Abstract
Repeated intratracheal injection of Pseudomonas aeruginosa (PA) in male Wistar rats was used to investigate the role of chronic infection in the development of chronic obstructive pulmonary disease (COPD) and the possible involvement of connective tissue growth factor (CTGF) and bone morphogenetic protein-7 (BMP-7) in this process. Injections of PA or normal saline solution were given for 8 weeks and the rats observed for a further 8 weeks. In addition to arterial blood gas, lung function and lung pathology measurement during this time period, protein and mRNA expression of CTGF and BMP-7 were measured, and the correlation of expression of CTGF and BMP-7 with pathological changes in the lung was evaluated. Repeated intratracheal PA infection in rats caused reduction in body weight, hypoxia, carbon dioxide retention, compromised lung function, chronic inflammation, thickening of the tracheal and arterial walls, and emphysema, changes consistent with those of COPD. Rats with PA infection also had increased CTGF and decreased BMP-7 expression, suggesting that both CTGF and BMP-7 are involved in the occurrence and development of airway remodeling. Our findings suggest that repeated airway infection is not only a factor resulting in deterioration of COPD, but is also a risk factor for its development, and that CTGF and BMP-7 are involved in the pathogenesis of this condition.
Introduction
Questions about whether bacterial infection is able to initiate or only to cause exacerbation of chronic obstructive pulmonary disease (COPD) have been present for several decades. Previous studies have shown that bacterial infection is a major cause of acute deteriorations in COPD, and that smoking and inhalation of harmful dust are the main causes of COPD. But in the 1950s, English investigators proposed that although smoking was a major cause of COPD, repeated airway infection and excessive secretion of mucus were also causative factors for COPD (Citation1).
In the 1990s, Sethi et al. (Citation2, 3) proposed a vicious circle hypothesis: -smoking, inhalation of harmful dust, respiratory diseases and other events are initiators that may impair mucociliary clearance. Then, after pathogens colonize the airway, the products of these pathogens induce a series of inflammatory reactions that result in an increase in mucus secretion, compromise in mucociliary clearance, degradation of elastic tissue and further damage to the respiratory mucosa. The sequential positive feedback of this cycle finally results in the development of COPD.
In clinical practice, COPD patients often have a history of chronic bronchitis or repeated lung infections that cause deterioration in the pathophysiology of COPD. However, in a fraction of COPD patients, known causes of COPD such as smoking and inhalation of harmful dust are not identified. We have previously shown that cigarette smoke and bacterial infection can be used together to establish a stable COPD model in rats (Citation4). But currently, there is no experimental and theoretical evidence to determine whether repeated pulmonary bacterial infection by itself can induce airway remodeling and subsequent progression into COPD.
Pseudomonas aeruginosa (PA) is an opportunistic pathogen that is also a major pathogen causing acute deterioration in COPD (Citation5). PA infection may also result in long-term colonization in a bacterial biofilm (Citation6, 7). Currently, whether PA can cause COPD is still unknown.
Connective tissue growth factor (CTGF) is a fibrogenic growth factor that belongs to the immediate early response gene family. The fibrogenic effect of transforming growth factor-β1 (TGF-β1) is mainly mediated by CTGF.
Bone morphogenetic protein-7 (BMP-7) is a member of the TGF superfamily. Studies have confirmed that BMP-7 can inhibit glomerular interstitial fibrosis and can reverse renal glomerular injury in mice (Citation8, 9). Izumi et al. (Citation10) showed that BMP-7 could protect rats against TGF-β1 induced pulmonary fibrosis by inducing the expression of Id2 and Id3.
In the present study, repeated intratracheal injection of PA was used to induce chronic lung infection in rats. Basic pathophysiological characteristics of COPD were examined and, at the same time, pulmonary expression of CTGF and BMP-7 was measured. In this way, the role of bacterial infection in producing the pathophysiological changes of COPD and the potential molecular mechanisms involved were investigated.
