. The incremental cost-effectiveness ratio (ICER) was computed at each CED level and compared with the willingness-to-pay (WTP) threshold. We use the commonly cited WTP = $50K/QALY (Citation10). The maximum cost in this model is the boundary price point for the program to be considered cost-effective (ICER ≤ WTP). In this analysis, the maximum monthly service fee for a cost-effective home management program for Cohort 1 was found to be $608, $290, and $99 for the best-, base-, and worst case scenarios. In contrast, for the higher risk Cohort 2, the best-, base-, and worst case boundary prices were found to be $1991, $1139, and $457.
The boundary conditions for the technology to be cost-saving for Cohort 1 and Cohort 2 are significantly different, as indicated in . Using the base case scenario as an example, the maximum monthly service fee should not exceed $95.60 for Cohort 1, or it would incur cost for the payers. In contrast, the maximum monthly service for Cohort 2 could be as high as $755.
Monte Carlo simulation
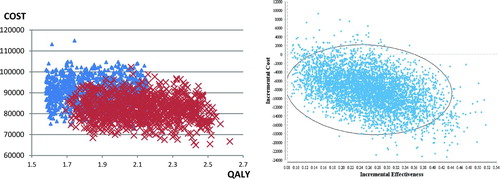
Second-order Monte Carlo simulations were conducted to address the joint uncertainty of the technical and clinical efficacies of home-based intervention as well as patients heterogeneity. The simulation was conducted in Cohort 2 by sampling the following distributions: 1) Monthly costs for home monitoring were sampled from a Gamma distribution with a mean of $500 and standard deviation of $150. 2) Costs of acute exacerbation were sampled from a Gamma distribution with a mean $11777 and standard deviation $1000; 3) Sensitivity, specificity, LRR, ERR were respectively sampled from triangular distributions, with the likeliest value being set to the base case scenario value, and the min/max value being set to worst/best scenarios values; 3) the utility of stable patients was sampled from a uniform distribution based on the quality of life variability among patients (see ). In both the control and intervention groups, we modeled patient deterioration by including a time-dependent mortality rate and AE rate where we assumed that the rates would increase over time. Three 5000-trial simulations were conducted. This number of simulations yielded highly stable results in terms of both expected value and variability (see ). The scatter plot reveals that with an average charge of $500 per month (CED), home-based COPD program could still demonstrate added clinical effectiveness (QALY) and cost-saving capabilities for the patients with one prior index admissions.
Figure 5. Monte Carlo simulation. Left: Cost effectiveness scatter plot. Simulated patients undergoing the usual standard-of-care are shown in blue triangles while patients undergoing the proposed home-based intervention are shown in red crosses. Right: incremental cost-effectiveness scatter plot. Cost saving has been achieved in > 95% of the simulations, and QALY gain has been achieved in 100% of the simulations.
Discussion
In this paper, we describe a methodology for examining the potential cost consequences of new home-based approaches that attempt to reduce the frequency/severity of exacerbations in COPD. We investigated multiple scenarios for cost and clinical performance for the proposed exacerbation management program and assessed the potential cost-savings from the perspective of a payer. Through these analyses, we demonstrated the likely cost-saving capabilities of the proposed COPD telehealth program and report on the technical and cost boundaries within which the program should operate. Clinical trial data are still required to fully demonstrate both the clinical and economic benefits of emerging COPD exacerbation management programs. However, our analysis suggests that, under the base case system performance and cost assumptions, a telehealth program has the potential to achieve long-term cost-savings. This cost-savings is most significant in the high-risk cohort. We envision that the results of this study and the broad approach can aid payers in technology acquisition decisions. Moreover, we believe that this framework and methodology can be applied broadly to other potential interventions as well. We also suggest that the results of this study can be used to set performance and price targets for those engaged in the development of COPD exacerbation management programs.
McLean et al (Citation33) have shown through systematic literature review and meta-analysis of ten randomized controlled trials that telehealth programs lead to significant reduction in COPD hospitalizations and emergency department visits. These results correspond to our best case scenario (with reduction of admission odds ratio = 0.46 for the telehealth group). In terms of economic benefits, past economic studies were focused on pharmaceutical treatments. For example, Mapel et al (Citation34) have described a simulation method to examine the potential cost consequences of treatments that reduced exacerbations. Through sensitivity analysis, they identify the cost-neutral (or “break-even” cost) point for a new exacerbation controller therapy for different risk reduction ratios and daily prices. This framework of early technology assessment (Citation35) and determination of price potential under coverage uncertainty (Citation36) can be useful to inform value-based pricing decisions.
