Abstract
Background: Tidal expiratory flow limitation (EFL) is a step of paramount importance in the functional decline in COPD. Among mechanisms contributing to EFL, loss of airway-parenchymal interdependence could mostly be involved. Aim: To assess if EFL is a functional marker more frequently linked to prevalent pulmonary emphysema rather than to prevalent chronic bronchiolitis in COPD patients with moderate-to-severe airflow obstruction. Methods: Forty consecutive stable COPD patients with FEV1 between 59 and 30% of predicted were functionally evaluated by measuring spirometry, maximal flow-volume curve and lung diffusion capacity (DLCO) and coefficient of diffusion (KCO). EFL was assessed by the negative expiratory pressure (NEP) method both in sitting and supine position. Chronic dyspnea was also scored by modified Medical Research Council (mMRC) scale. Results: In sitting position 13 patients (33%) were flow limited (FL) and 27 were non-flow limited (NFL). Only FEV1/FVC, FEV1 and MEF25–75% were different between FL and NFL patients (p < 0.01). In supine position, however, among NFL patients in sitting position those who developed EFL, had significantly lower values of DLCO and KCO (p < 0.05) and higher mMRC score (p < 0.01), but similar values of FEV1 as compared to those who did not have EFL. Conclusions: In COPD EFL in sitting position is highly dependent by the severity of airflow obstruction. In contrast, the occurrence of EFL in supine position is associated with worse DLCO and KCO and greater chronic dyspnea, reflecting a prevalent emphysematous phenotype in moderate-to-severe COPD patients.
Introduction
Patients suffering from chronic obstructive pulmonary disease (COPD) should first be characterized according to the underlying disease leading to their functional disorder. Two main diseases are responsible for COPD to develop: chronic bronchiolitis, a proliferative and fibrosing small airway disease (Citation1) that can occur alone or more often associated to centrilobular emphysema (more or less extensive) and panlobular emphysema, a rarer, destructive and non-regenerative disease of lung parenchyma (Citation2). Although in general the risk factors are similar, genetic background has to play an important role on development of one disease instead of other (Citation3). It is increasingly recognized that such fundamental distinction has different and relevant prognostic and therapeutic implication in these patients (Citation4–6).
In the past two different phenotypes were clearly described in patients with more advanced COPD: type A or pink puffer with lower DLCO, higher TLC, no severe gas exchange abnormalities at rest and greater dyspnea (chronic and exertional) vs type B or blue bloater with higher DLCO, normal TLC, severe gas exchange abnormalities at rest and much less chronic dyspnea. The pathological aspects revealed prevalent emphysema in type A and prevalent small airway disease (i.e.: chronic bronchiolitis) in type B. Some patients showed intermediate functional, clinical and pathological characteristics (Citation7).
Recently, in COPD patients DLCO has been validated as a functional respiratory parameter mostly related to inspiratory high resolution computerized tomography (HRCT) attenuation parameters, reflecting the extent of parenchymal destructive changes compatible with emphysema (Citation8) and both DLCO and KCO were inversely associated with qualitative and quantitative HRCT measurements of significant pulmonary emphysema in a prospective observational study conducted in a large cohort of COPD patients (Citation4). Moreover, greater baseline reduction in DLCO and KCO was found as predictor of both faster progression of emphysematous lesions (as% LAA increase in HRCT scan) and worse functional decline (as FEV1 loss in ml/yr) in longitudinal studies in COPD (Citation4, Citation9).
During the natural history of COPD, the occurrence of tidal expiratory flow limitation at rest (EFL) marks a step of paramount importance, favoring dynamic pulmonary hyperinflation and its consequence such as more severe chronic dyspnea (Citation10) orthopnea (Citation11) and greater exercise limitation (Citation12, 13).
The aim of this study was to assess if in COPD patients with moderate-to-severe airflow obstruction, EFL could be a functional marker mostly associated with type A phenotype, suggesting underlying pulmonary emphysema rather than with type B phenotype, suggesting underlying chronic bronchiolitis. The secondary aim was to identify the predictors of EFL in these patients.