Materials and Methods
Animal model and grouping
A total of 96 male Wistar rats (specific pathogen free) weighing 250 ± 10 g were purchased from Slac Experimental Animal Center in Shanghai and fed with basic food and water for an accommodation period of 1 week. The protocol has the approval of the institutional animal committee on animal use. Animals were randomly assigned to a normal saline (NS) group (n = 48) and a PA group (n = 48). Six rats were sacrificed at each of the following time points: 0 days, 1 day, 1 week, 2 weeks, 4 weeks, 8 weeks, 12 weeks and 16 weeks for pathological and biochemical studies. In the NS group, rats received repeated intratracheal injection of normal saline (0.1 ml) twice weekly (on day 1 and day 4 of each week) for 4 weeks and then once weekly (on day 1 of the week) for the next 4 weeks.
In the PA group, rats received intratracheal injection of PA (ATCC27853; 0.1 ml; Clinical Laboratory Center of the Ministry of Health) at 0.7 × 108 CFU/ml in the same sequence. At the end of the 8 week PA or saline treatment period, the injections were stopped and the rats observed for a further 8 weeks. At each of the 8 time points described above, 6 rats from each group were selected for measurement of body weight and lung function, followed by sacrifice for the detection of blood gases, evaluation of pulmonary pathophysiology, and measurement of CTGF and BMP-7.
Body weight and arterial blood gas
After anesthesia, rats were placed on an electronic balance for the measurement of body weight and were then sacrificed. The abdominal aorta was exposed and arterial blood obtained (0.5 ml) for blood gas analysis (ABL800; Radiometer, Finland) to measure partial pressure of oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2).
Measurement of lung function
The AniRes2005 animal lung function analysis system (Beijing Bestlab Biotech Co., Ltd) was used to measure lung function. Animals were intraperitoneally anesthetized with 3% pentobarbital sodium at 0.75 mg/kg, followed by tracheal intubation and connection to a plethysmograph. Expiratory resistance (Re), forced expiratory peak flow (PEF), and ratio of forced expiratory volume in 0.3 second to forced vital capacity (FEV0.3/FVC%) were measured. FEV0.3/FVC% is a measurement reflecting airflow obstruction and is similar to FEV1/FVC%, a measurement that cannot be used in rats due to their rapid breathing rate.
Pathological examination
At the pre-designated time points, rats were sacrificed and the trachea, left lung, and middle and posterior lobes of right lung were collected and fixed in 10% neutral formalin followed by processing for haematoxylin/eosin staining.
Immunohistochemistry for CTGF and BMP-7
Immunohistochemistry was performed with a two-step method according to the manufacturer's instructions (EnvisionTM plus; Beijing Zhongshan Golden Bridge Co., Ltd), using HRP conjugated anti-rabbit (PV-6001) and anti-goat (PV-6003) secondary antibodies. After deparaffinization and dehydration, lung sections were treated with citrate buffer at high temperature and high pressure for antigen retrieval. Goat anti-rat CTGF (1:100) polyclonal antibody (Santa Cruz, CA, USA) and rabbit anti-rat BMP-7 (1:250) polyclonal antibody (Abcam, UK) were used as the primary antibodies. Visualization was done with diaminobenzadine staining and counterstaining with haematoxylin. In the negative control, the primary antibody was replaced with IgG.
On each slide (×200), 20 fields were selected and positive cell numbers were counted by 2 blinded observers. According to the percentages of positive cells that were recorded, the ICH results were graded from 0–4 using the following scoring scheme: 0: no positive cells; 1: <25% positive; 2: 25%–50% positive; 3: 50%–75% -positive; 4: >75% positive.
Quantitative analysis of pathological findings
Sections (∼3 mm in thickness) of the middle lobe of right lung were collected in the plane vertical to the bronchi and bronchioles and with maximal perimeter at the sagittal plane, followed by fixation and sectioning. Sections were observed under a microscopic micrometer (107JC, Shangguang Instrument Co., Ltd.) at ×400. The thickness of the tracheal wall and vessel wall was measured at the cross-section of bronchi and bronchioles. A total of 5 bronchial rings were measured and the data were averaged to calculate the thickness of the tracheal and vessel walls.
For goblet cell analysis, the lungs were fixed by inflation with 4% paraformaldehyde and embedded in paraffin. Sections were cut 5 mm thick and stained with periodic acid-Schiff/Alcian-blue. Assessments were performed on stratified random fields (n55) in each animal, as described by Nagai et al. (Citation11). Goblet cell metaplasia was assessed in bronchi with internal diameter measuring .200 mm in cross section, using a mucus grading system (scores 0–3), based on the ratio of goblet cell area to whole cross-sectional epithelial area in each round bronchus. In this grading system, a score of 0 indicates no goblet cells; a score of 1 indicates occupation of up to 1/3 of the epithelial area; a score of 2 indicates occupation of 1/3 to 2/3 of the epithelial area; and a score of 3 indicates occupation of 2/3 or more of the epithelial area. The mucus score was obtained by averaging the scores of the measured bronchi.
