Abstract
Background: This randomized, double-blind, Phase IIIb study evaluated the 24-hour bronchodilatory efficacy of aclidinium bromide versus placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). Methods: Patients received aclidinium 400 μg twice daily (morning and evening), tiotropium 18 μg once daily (morning), or placebo for 6 weeks. The primary endpoint was change from baseline in forced expiratory volume in 1 second area under the curve for the 24-hour period post-morning dose (FEV1 AUC0–24) at week 6. Secondary and additional endpoints included FEV1 AUC12–24, COPD symptoms (EXAcerbations of chronic pulmonary disease Tool-Respiratory Symptoms [E-RS] total score and additional symptoms questionnaire), and safety. Results: Overall, 414 patients were randomized and treated (FEV1 1.63 L [55.8% predicted]). Compared with placebo, FEV1 AUC0–24 and FEV1 AUC12–24 were significantly increased from baseline with aclidinium (∆ = 150 mL and 160 mL, respectively; p < 0.0001) and tiotropium (∆ = 140 mL and 123 mL, respectively; p < 0.0001) at week 6. Significant improvements in E-RS total scores over 6 weeks were numerically greater with aclidinium (p < 0.0001) than tiotropium (p < 0.05) versus placebo. Only aclidinium significantly reduced the severity of early-morning cough, wheeze, shortness of breath, and phlegm, and of nighttime symptoms versus placebo (p < 0.05). Adverse-event (AE) incidence (28%) was similar between treatments. Few anticholinergic AEs (<1.5%) or serious AEs (<3%) occurred in any group. Conclusions: Aclidinium provided significant 24-hour bronchodilation versus placebo from day 1 with comparable efficacy to tiotropium after 6 weeks. Improvements in COPD symptoms were consistently numerically greater with aclidinium versus tiotropium. Aclidinium was generally well tolerated.
Introduction
Circadian variation in lung function, driven in part by changes in cholinergic tone, has been documented in patients with chronic obstructive pulmonary disease (COPD) (Citation1–3). Variation in COPD daily symptoms has also been reported, with the most severe symptoms generally experienced in the morning followed by during the nighttime (Citation4, 5). Consequently, the importance of identifying and managing early-morning symptoms is generally accepted. However, unlike in asthma where variation in lung function and symptoms has been better characterized, the clinical relevance of nighttime symptoms can sometimes be underestimated in COPD and a lack of routine assessment means they can be under-reported (Citation6). As symptoms throughout the day impact on patient quality of life (Citation5), maintaining significant bronchodilation and symptom control over 24 hours should be an important goal of therapy.
Inhaled bronchodilatory therapies, including long-acting β2-agonists and long-acting muscarinic antagonists (LAMAs), are central to COPD management (Citation7); however, until recently, tiotropium bromide was the only available agent in the LAMA class (Citation2, Citation8). Aclidinium bromide is a LAMA that has recently been approved as a maintenance bronchodilator treatment for patients with COPD (Citation9, 10). In a Phase IIa study, twice-daily (BID) treatment with aclidinium 400 μg was demonstrated to provide significant improvements in 24-hour bronchodilation versus placebo that were generally similar to once-daily (QD) treatment with tiotropium 18 μg after 2 weeks, although significant differences in favor of aclidinium were observed for the nighttime period (Citation11).
The Phase IIIb study reported in this paper was conducted to confirm the 24-hour bronchodilatory efficacy of aclidinium versus placebo and tiotropium over a longer treatment period (6 weeks) and in a larger population. The effects of treatment on COPD symptoms, inhaler preference, and safety were also evaluated in this Phase IIIb study.
Methods
Study design and treatment
This randomized, double-blind, double-dummy, placebo- and active comparator-controlled, multicenter Phase IIIb study was conducted in the Czech Republic, Germany, Hungary, and Poland between October 2011 and March 2012 (clinicaltrials.gov identifier NCT01462929).