Patient risk stratification has been recognized as an important step in delivering targeted medical care. Patient utilization of the proposed COPD management services is based on frequency of exacerbation; this frequency can be used to define patient risk levels. As such, we analyzed cost boundaries for economically viable disease management programs for stratified patients cohorts, accounting for different possible system performance levels. It was found that the payers would be able to maximize cost savings by focusing the proposed COPD management program on patients with deteriorating conditions. These patients are subject to frequent hospital admissions. Consequently, they have the greatest potential to benefit from reducing the number of AE or reducing the severity and cost for those exacerbations that do occur. The maximum viable service price was also found for these high risk patients. We demonstrate that the economics of the proposed COPD management program varies significantly with the complexity and intensity of services at different patient risk levels.
One limitation of the current model is that it does not consider the potential of the COPD management program to change the frequency and costs of general practitioner and specialist visits and consultations. In some trials, reduction of primary care contacts has been found to be the major benefit of telehealth (Citation31). These results were found for a highly specific patient group and further study is needed to generalize the finding. Nevertheless, it represents a potential benefit of a telehealth program. Since outpatient visits account for only a small fraction of the overall cost, our model focused instead on hospital admission reduction. In addition, indirect costs such as loss of productivity and the increase in sick days were not assessed as we focused solely on direct health care costs. In both cases, these represent additional potential cost savings. As such, they do not change the fundamental conclusion of this work that the proposed program is economically viable under the base case cost and performance assumptions.
A further limiting assumption of the current study is in the modeling of the frequency of exacerbation, particularly in our low-risk cohort (Cohort Citation1). We assume that the patient risk stratification is stable over time; that is, we assume that a patient will stay in their risk category with a constant frequency (or frequency range) of exacerbation over time. This assumption was made on the basis of the ECLIPSE study results (Citation7) which identified COPD patients according to their susceptibility to exacerbation and further demonstrated the stability of those phenotypes over the trial period. If this model assumption were relaxed, simulated low-risk patients would be allowed to evolve into the high-risk categories. This would increase the cost-effectiveness and headroom for the potential service pricing for the proposed COPD management program for those patients, thereby strengthening the conclusion that such a program would be economically viable. Moreover, changing the assumption of stable risk stratification does not significantly affect our conclusion on high-risk cohort (Cohort 2) as these patients’ exacerbation patterns have already reached the peak; therefore the primary cost-saving benefit is for the high-risk patients (Cohort 2) still holds true.
The stakeholders primarily addressed in this paper are public payers. With healthcare reform in the U.S., more advanced implementation of outcome-based reimbursement is being proposed in the Affordable Care Act in the form of Accountable Care Organizations (ACOs) - networks of doctors and hospitals that share clinical and financial responsibility for providing care to patients. ACOs are incentivized to manage long term health outcomes and the overall cost of their healthcare, potentially blending the cost implications of hospital- and home-based care. The findings of our study may be of interest to these organizations as well. Similarly, individual physicians, patients, private payers, and medical innovators are increasing their efforts to obtain reliable cost effectiveness data for care, research and development, and reimbursement decisions (Citation37, Citation38–41). The current study acknowledges these trends by explicitly modeling the relationship between technical and clinical performance and downstream costs.
While the focus in this study was on home-based COPD exacerbation management, the general approach can be applied during the early development of any disease management program. When complete clinical trial data is not yet available, this approach may help set priorities for the investment, provide performance envelope requirements for research and development teams, anticipate reimbursement coverage, and suggest price potential.
In conclusion, we have demonstrated the potential economic benefits of home-based COPD exacerbation management under reasonable technical and cost assumptions. This simulation study highlights the importance of risk and service stratification for COPD patients given the differential benefits within these risk strata. Additionally, the study has quantified the link between technical performance and downstream costs, though, again, the viability of such an exacerbation management program was seen under a wide range of conditions. These results should be further validated through trial data and relaxing of the technical assumptions, but it is hoped that the current study provides valuable input for the wide range of stakeholders interested in new COPD exacerbation management programs.
Declaration of Interest
SXL, MCL, MA, JT, NH, and DPW are employees of Royal Philips Electronics, the parent company of Philips Healthcare.
Acknowledgement
The corresponding author would like to thank John Bell, Jeroen Wals, Chris Hall, John Prichard, Sharon Baer, and Emiel Wouters for the useful discussions and generous support.
References
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000; 117(2 Suppl):5S–9S.
- National Institutes of Health National Heart Lung and Blo-od Institute Morbidity & Mortality: Chart Book on Cardio-vascular, Lung, and Blood Diseases. 2009 [cited 2013 March 1, 2013]; Available from: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm.
- Simoens S. The economic burden of COPD exacerbations. COPD, 2010; 7(3):159–61.
- Andersson F, The costs of exacerbations in chronic obstructive pulmonary disease (COPD). Respir Med 2002; 96(9):700–8.
- Seemungal TA, Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157(5 Pt 1):1418–22.
- Menn PN, Weber, Holle R. Health-related quality of life in patients with severe COPD hospitalized for exacerbations - comparing EQ-5D, SF-12 and SGRQ. Health Qual Life Outcomes 2010; 8:39.