Quantitative analysis of neutrophil count
Sections (∼3 mm in thickness) of 3 regions of lung were fixed and haematoxylin/eosin stained. Sections were observed under a microscopic micrometer (107JC, Shangguang Instrument Co., Ltd.) at ×400, and the neurophils were counted. A total of 5 fields were measured and the data were averaged to calculate the neutrophil count.
Real-time RT-PCR
Total RNA was extracted with Trizol (Invitrogen) and used to reverse transcribe into cDNA according to the manufacturer's instructions (Reverse Transcription System; Promega). The gene sequences of GAPDH, CTGF and BMP-7 were obtained from the NCBI Genebank and the primers were designed with Primer 3 and synthesized by Shanghai Sangon Biotech Co., Ltd (). Real-time PCR was done in a Bio-Rad iCycler thermal cycler. In brief, 1 μl of cDNA was mixed with 6.25 μl of SYBR Premix ExTaqTM, each primer (0.25 μl), and sterilized ultrapure water (final volume: 12.5 μl).
Table 1. Primer sequences
The PCR conditions were as follows: predenaturation at 94°C for 3 min, 40 cycles of denaturation at 94°C for 30 s, annealing at 55° for 30 s, and extension at 72°C for 30 s, followed by a final extension at 72°C for 7 min. The mixture without cDNA served as a negative control. Experiments were done twice and in triplicate. The gene expression in the normal control group was used as a standard and was compared with that in the NS and PA groups. In addition, gene expression was compared between the NS and PA groups.
Statistical analysis
Data were presented as mean with standard deviation, which were compared between NS nail and PA groups by the Student's t-test. Comparisons were performed with repeated ANOVA with post-hoc comparison adjusted by the Bonferroni method. Data were analyzed with SPSS 15.0 statistics software (SPSS Inc, Chicago, IL, US), and a p-value < 0.05 was considered statistically significant.
Results
Body weight
Four weeks after PA infection, body weight in the PA group was significantly lower than that in the NS group (p = 0.0076). Twelve weeks later, at the end of study, the body weight in the PA group was even more profoundly reduced compared to the NS group (p < 0.0001) ().
Table 2. Body weight at different time points
Arterial blood gas analysis
Changes in PaO2 and PaCO2 in the two groups are shown in . By 12 weeks after PA infection, PaO2 was significantly reduced (p < 0.0001), and PaCO2 significantly increased (p = 0.0003) compared to the NS group. And at the end of study, the PaO2 in the PA group was also significantly lower and the PaCO2 significantly higher than that in the NS group (p < 0.0001 and p = 0.0004, respectively).
Table 3. PaO2 and PaCO2 at different time points
Lung function
Eight weeks after PA infection, the FEV0.3/FVC had a decreasing tendency, and by 16 weeks after PA infection, it was significantly reduced compared to both baseline (p < 0.0001) and NS group (p = 0.0017) levels. PEF started to become reduced 1 week after PA infection, and by 8 weeks after PA infection was significantly reduced compared to the NS group (p < 0.0001). Re began to increase 1 week after PA infection and was significantly different from that in the NS group at this time (p = 0.0007). The changes seen in lung function in the PA rats supported a diagnosis of COPD ().
Table 4. Lung function at different time points
Pathological examination of lung
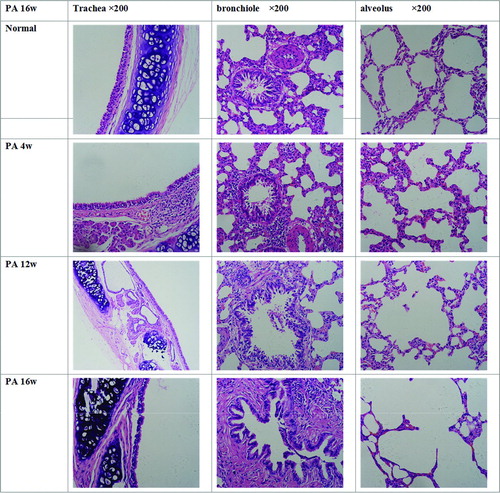
In the normal control group, the mucosal cilia in the trachea and bronchus were intact (not shedding), the ciliated columnar epithelial cells were regularly arranged, and no congestion or infiltration of inflammatory cells were seen in the tracheal wall and surrounding tissues. Bronchioles and alveoli had complete structures, and the alveolar septum was intact.