Following screening and a 2- to 3-week run-in period, during which disease stability was assessed, patients were randomized (2:2:1) via an interactive voice-response system and computer-generated schedule to receive aclidinium bromide 400 μg (metered dose; equivalent to aclidinium 322 μg delivered dose) BID in the morning and evening via the Genuair®/PressairTM multidose dry powder inhaler, tiotropium 18 μg QD in the morning via the HandiHaler®, or placebo for 6 weeks.
Two Genuair inhalers (pre-loaded with 1 month's supply [60 doses] of either aclidinium or matched placebo) and one HandiHaler (with 60 capsules of tiotropium or matched placebo) were supplied. To maintain blinding, patients were instructed to use both inhalers each morning (9:00 ± 1 hour) and Genuair only each evening (21:00 ± 1 hour). Patients and study personnel remained blinded to treatment allocation throughout the study. Training on the correct use of the inhalers was provided at screening and before randomization on day 1.
Relief medication (salbutamol pressurized metered-dose inhaler 100 μg/puff) was provided for additional symptom control as needed (except ≤6 hours before each visit). Patients were permitted to continue stable use of oral sustained-release theophylline (use of other methylxanthines was not permitted), inhaled corticosteroids, and oral or parenteral corticosteroids (prednisone ≤10 mg/day or 20 mg/every other day, or equivalent), except ≤6 hours before each visit. Oxygen therapy (except ≤2 hours before each visit) was allowed.
The study was approved by an independent ethics committee at each site and was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization and Good Clinical Practice. Patients provided written informed consent before participating in any study procedures.
Patients
Eligible patients were aged ≥40 years with a clinical diagnosis of stable moderate-to-severe COPD (post-bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] <70%, and FEV1 ≥30% and <80%) (Citation12) and were either current or former cigarette smokers (smoking history of ≥10 pack-years). Patients with a history or current diagnosis of asthma or other clinically significant respiratory or cardiovascular conditions were excluded, as were those who had experienced any respiratory tract infection or COPD exacerbation ≤6 weeks before screening (≤3 months if exacerbation resulted in hospitalization), or for whom the use of muscarinic antagonists was contraindicated. Additional relevant exclusion criteria included hypersensitivity to inhaled muscarinic antagonists and inability to use the study inhalers properly.
Patients could be discontinued from the study at any time at their own request or in the event of ineligibility, non-compliance, lack of efficacy, loss to follow-up, safety concerns (including moderate or severe COPD exacerbation), or any other reason at the investigator's discretion.
Study Assessments
Lung function
Lung function was assessed over 24 hours following morning treatment on day 1 and at week 6. FEV1 and FVC were measured using procedures and spirometers that met European Respiratory Society (ERS) and American Thoracic Society (ATS) standards (Citation13). Three manoeuvres were performed at each time point to provide three measurements that met ATS/ERS acceptability and repeatability criteria. Additional measurements could be made (up to a total of eight tests) if the first three were not acceptable.
The primary endpoint was change from baseline in normalized FEV1 area under the curve over the 24-hour period post-morning dose (AUC0–24) at week 6 (tiotropium administered QD in the morning; aclidinium administered BID in the morning and evening). Change from baseline in normalized FEV1 AUC over the nighttime period (AUC12–24) at week 6 was the secondary endpoint. Changes from baseline in normalized FEV1 AUC for the 12-hour period post-morning treatment (AUC0–12), morning pre-dose (trough) and peak FEV1 and FVC were additional endpoints.
COPD daily symptoms and relief medication use
Patients completed the 14-item EXAcerbations of Chronic pulmonary disease Tool (EXACT) each evening before bedtime (recall period of ‘today’) via electronic diaries. Responses to 11 of 14 EXACT questions that captured changes in specific respiratory symptoms (grouped into breathlessness, cough and sputum, and chest symptom domains) were used to calculate an EXACT-Respiratory Symptoms (E-RS) (Citation14) total score (range 0–40; a higher score indicates more severe symptoms).
An additional COPD symptoms questionnaire (developed by the study sponsor) was completed by patients each morning via electronic diaries to capture the severity of early-morning and nighttime symptoms (5-point scale: 1 = ‘did not experience symptoms’; 5 = ‘very severe’), and individual morning symptoms of cough, wheeze, shortness of breath, and phlegm (5-point scale: 0 = ‘no symptoms’; 4 = ‘very severe symptoms’), as well as limitation of morning activities (5-point scale: 1 = ‘not at all’; 5 = ‘a very good deal’) and frequency of nocturnal awakenings as a result of COPD symptoms. Relief medication use was also recorded daily via electronic diaries.