Methods
Forty consecutive patients suffering from COPD with moderate-to-severe airway obstruction (FEV1 < 60 and ≥ 30% of predicted value) were studied in stable clinical condition. COPD was defined according to the presence of risk factors with a FEV1/VC ratio lower than 5th percentile of predicted and an increase less than 10% predicted and 200 ml after 400 mcg of inhaled albuterol (MDI + spacer) (Citation14). All patients had no other respiratory, cardiac, neuromuscular or endocrine disease. The patients were investigated at 8:30 AM after withdrawing any pharmacological treatment at least 24 hours before. They performed pulmonary function testing (PFT) at rest to assess maximal expiratory and inspiratory flow rates, lung volumes and lung diffusion capacity for carbon monoxide (DLCO) with determination of alveolar volume (VA) and coefficient of diffusion (KCO). Spirometry and maximal flow/volume curve were performed to measure dynamic lung volumes and maximal flow-rates. Multibreath helium dilution technique in a close circuit was used to measure lung volumes. A computerized water-sealed light-bell Stead-Wells spirometer was used and the operator was assisted during the test with a software able to verify online both acceptability and repeatibility of spirometric manoeuvres (Biomedin system, Padua, Italy). All the spirometric tests employed in the study fulfilled the ATS recommendations (Citation15). DLCO was measured twice by the single-breath method (DIMO module, Biomedin, Padua). The mean values of DLCO, VA and KCO were retained for analysis, providing the single values differed less than 5%. The predicted values of the lung function parameters were from ECCS equations (Citation16); the IC predicted values were from Tantucci et al. (Citation17). In these stable COPD patients a prevalent emphysematous phenotype was defined by the presence of DLCO and KCO values lower than 60% of predicted, while DLCO and KCO values normal or higher than 60% of predicted were considered as indicative of a prevalent chronic bronchiolitic phenotype. Subsequently, to detect EFL at rest each patient underwent the application of NEP test (Direc/NEP/Model 200B/Raytech) (Citation18). The patients breathed quietly wearing a nose clip through a flanged mouthpiece and a heated pneumotachograph (series 3700; Hans Rudolph; Kansas City, MO) that was connected to a differential pressure transducer (Raytech DP55 ± 3 cmH2O; Raytech Instruments; Vancouver, BC, Canada) to measure the flow. Pressure was measured at the mouth by a differential pressure transducer (Raytech DP55 ± 100 cmH2O; Raytech Instruments). The pneumotachograph was assembled in series with a Venturi device that created in the circuit a negative pressure, the magnitude of which could be precisely fixed. The Venturi device was connected to a solenoid valve (electrical valve model 8262G208; Ascoelectric; Brantford, ON, Canada) controlled by a computer and activated automatically when the expiratory flow reached a preset threshold value (usually 50 ml/sec) and after a preset time delay in order to apply NEP (−5 cmH2O) immediately after the peak of the tidal expiratory flow and to maintain it throughout the ensuing expiration. Subsequently, NEP test was performed after 10 minutes of recumbency in the supine position. NEP test was repeated at least 5 times in each condition. We also measured the inspiratory capacity (IC) in either position to assess indirectly the variations in the end expiratory lung volume (EELV) in the supine position. Chronic dyspnea was scored using the modified Medical Research Council (mMRC) scale (Citation19).
Statistical analysis
Continuous variables were expressed as mean ± SD. Mann-Whitney test was performed to research statistical significance among unpaired functional data obtained in patients with and without EFL, according to NEP test. Multivariate logistic regression analysis was performed with anthropometric and functional parameters, using the presence of EFL as independent variable. Chi-square test was performed when appropriate. Statistical significance was considered if p values resulted less than 0.05. Statistical software (SPSS version 12.0.1, Chicago, IL.) was used for data processing and statistical analysis.
Results
The anthropometric and functional parameter of all patients are shown in . Twenty-four patients (60%) had DLCO and KCO lower than 60% of predicted. Subsequently, the patients were divided according to the presence of EFL in sitting position. 13 patients showed flow limitation (FL) whereas 27 patients did not (NFL). The two groups were comparable for age, BMI and dyspnea severity score (). FL patients showed greater airflow obstruction (significantly lower values of FEV1/FVC, FEV1 and MEF25–75%), while lung volumes were similar and IC no significantly different. Moreover, no difference was observed for both DLCO and KCO ( and )
Figure 1. Comparison of most relevant functional parameters between COPD patients without (NFL; n = 27) and with (FL; n = 13) resting tidal expiratory flow limitation in sitting position. Only FEV1 is significantly lower in FL patients (* = p < 0.05).