In the PA group at 4 weeks after PA infection, many glands were observed in the tracheal submucosa, goblet cell numbers were increased, and mucous plugs were present in the glandular ducts. Infiltration of lymphocytes and neutrophils was found in the submucosa of bronchioles and surrounding tissues, the alveolar septum was slightly thickened, congestion and edema were seen in the interstium, and proliferation of alveolar epithelial cells was noted.
In the PA group at 12 weeks after PA infection, the tracheal epithelial cells were irregularly arranged, hyperplasia of glands and rupture of cartilages were present, the lumen of the bronchioles was irregular, hyperplasia of surrounding smooth muscle was seen, and shedding epithelial cells were found in the lumen. Tracheal remodeling began to occur, the alveolar structure was irregular, and the alveolar wall was thinned, its volume increased, and in some places it had begun to rupture.
At 16 weeks after PA infection, the tracheal epithelial cells were irregularly arranged and shedding, hyperplasia of submucosal smooth muscle was evident, vascular congestion was found, proliferative goblet cells were observed in the bronchi, fibrosis of the submucosa was present, and smooth muscle had begun to thicken. Deposition of extracellular matrix was noted accompanied by airway remodeling. Bronchioles and alveoli showed tube-like or cyst-like expansion, and emphysematous changes were seen. The alveolar space was irregularly enlarged, the alveolar septum ruptured, and some alveoli had merged to form bullae (). Goblet cell scoring in the 2 groups is shown in . From 4 weeks onward, significantly more goblet cells were seen in the PA group than in the NS group (all p ≤ 0.0104).
Table 5. Goblet cell scoring of PA and NS groups (n = 6)
Quantitative analysis of pathological findings
As seen in the pathological examination, the tracheal wall began to thicken in the PA group as the duration of the PA infection lengthened, and this change was accompanied by hyperplasia of glands. Two weeks after PA infection, the tracheal wall was significantly thicker than that in the NS group (p = 0.0232). The walls of small blood vessels also thickened, and were significantly thicker than in the NS group at 4 weeks after PA infection (p = 0.0178). At the end of study, both the tracheal wall and the blood vessel wall in the PA group were significantly thicker than those in the NS group (p < 0.0001 and p = 0.0004, respectively) ().
Table 6. Thickness of tracheal wall and vessel wall at different time points
Neutrophil counts in trachea, bronchiole and alveolus
Changes in neutrophil count in the two groups are shown in . From 4 weeks after the beginning of PA inhalation until the end of the study, the neutrophil count in the PA group was significantly higher than that in the NS group in the trachea (all p ≤ 0.0002), the bronchiole (all p ≤ 0.0007), and the alveolus (all p ≤ 0.0008).
Table 7. Neutrophil counts in trachea, bronchiole and alveolus
Protein expression of CTGF and BMP-7 in the lung
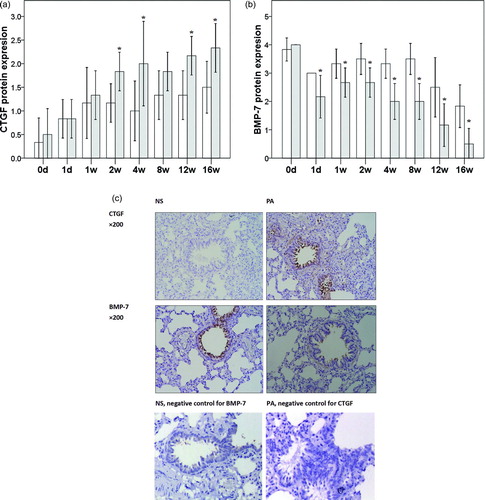
CTGF-positive staining was mainly found in the airway in ciliated epithelial cells, blood vessels and alveolar interstitial cells. In the NS group, only a few airway ciliated epithelial cells and alveolar interstitial cells were positive for CTGF and the staining was light. In the PA group, CTGF expression in the airway ciliated epithelial cells and alveolar interstitial cells began to increase at 2 weeks after PA infection, especially in the airway ciliated epithelial cells. The CTGF expression in the PA group was higher than that in the NS group. At the end of study, the CTGF expression in the PA group was significantly higher than that in the NS group. BMP-7 positive staining was mainly found in the airway ciliated epithelial cells and a few smooth muscle cells surrounding the airway.