Inhaler preference and willingness to continue
After 6 weeks, overall inhaler preference and preference based on a number of specific inhaler attributes were assessed. Patients were first asked ìwhich inhaler do you prefer?î (Genuair, HandiHaler, or no preference) then, ìwhich device do you prefer in terms of the following attributes: ease of use, convenience, ease of learning to use, ease of holding, ease of operating, ease of preparation of the dose, and feedback to indicate correct inhalation?î Willingness to continue using each inhaler was also rated on a scale from 0 = ‘not willing’ to 100 = ‘definitely willing’.
Safety
Adverse events (AEs) were recorded throughout the study and assessed for severity and relationship to study treatment by the investigator. Other safety assessments included a physical examination and vital signs measurement at screening and week 6.
Statistical Analysis
A target population of approximately 405 patients was planned to provide a sample size of 385 patients, taking into account a 5% dropout rate. This provided >90% power to detect a 130 mL difference between aclidinium and placebo for the primary and secondary endpoints, and >80% power to detect a 70 mL difference between aclidinium and tiotropium for the secondary endpoint, with a two-sided significance level of p < 0.05. Assumptions about treatment group differences were made based on observations from the previous Phase IIa study that compared the 24-hour bronchodilatory efficacy of aclidinium with placebo and tiotropium (Citation11).
Efficacy analyses were based on the intent-to-treat population, which included all treated patients (≥1 dose) who had ≥1 baseline and post-baseline FEV1 value. Endpoints were assessed using an analysis of covariance model with treatment and sex as factors, and age and baseline values as covariates. Between-group least squares mean differences and 95% confidence intervals were calculated for all treatment-group comparisons. The primary and secondary endpoint analyses were conducted in a stepwise manner to control for multiplicity: for FEV1 AUC0–24, the primary comparison was aclidinium versus placebo; for FEV1 AUC12–24, the primary comparison was aclidinium versus placebo, and the secondary comparison was aclidinium versus tiotropium. Other comparisons were considered additional.
Inhaler preference was summarized descriptively and the percentage of patients preferring Genuair was assessed using an exact binomial test. Willingness to continue inhaler use was assessed using an analysis of variance. Safety analyses included all treated patients (safety population) and were descriptive in nature.
Results
Patients
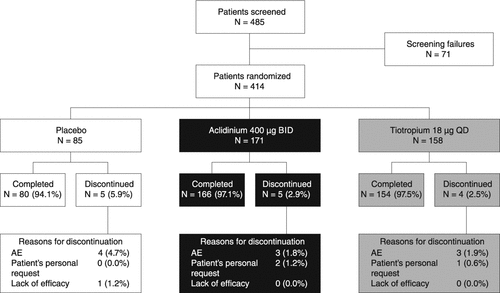
Of 485 patients screened, 414 patients from 41 sites (2.7% from 3 sites in the Czech Republic, 49.5% from 20 sites in Germany, 10.6% from 5 sites in Hungary, and 37.2% from 13 sites in Poland) were randomized, treated, and included in the study analyses, and 400 completed the study (). The discontinuation rate was slightly higher in the placebo group (5.9%) compared with the aclidinium (2.9%) or tiotropium (2.5%) groups. In the placebo group, reasons for discontinuation were AEs and lack of efficacy, whereas in the aclidinium and tiotropium groups, reasons for discontinuation were AEs and patient's personal request (). No patients were lost to follow-up. Treatment compliance, as assessed by the investigator based on information provided in patients’ electronic diaries and the Genuair dose indicator or number of tiotropium pierced capsules, was 94.1%, 98.2%, and 96.8% in the placebo, aclidinium, and tiotropium groups, respectively.