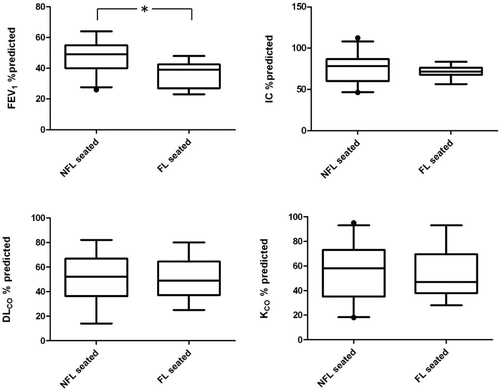
Table 1. Anthropometric and functional parameters of all patients studied and of the two groups of patients divided according to the presence of expiratory flow limitation in sitting position
In supine position, 27 patients exhibited EFL, while 13 were still NFL. As compared to supine NFL patients, supine FL patients had higher mMRC scores (p < 0.01), diminished FVC (p < 0.05) and decreased FEV1 and MEF25–75% (p < 0.01). Lung volumes were similar and IC was lower but non significantly different. They showed lower values of DLCO and KCO (p < 0.05) () and exhibited no increase of IC when supine (ΔIC = −0.01 L ± 0.20 L vs 0.28 ± 0.27 L (p < 0.001).
Table 2. Functional parameters and dyspnea score of the two groups of patients divided according to the presence of expiratory flow limitation in supine position
Among NFL patients in sitting position (n = 27), those who became FL in supine position (n = 14) had worse dyspnea, (p < 0.01), reduced MEF25–75% (p < 0.05) and lower DLCO and KCO values (p < 0.05) and less increase of IC when supine (ΔIC = 0.02 L ± 0.18 L vs 0.28 ± 0.27 L (p < 0.01), as compared to patients who remained NFL in supine position (n = 13), while FEV1 was not significantly different between the two groups as well as lung volumes and IC ( and ). Among 14 patients who were FL in supine position, being NFL in sitting position, 11 (79%) had DLCO or KCO lower than 60% predicted and 9 (64%) had both DLCO or KCO lower than 60% predicted.
Figure 2. Comparison of most relevant functional parameters in COPD patients who are NFL in sitting position (n = 27) between those without (NFL; n = 13) and with (FL; n = 14) tidal expiratory flow limitation in supine position. Both DLCO and KCO are significantly lower in supine FL patients (* = p < 0.05), while FEV1 is not.
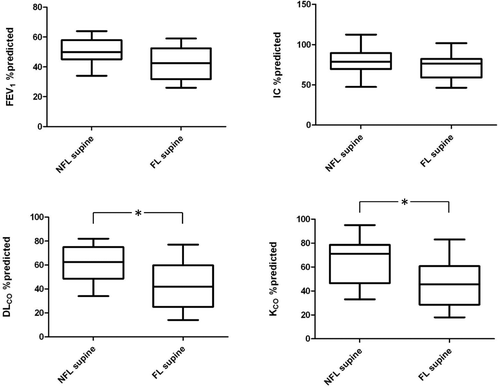
Table 3. Functional parameters and dyspnea score of the two groups of patients divided according to the presence of expiratory flow limitation only in supine position
Looking at the COPD patients with DLCO lower than 60% predicted (n = 26) as compared to those with DLCO higher than 60% predicted (n = 14), we found a significant difference in supine EFL (7 NFL and 19 FL vs 7 NFL and 7 FL, respectively; p < 0.01) and an almost significant difference in seated EFL (17 NFL and 9 FL vs 10 NFL and 4 FL, respectively; p = 0.06).