- Hurst JR, Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010. 363(12): p. 1128–38.
- Bourbeau J. Disease management for COPD: avoiding hospitalizations and controlling cost? COPD 2011; 8(3):143–144.
- Newhouse JP. Financing Medicare in the next administration. N Engl J Med 2004; 351(17):1714–6.
- Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med 2005; 353(14): 1516–22.
- Warren PM, Respiratory failure revisited: acute exacerbations of chronic bronchitis between 1961–68 and 1970–76. Lancet 1980; 1(8166):467–70.
- Connors AF Jr, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med, 1996; 154(4 Pt 1):959–67.
- Hoogendoorn M, Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis 2010; 5:435–44.
- Halpin DM. Health economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc, 2006; 3(3): 227–33.
- Rutten-van Molken M, Lee TA. Economic modeling in chronic obstructive pulmonary disease. Proc Am Thorac Soc, 2006; 3(7):630–4.
- Seemungal TA, Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med, 2000; 161(5):1608–13.
- Fuso L, Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med, 1995; 98(3):272–7.
- Atsou K, Chouaid C, Hejblum G. Simulation-based estimates of effectiveness and cost-effectiveness of smoking cessation in patients with chronic obstructive pulmonary disease. PLoS One 2011; 6(9):e24870.
- Oostenbrink JB, Probabilistic markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value in Health 2005; 8(1): 32–46.
- Dalal AA, Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis 2010; 5:341–9.
- Yu AP, Incremental third-party costs associated with COPD exacerbations: a retrospective claims analysis. J Med Econ 2011; 14(3):315–23.
- Borg S, A computer simulation model of the natural history and economic impact of chronic obstructive pulmonary disease. Value Health, 2004; 7(2):153–67.
- Mittmann N, The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med, 2008; 102(3):413–21.
- Paterson C, Assessing patient outcomes in acute exacerbations of chronic bronchitis: the measure your medical outcome profile (MYMOP), medical outcomes study 6-item general health survey (MOS-6A) and EuroQol (EQ-5D). Qual Life Res 2000; 9(5):521–7.
- Dalal AA, Costs of COPD exacerbations in the emergency department and inpatient setting. Respir Med 2011; 105(3):454–60.
- Stanford RH, Shen Y, McLaughlin T. Cost of Chronic Obstructive Pulmonary Disease in the Emergency Department and Hospital: An Analysis of Administrative Data from 218 US Hospitals. Treat Respir Med, 2006; 5(5):343–9.
- Spencer S, Jones PW. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax, 2003; 58(7):589–93.
- National Institute for Health and Clinical Excellence: Guide to the methods of technology appraisal. [cited 2011; Available from: http://www.nice.org.uk/aboutnice/howwework/devnicetech/technologyappraisalprocessguides/guidetothemethodsoftechnologyappraisal.jsp.
- Siegel J, Guidelines for Pharmacoeconomic Studies. PharmacoEconomics, 1997; 11(2):159–168.
- Lewis KE, Home telemonitoring and quality of life in stable, optimised chronic obstructive pulmonary disease. J Telemed Telecare, 2010; 16(5):253–9.
- Lewis KE, Does home telemonitoring after pulmonary rehabilitation reduce healthcare use in optimized COPD? A pilot randomized trial. COPD, 2010; 7(1):44–50.
- Hanna B. Evaluation Report for COPD Telehealth Project. 2008.
- McLean S, Telehealthcare for chronic obstructive pulmonary disease: Cochrane Review and meta-analysis. Br J Gen Pract. 62(604):e739–49.
- Mapel DW, A new method for examining the cost savings of reducing COPD exacerbations. Pharmacoeconomics. 28(9): 733–49.
- Ijzerman MJ, Steuten LM. Early assessment of medical technologies to inform product development and market access: a review of methods and applications. Appl Health Econ Health Policy. 9(5):331–47.
- Girling, AJ, Lilford RJ, Young TP. Pricing of medical devices under coverage uncertainty-a modelling approach. Health Econ. 21(12):1502–7.
- Ginsburg ME. Cost-effectiveness: will the public buy it or balk? Health Aff (Millwood), 2004. Suppl Web Exclusives: p. W4-297–9.
- Bloom BS. Use of formal benefit/cost evaluations in health system decision making. Am J Manag Care, 2004; 10(5): 329–35.
- Statement for Ways and Means hearing to examine comparative effectiveness. 2007 [cited 2011; Available from: www.bcbs.com/news/bcbsa/statement-on-ways-and-means-hearing-to-examine-comparative-effectiveness-research.html.
- Vallejo-Torres L, Integrating health economics into the product development cycle: a case study of absorbable pins for treating hallux valgus. Med Decis Making, 2011; 31(4): 596–610.
- Ijzerman MJ, Steuten LM. Early assessment of medical technologies to inform product development and market access: a review of methods and applications. Appl Health Econ Health Policy, 2011; 9(5):331–47.