Demographics and baseline characteristics were similar across treatment groups, with the exception of higher proportions of male patients in the aclidinium and tiotropium groups than in the placebo group (). Mean post-bronchodilator percent predicted FEV1 at screening (adjusted for gender-related differences) was similar in each treatment group but, reflective of the higher proportion of male patients in the active treatment groups, mean FEV1 was slightly higher in these groups versus the placebo group. In total, 86.7% of patients had used COPD medications prior to the start of the study. Anticholinergic therapies (LAMA, short-acting muscarinic antagonist [SAMA], or short-acting β-2 agonist plus SAMA) had been used by 25.6%, 18.8%, and 5.3% of patients, respectively.
Table 1. Demographics and baseline characteristics of the study population (safety population)
Lung function
On day 1 of treatment, both aclidinium and tiotropium improved 24-hour, nighttime, and day time bronchodilation, as demonstrated by statistically significant increases in FEV1 AUC0–24, FEV1 AUC12–24, and FEV1 AUC0–12 from baseline versus placebo (p < 0.001; ). Improvements in FEV1 AUC0–24 and FEV1 AUC12–24 were significantly greater with aclidinium versus tiotropium (p < 0.05 and p < 0.01, respectively) on day 1.
Figure 2. Change from baseline in FEV1 AUC0–24, FEV1 AUC12–24, and FEV1 AUC0–12 compared with placebo (A) on day 1 and (B) at week 6 (ITT population). Data reported as LS mean (95% CI) differences from placebo (ANCOVA). ‡p < 0.001; §p < 0.0001 for aclidinium or tiotropium versus placebo. ¶p < 0.05; #p < 0.01 for aclidinium versus tiotropium. ANCOVA, analysis of covariance; AUC, area under the curve; AUC0–24, AUC over the 24-hour period post-morning treatment; AUC12–24, AUC over the nighttime period; AUC0–12, AUC for the 12-hour period post-morning treatment; BID, twice daily; CI, confidence interval; FEV1, forced expiratory volume in 1 second; ITT, intent-to-treat; LS, least squares; QD, once daily.
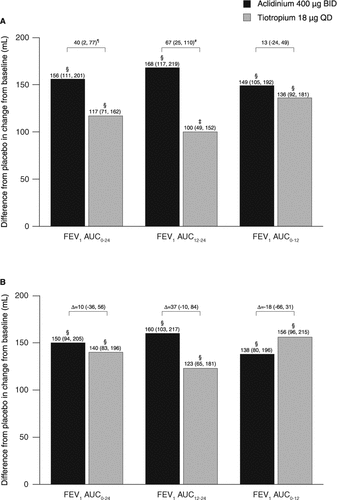
In the primary endpoint analysis, FEV1 AUC0–24 was significantly improved with aclidinium compared with placebo at week 6 (p < 0.0001; ). Compared with placebo, tiotropium also significantly increased FEV1 AUC0–24 from baseline to week 6 (p < 0.0001; ); the effects of aclidinium and tiotropium over 6 weeks were similar.
Over 6 weeks, FEV1 AUC12–24 and FEV1 AUC0–12 were also significantly increased from baseline with both aclidinium and tiotropium versus placebo (p < 0.0001) (). Although the improvement in FEV1 AUC12–24 was numerically greater with aclidinium versus tiotropium, and the improvement in FEV1 AUC0–12 was numerically greater with tiotropium versus aclidinium, there were no significant differences between active treatments (p = 0.12 and p = 0.48, respectively).
Both aclidinium and tiotropium produced significant increases from baseline in FEV1 versus placebo at each observation time point from 15 minutes to 24 hours post-dose on day 1 and at week 6 (). Both aclidinium and tiotropium also significantly improved morning pre-dose (trough) and peak FEV1 and FVC compared with placebo on day 1 and at week 6 (). Improvements were numerically greater with aclidinium versus tiotropium, with significant differences in favor of aclidinium for trough FEV1 and FVC on day 1.
Figure 3. Change from baseline in FEV1 over 24 hours (A) on day 1 and (B) at week 6. Data reported as LS mean change from baseline (ANCOVA). p < 0.01 for aclidinium and tiotropium versus placebo at each time point. ¶p < 0.05; #p < 0.01; ∥p < 0.001; ¶¶p < 0.0001 for aclidinium versus tiotropium. ANCOVA, analysis of covariance; BID, twice daily; FEV1, forced expiratory volume in 1 second; LS, least squares; QD, once daily.