In the NS group, the majority of airway ciliated epithelial cells were positive for BMP-7. In the PA group, BMP-7 expression decreased gradually with the prolongation of PA infection. At the end of study, CTGF protein expression in the PA was significantly higher than that in the NS group at 2, 4, 12 and 16 weeks (all p < 0.0493, ). In the PA group, from the first day after PA infection until the end of the study, BMP-7 protein expression was significantly lower than that in the NS group (all p < 0.0493, ). Representative images are shown in .
mRNA expression of CTGF and BMP-7 in the lung
Findings in real-time RT-PCR
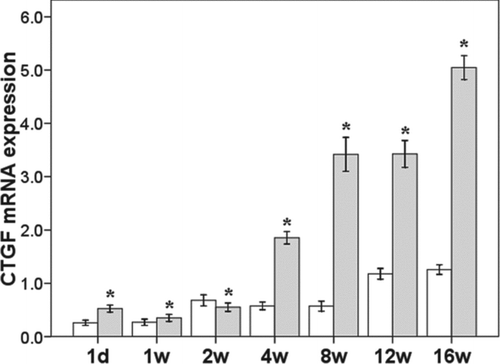
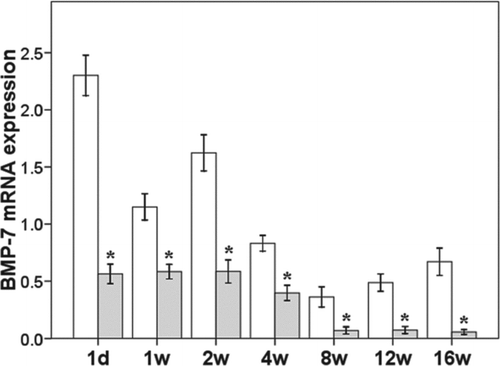
CTGF mRNA expression increased progressively with the prolongation of PA infection. In the PA group, from 1 day after PA infection onward, CTGF expression was significantly increased compared to the NS group (p < 0.0001). By the end of study, CTGF expression in the PA group had increased to approximately 5 times that in the NS group (p < 0.0001) (). BMP-7 mRNA expression decreased gradually in the PA group with the prolongation of infection, and was significantly lower than that in the NS group from 1 day after PA infection (p = 0.0219) until the end of the study (p = 0.0056, ).
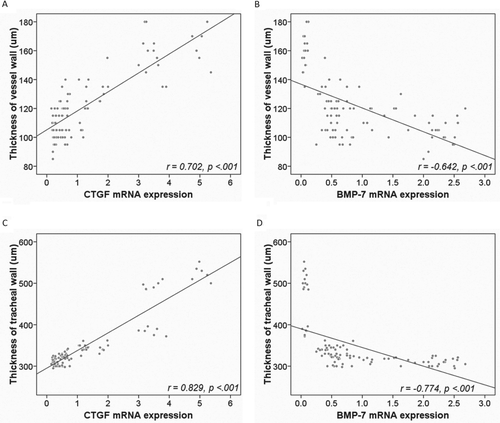
Association of thickness of tracheal wall and vessel wall with expression of CTGF and BMP-7
CTGF was positively and BMP-7 negatively correlated with tracheal and vessel wall thickness (, all p < 0.0001). In addition, CTGF expression was significantly negatively correlated to BMP-7 expression (r = −0.8911, p < 0.0001).