Multivariate logistic regression analysis showed MRC as the strongest predictor of supine EFL either in all patients (n = 40) or in patients without EFL in sitting position (n = 27) with odds ratio (OR) = 6.60 (95% CI = 1.12-38.80) and 3.62 (95% CI = 1.01−12.98), respectively. The stepwise (backward) logistic regression in either instance retained MRC, MEF25–75% and KCO in the best explanatory model for the occurrence of EFL in supine position.
Discussion
The results of this study show that in moderate-to-severe COPD patients, EFL in sitting position does not distinguish between prevalent chronic bronchiolitis and prevalent pulmonary emphysema. However, the presence of EFL only in supine position is highly suggestive of prevalent emphysema, as documented by the significantly lower DLCO, KCO and higher chronic dyspnea in supine FL COPD patients. This indicates that EFL occurs earlier when the loss of alveolar-airway interdependence (which is a typical abnormality of emphysema) is a contributory mechanism of airflow reduction. Interestingly, this condition is associated with worse chronic dyspnea that characterizes the pink puffers.
In the absence of HRCT lung scan for detecting and quantifying pulmonary emphysema in many of our patients, we used DLCO and KCO, when both were markedly reduced (less than 60% of predicted), as functional marker to identify the presence of relevant emphysema and distinguish the prevalent COPD phenotype. This proxy is strongly supported by several cross-sectional and longitudinal studies in which DLCO and KCO have been shown in COPD patients to closely reflect the loss of lung parenchyma, as assessed by% of low attenuation area in HRCT scan quantitative analysis at full inspiration (4, 8, 20).
Although EFL cannot be predicted by the severity of baseline airway obstruction based on FEV1 reduction, it is conceivable that FL patients in sitting position had on average a lower FEV1 as compared with NFL patients in sitting position, even if the patients were all recruited in a quite narrow FEV1 range (59–30% predicted). In any case, lower maximal expiratory flow rates are crucial to promote EFL, especially when they impinge in the tidal volume range.
On the contrary, we were surprised that DLCO, KCO and chronic dyspnea were not different between FL and NFL patients in sitting position. In this respect, EFL in sitting position seems to be strongly associated with the severity of airway obstruction.
However, this was not the case when were compared FL and NFL patients in supine position. In fact, FL patients in supine position had significantly worse DLCO, KCO and chronic dyspnea, suggesting a prevalent emphysematous phenotype. This was also true and even more interesting if only patients who became FL in supine position, being NFL in sitting position, were compared to those who remained NFL when supine ().
In COPD patients EFL occurs first in supine position because of physiological reduction of FRC and ERV with recumbency that per se implies a lower expiratory flow reserve in the tidal volume range. Thus, supine EFL can be found in the presence of less severe airway obstruction, as in fact was the case in our FL patients only in supine position (FEV1 = 43 ± 11% pred.) as compared with FL patients in sitting position (FEV1 = 36 ± 8% pred.) (p < 0.05). Under these circumstances, it is likely that mechanisms mostly involved in airflow reduction when emphysema is prevalent, such as airway-parenchyma uncoupling and some loss of elastic recoil, may play a relatively major role than airway wall remodeling, in the EFL development. In our opinion this can explain why EFL in supine position is observed in COPD patients with lower DLCO, and KCO.
This finding is important because, favoring identification of COPD patients with prevalent emphysema, may suggest a different therapeutic strategy based more on different long-term bronchodilators and respiratory rehabilitation rather than on combination with high dose of inhaled corticosteroids or other anti-inflammatory drugs such as PDE4-inhibitors. In addition, it gives a reasonable patho-physiological clue for greater breathlessness that a number of moderate-to-severe COPD patients refers in the morning, since EFL in supine position that is usually assumed during the night-time, can predispose to dynamic pulmonary hyperinflation with its deleterious functional and clinical consequences (21).
These data were obtained in a relatively small sample of COPD patients and using DLCO and KCO less than 60% predicted as indicators of prevalent pulmonary emphysema instead of measuring% LAA by HRCT lung scan at full inspiration that is the gold standard to assess and quantify pulmonary emphysema in vivo. It is highly improbable, however, that a type I error occurred in this study because the patients were adequately selected and their lung function carefully investigated. On the other hand, large evidence has been provided for low DLCO and KCO as effective markers of lung parenchyma destruction in COPD (4, 8, 9, 21). Therefore, within the above mentioned limits, we are confident that our results may well fulfill the claim of the study.