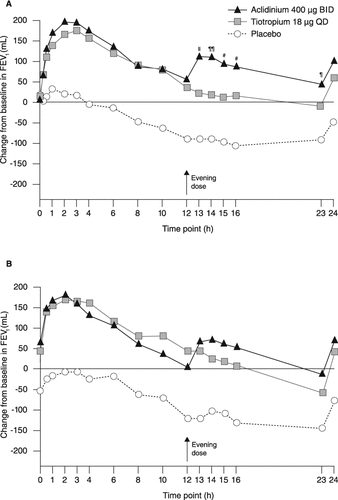
Table 2. Change from baseline (difference from placebo) in additional lung-function variables (ITT population)
COPD Symptoms and Relief Medication Use
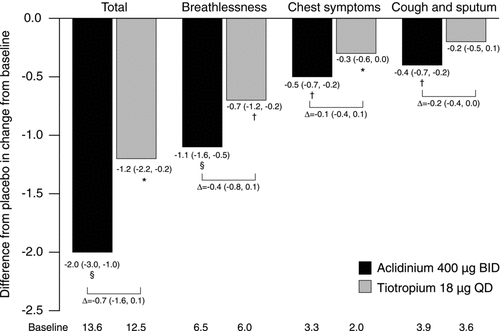
Over 6 weeks, E-RS total scores were significantly reduced from baseline with both aclidinium (p < 0.0001) and tiotropium (p < 0.05) versus placebo (). Improvements in individual domain scores were numerically greater with aclidinium than tiotropium, and the improvement for cough and sputum was significant versus placebo for aclidinium only (p < 0.05). Comparisons of aclidinium versus tiotropium yielded no significant differences.
Figure 4. Change from baseline in E-RS total and domain scores over 6 weeks. Data reported as LS mean (95% CI) difference from placebo (ANCOVA). *p < 0.05; †p < 0.01; §p < 0.0001 for aclidinium or tiotropium versus placebo. ANCOVA, analysis of covariance; BID, twice daily; CI, confidence interval; E-RS, EXAcerbations of Chronic pulmonary disease Tool (EXACT)-Respiratory Symptoms; LS, least squares; QD, once daily.
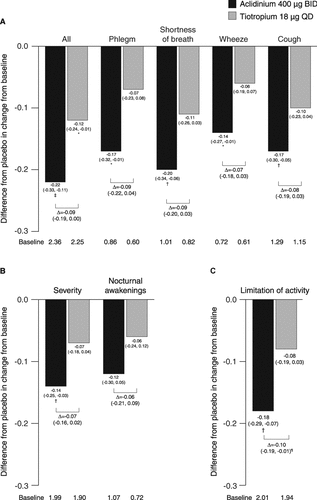
The severity of early-morning symptoms, overall, was significantly reduced over 6 weeks with aclidinium (p < 0.001) and tiotropium (p < 0.05) versus placebo (); the difference between active treatments was not statistically significant. Only aclidinium produced significant improvements in individual early-morning symptoms of phlegm, shortness of breath, wheeze, and cough compared with placebo at week 6. Nighttime symptom severity was significantly reduced from baseline over 6 weeks with aclidinium versus placebo but not with tiotropium versus placebo (); the difference between active treatments was not statistically significant. There were no significant changes from baseline in number of nocturnal awakenings in any treatment group. Limitation of activity caused by COPD symptoms was also significantly reduced from baseline over 6 weeks with aclidinium versus placebo but not with tiotropium versus placebo (). This improvement in limitation of activity was significantly greater for aclidinium versus tiotropium (p < 0.05).