Discussion
In earlier studies, the reported evidence was not considered sufficient to support an important role of bacterial infection in chronic bronchitis. And in COPD, -infection (particularly bacterial infection) is usually regarded as a concomitant phenomenon that has no role in the -pathogenesis of this condition. In the last 20 years, however, molecular, cell and immunological techniques have been used to investigate the role of bacterial infection in COPD. Sethi et al. (Citation2, 3) found that infection, especially bacterial infection, was not only a major cause of acute deterioration of COPD, but an important factor in the pathogenesis of COPD. In the current study, PA, a pathogen known to be involved in the pathogenesis of COPD (Citation5, 6) and to be susceptible to colonization in the lung (Citation7), was used to induce chronic lung infection.
In previous studies, sulfur dioxide or smoking in combination with lipopolysaccaride and elastase were usually used to establish a COPD animal model. In the present study, these classical methods were not employed, but instead bacterial infection alone was used to establish the COPD model using the method described by Xu et al. (Citation12) with slight modifications. This method, in which intratracheal injection of a PA solution is performed under anesthesia to induce COPD, has the advantages that the amount of bacteria given is controlled and possible infection of researchers is avoided. In a pilot study, we determined that 0.7 × 108 CFU/ml PA was a dose at which the bacteria could induce chronic lung infection but at which an acceptable survival rate of the infected rats was ensured.
Currently, pathological examination and lung function measurements are used to confirm whether or not a COPD animal model is successful. In the present study, our results showed that repeated PA infection at the dose selected caused reduction in body weight, hypoxia, carbon dioxide retention and compromised lung function, accompanied by chronic airway inflammation and lung and airway remodeling. These findings are consistent with the pathophysiology seen in COPD.
The CTGF gene was first cloned from the cDNA library of human umbilical vein endothelial cells. CTGF is a polypeptide rich in cysteine containing 349 amino acids (Citation12, 13). It is known to be increased in COPD (Citation14) and causes cells to shift from an epithelial to a mesemchymal phenotype (Citation15) and in this way to promote remodeling. If CTGF is blocked, the vascular remodeling seen in cigarette smoke-induced pulmonary hypertension is reduced (Citation16).
Our results showed that in the absence of bacterial injection, CTGF expression was not found in the lung. With the prolongation of PA infection, pulmonary CTGF expression increased gradually. This expression was found mainly in the airway ciliated epithelial cells, trachea and surrounding interstium, findings consistent with those previously reported. When the correlation between CTGF expression and tracheal and vessel wall thickness was evaluated, the results showed CTGF expression to be closely correlated with changes in both thicknesses, a finding that suggests that CTGF is involved in PA-induced airway remodeling.
We speculated that PA infection might increase expression of TGF-β which then induced CTGF expression. CTGF would then act on downstream nuclear factors that promote the proliferation of smooth muscle cells in the airway and blood vessels and the transformation of smooth muscle cells into fibroblasts, increase the synthesis of extracellular matrix, mediate the infiltration of leukocytes, thicken the tracheal wall and vessel wall, and, in this way, finally induce airway remodeling. This hypothesis should be confirmed in future studies.
BMP-7, also known as osteopontin-1, is a member of the TGF-β superfamily. In the kidney, it reverses TGF-β1 induced epithelial to mesenchymal cell transformation and reverses renal injury (Citation8, 9, Citation17). In the liver, it inhibits hepatic fibrosis and promotes liver regeneration (Citation18).
Pulmonary fibrosis is similar in pathology to fibrosis of other organs. Thus, we speculate that BMP-7 might exert a protective effect on the lung. There is evidence showing that the balance between TGF-β and BMP signaling pathways is crucial for the repair, regeneration and fibrosis of the lung (Citation19, 20).
In the present study, with prolongation of PA infection, the BMP-7 expression in the airway mucosal epithelial cells and a few peri-tracheal smooth muscle cells became gradually reduced. In addition, correlation analysis showed BMP-7 expression to be negatively associated with the thickness of tracheal wall and vessel and negatively related to CTGF expression.
These findings suggest that the role of BMP-7 in the lung might be similar to that in the liver and kidney, and that BMP-7 can inhibit the development of pulmonary fibrosis and protect the lung.
A curious finding () was that lung function in PA rats was significantly worse than NS rats at 1 week after initiation of PA inhalation, but did not become significantly worse again until the 8- to 16-week period. This finding might be due to the small sample size. Another explanation might be that 1 week is the period of acute inflammation and the edema present at that time might be greter than at 2 or 4 weeks and be responsible for the significant difference seen at 1 week.