In conclusion, in moderate-to-severe COPD patients in stable conditions sitting EFL is associated with lower values of FEV1, FEV1/FVC and reduced maximal expiratory flow rates. In these patients, however, when EFL occurs in supine position it is more frequently and significantly associated with functional and symptomatic markers such as lower DLCO and KCO and greater chronic dyspnea, reflecting the emphysematous rather than bronchiolitic prevalent phenotype of COPD.
Declaration of Interest
Acknowledgments
The authors are very grateful to Michele Guerini for the remarkable technical assistance.
References
- McDonough JE, Yuan R, Suzuki M, Small airways obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011; 365:1567–1575.
- Kim KD, Eidelman DH, Izquierdo JL, Centrilobular and panlobular emphysema in smokers. Two distinct morphologic and functional entities. Am Rev Respir Dis 1991; 144:1385–1390.
- Patel BD, Coxson HO, Pillai SG, Airway Wall Thickening and Emphysema Show Independent Familial Aggregation in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2008; 178:500–505.
- Nishimura M, Makita H, Nagai K, Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 185:44–52.
- Vestbo J, Edwards LD, Scanlon PD, Changes in forced expiratory volume in 1 second overtime in COPD. NEJM 2011; 365:1184–1192.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomized clinical trials. Lancet 2009; 374:685–694.
- Burrows B, Fletcher CM, Heard BE, Jones NL, Wootliff JS. The emphysematous and bronchial types of chronic airways obstruction. A clinico-pathological study of patients in London and Chicago. Lancet 1966; 87:830–805.
- Camiciottoli G, Bartolucci M, Maluccio NM, Spirometrically gated high-resolution CT findings in COPD: lung attenuation vs lung function and dyspnea severity. Chest 2006; 129:558–564.
- Mohamed Hoesein FAA, Zanen P, van Ginneken B Association of transfer coefficient of the lung carbon monoxide with emphysema progression in male smokers. Eur Respir J 2011; 38:1012–1018.
- Eltayara L, Becklake MR, Volta CA, Milic-Emily J. Relationship between chronic dyspnoea and expiratory flow-limitation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 154:1726–1734.
- Eltayara L, Ghezzo H, Milic-Emili J. Orthopnea and tidal expiratory flow limitation in patients with stable COPD. Chest 2001; 119:99–104.
- Boni E, Corda L, Franchini D, Volume effects and exertional dyspnoea after bronchodilator in patients with COPD with and without expiratory flow limitation at rest. Thorax 2002; 6:528–532.
- Koulouris NG, Dimopoulou I, Valta P, Finkelstein R, Cosio MG, Milic-Emily J. Detection of expiratory flow limitation during exercise in COPD patients. J Appl Physiol 1997; 82:723–731.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R e altri. Interpretative strategies for lung function test. Eur Respir J. 2005; 26:948–968.
- ATS. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152:S77–S120.
- Quanjer Ph.H, Tammeling GJ, Cotes JE, Lung volumes and forced ventilatory flows. Eur Respir J 1993; 6, Suppl. 16:5–40.
- Tantucci C, Pinelli V, Cossi S, Reference values and repeatability of inspiratory capacity for men and women aged 65–85. Respir Med. 2006; 100:871–877.
- Koulouris NG, Valta P, Lavoie A, A simple method to detect expiratory flow limitation during spontaneous breathing. Eur Respir J 1995; 8:306–313.
- Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978; 118:1–120.
- Camiciottoli G, Bigazzi F, Paoletti M, Cestelli L, Lavorini F, Pistolesi M. Pulmonary function and sputum characteristics predict CT phenotype and severity of COPD. Eur Respir J 2102; Dec 20 Epub ahead of print as doi: 10.1183/09031936.00133112.
- O'Donnell DE, Bertley JC, Chau LKL, Qualitative aspects of exertional breathlessness in chronic airflow limitation. Pathophysiologic mechanisms. Am J Respir Crit Care Med 1997; 155:109–115.