Figure 5. Difference from placebo in change from baseline in (A) severity of early-morning symptoms, (B) severity of nighttime symptoms and number of nocturnal awakenings due to COPD symptoms, and (C) limitation of activity caused by COPD symptoms (COPD additional symptoms questionnaire) over 6 weeks (ITT population). Data reported as LS mean differences from placebo (ANCOVA). *p < 0.05; †p < 0.01; ‡p < 0.001 for aclidinium or tiotropium versus placebo. ¶p < 0.05 for aclidinium versus tiotropium. Severity of overall early-morning and nighttime symptoms rated on a 5-point scale from 1 = ‘did not experience any symptoms’ to 5 = ‘very severe’; individual morning symptoms rated on a 5-point scale from 0 = ‘no symptoms’ to 4 = ‘very severe’; limitation of activity rated on a 5-point scale from 1 = ‘not at all’ to 5 = ‘a very good deal’. ANCOVA, analysis of covariance; BID, twice daily; COPD, chronic obstructive pulmonary disease; ITT, intent-to-treat; LS, least squares; QD, once daily.
Over 6 weeks, there was a significant increase in relief medication-free days with aclidinium and tiotropium versus placebo (difference of 9.6% and 8.9%, respectively; p < 0.05).
Inhaler preference
When asked ìwhich device do you prefer?î at week 6, significantly more patients overall preferred Genuair to HandiHaler (80.1% vs 10.7%; p < 0.0001). The option of ‘no preference’ was chosen by 9.2% of patients. Additionally, >75% of patients preferred Genuair to HandiHaler for each of the individual inhaler attributes assessed (). Inhaler preference appeared to be independent of whether active medication or placebo was administered via the inhalers: when the aclidinium, tiotropium, and placebo groups were analyzed separately, ≥79% of patients preferred Genuair compared with ≤13% who preferred HandiHaler in each group. Patients were more willing to continue using Genuair than HandiHaler, as indicated by a significant difference in mean ratings at week 6 (88.8 vs 45.4; p < 0.0001).
Table 3. Patient preference for each inhaler based on specific inhaler attributes at week 6, n (%)
Safety
AE incidence was similar in the placebo (25.9%), aclidinium (27.5%), and tiotropium (29.7%) groups. Nasopharyngitis and headache were most common, each reported by 5.1% of patients overall. Nasopharyngitis occurred more frequently with aclidinium and tiotropium versus placebo (5.8% and 5.7% vs 2.4%, respectively); headache occurred more frequently with aclidinium than with either placebo or tiotropium (7.0% vs 3.5% and 3.8%, respectively). Other common AEs (≥2% of patients overall) were COPD exacerbation (2.4%) and cough (2.2%). The majority of AEs were mild or moderate in intensity. There were few serious AEs (1.7% overall) and no deaths. In total, 8 patients (1.9%) discontinued due to AEs, with COPD exacerbation being the most common cause (n = 2 in each treatment group). No AEs resulting in discontinuation were considered to be treatment-related.
With the exception of dry mouth, which was reported by three patients (0.7%) in total (aclidinium n = 1; tiotropium n = 2), no other treatment-related AEs occurred in >1 patient overall. The incidence of potentially anticholinergic AEs was similarly low across treatment groups (<1.5% in any group). Only dry mouth, pharyngitis (placebo n = 1; aclidinium n = 1; tiotropium n = 2), and constipation (tiotropium n = 2) occurred in >1 patient in any group. No potentially anticholinergic AEs were serious or led to discontinuation. There were no clinically significant physical examination or vital signs findings.
Discussion
This Phase IIIb study was designed to evaluate the 24-hour bronchodilatory efficacy of aclidinium 400 μg BID in patients with moderate-to-severe COPD. Findings from our primary endpoint analysis of change from baseline in FEV1 AUC0–24 after 6 weeks of treatment demonstrated a statistically significant improvement with aclidinium versus placebo, and changes from baseline in trough FEV1 with aclidinium exceeded the proposed minimally important difference for this parameter (100–140 mL) (Citation15, 16). Furthermore, aclidinium significantly improved day time and nighttime bronchodilation over placebo. These findings are consistent with an earlier 15-day Phase IIa cross-over study (n = 30) that also evaluated 24-hour bronchodilation with aclidinium versus placebo and tiotropium, although a larger difference between aclidinium and placebo for the change from baseline in FEV1 AUC0–24 (232 mL) was reported previously, potentially as a result of differences in trial design and duration (Citation11).