Taken together, repeated intratracheal injection of PA may cause reduction in body weight, decrease in arterial PaO2, increase in PaCO2, a compromise in lung function, chronic inflammation in the bronchi at different levels, thickening of tracheal wall, stenosis of trachea, airway remodeling and emphysema. These characteristics are consistent with the pathology of COPD. In addition, BMP-7 expression is reduced and CTGF expression increased in the airway epithelial cells and interstitial cells in the alveoli and surrounding the blood vessels, changes that may contribute to the above pathological changes. Thus, in addition to quitting smoking, measures such as timely and effective control of infection, strengthening of immune function, and prevention of chronic lung inflammation and airway remodeling are crucial for the prevention of COPD.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This study was supported by the Natural Science Foundation of Fujian province, China (Grant No. Z0516077).
References
- Fletcher CM. Chronic bronchitis, its prevalence, nature and pathogenesis. Am Rev Respir Dis 1959; 80:483–494.
- Sethi S. Bacterial infection and the pathogenesis of COPD. Chest 2000; 117 (5 Suppl 1):286S–91S.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359:2355–2365.
- Li Y, Li SY, Li JS, A rat model for stable chronic obstructive pulmonary disease induced by cigarette inhalation and repetitive bacterial infection. Biol Pharm Bull 2012; 35:1752–1760.
- Garcia-Vidal C, Almagro P, Romaní V, Pseudomonas aerugenosa in patients hospitalized for COPD exaberation: a prospective study. Eur Respir J 2009; 34:1072–1078.
- Murphy TF, Brauer AL, Eschberger K, Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177:853–860.
- Valderrey AD, Pozuelo MJ, Jiménez PA, Chronic colonization by Pseudomonas aerogenosa of patients with obstructive lung diseases: cycstic fibrosis, bronchiectasis, and chronic obstructive pulmonary disease. Diag Microb Infect Dis 2010; 68:20–27.
- Zeisberg M, Müller GA, Kallun R. Are there endogenous molecules that protect kidneys from injury? The case for bone morphogenic protein-7(BMP-7). Nephrol Dial Transplant 2004; 19:759–761.
- Zeisberg M, Hanai J, Sugimoto H, BMP-7 counteracts TGF-β1-induced epithelial to mesenchymal transition and reverses chronic renal injury. Nat Med 2003; 29:964–968.
- Izumi N, Mizuguchi S, Inagaki Y, BMP-7 opposes TGF-β1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am J Physiol Lung Cell Mol Physiol 2006; 290:L120–126.
- Nagai A, Inano H, Takizawa T. Morphologic changes in the airways induced by recurrent exposure of acetylcholine in the guinea pig. Am Rev Respir Dis 1990; 142:172–176.
- Xu H, Xiong M, Huang QH, The study on COPD rat model produced by bacterial infection. Chin J Tuberc Respir Dis 1999; 22:739–742. (in Chinese)
- Bradham DM, Igarashi A, Potter RL, Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC induced immediate early gene product CEF-10. J Cell Biol 1991; 114:1285–1294.
- Nishioka M, Ogawa E, Kinose D, Lipopolysaccaride induced connective tissue growth factor gene expression in human bronchial epithelial cells. Respirology 2010; 15:669–676.
- Ogawa E, Elliott WM, Hughes F, Latent adenoviral infection induces production of growth factors relevant in airway remodeling in COPD. Am J Physiol Lung Cell Mol Physiol 2004; 286:L189–197.
- Wang R, Xu Y, Liu XS, Knockdown of connective tissue growth factor by plasmid-based short hairpin RNA prevented pulmonary vascular remodeling in cigarette smoke-exposed rats. Arch Biochem Biophys 2011; 508:93–100.
- Morrissey J, Hruska K, Guo G, Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol 2002; 13 Suppl 1:S14–21.
- Sugimoto H, Yang C, LeBleu VS, BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J 2007; 21:256–264.
- Liu Y, Chen J, Yang Y, Molecular impact of bone morphogenetic protein 7, on lung cancer cells and its significance. Int J Mol Med 2012; 29:1016–1024.
- Pegorier S, Campbell GA, Kay AB, Bone Morphogenetic Protein (BMP)-4 and BMP-7 regulate differentially Trransforming Growth Factor (TGF)-β1 in normal human lung fibroblasts (NHLF). Respir Res 2010; 11:85.