Also consistent with previous findings are the numerically greater improvements in nighttime bronchodilation achieved with aclidinium versus tiotropium in this study, with statistically significant improvements favoring aclidinium on day 1 of treatment. These day 1 findings may be explained by differences in the pharmacokinetics of aclidinium and tiotropium, whereby aclidinium reaches steady state more quickly than tiotropium (Citation17, 18). After 6 weeks of treatment, the 24-hour bronchodilatory efficacy of aclidinium and tiotropium was considered to be comparable.
Many patients with COPD experience early-morning or nighttime peaks in symptom severity (Citation5). Although as many as 80%–90% of patients experience nighttime symptoms (Citation19) and sleep disturbance (Citation6), there is a lack of therapeutic options for their management. To date, a small number of studies have evaluated the efficacy of bronchodilatory therapies to improve nighttime lung function and/or sleep quality with mixed findings (Citation2, Citation20–23). For example, a 6-week study in patients with stable COPD found that evening dosing of tiotropium did not result in improved nighttime bronchodilation compared with morning dosing (Citation2).
Additionally, reduced daily variation in lung function and improved nighttime oxygen saturation achieved with evening administration of tiotropium in a separate 4-week study in patients with severe COPD did not translate into improved sleep quality (Citation21). Conversely, four-times-daily treatment with ipratropium (a SAMA) has been shown to improve oxygen saturation and sleep quality over 4 weeks in patients with moderate-to-severe COPD (Citation20).
The impact of treatment with aclidinium on sleep quality has not been evaluated in a sleep laboratory setting; however, it has been demonstrated to improve health status and early-morning and nighttime symptoms, and to reduce the frequency of nocturnal awakenings in Phase III studies (ATTAIN and ACCORD COPD I) (Citation24–27). In the present study, changes in COPD daily symptoms following BID dosing of aclidinium and QD dosing of tiotropium compared with placebo were assessed using the E-RS tool (Citation14, Citation28), and an additional symptoms questionnaire (currently undergoing validation), that was developed by the sponsor in the absence of a validated tool to capture the severity and impact of early-morning and nighttime symptoms.
Our results suggest that aclidinium provides statistically significant improvements in early-morning and nighttime symptoms compared with placebo that were consistently numerically greater than those observed with tiotropium. Improvements in nighttime symptom severity were significantly different versus placebo for aclidinium only, which could suggest that the numerical advantage of aclidinium over tiotropium for greater nighttime bronchodilation may translate into statistically significant changes in patient-reported outcomes. Furthermore, only aclidinium significantly reduced the limitation of activity caused by COPD symptoms compared with placebo. This is the first report of this therapeutic effect with a LAMA but should, along with the other COPD additional symptoms findings, be interpreted with some caution given the generally mild symptoms that patients reported at baseline, the small magnitude of the reductions observed, and the as yet unvalidated state of this tool.
The trend towards greater symptomatic improvement with aclidinium over tiotropium observed in this study may be related to differences in dosing frequency. However, while a second evening dose of aclidinium may be beneficial in terms of improving nighttime and early-morning symptoms under clinical trial conditions, the potential disadvantages of BID versus QD dosing should also be considered. Findings from an observational study have suggested that treatment adherence among patients with COPD declines with increasing dosing frequency (Citation29), but this appears to be a greater concern for three- and four-times-daily regimens, and there is a lack of evidence to support greater adherence to QD versus BID treatment in practice.
Furthermore, poor adherence to prescribed treatment in COPD is multifactorial: it can also be influenced by perceived efficacy of treatment, side effects, number of concomitant medications, patient understanding, inhaler satisfaction or preference, and a number of other issues (Citation30–32). Due to the double-blind, double-dummy design of this study, adherence to tiotropium QD compared with aclidinium BID could not be assessed but may be of interest for future work.
Although not powered for this purpose, patients’ preference for Genuair compared with HandiHaler was assessed as an additional endpoint in this study and our findings were consistent with those of a previous study that reported greater preference for, greater satisfaction with, and fewer errors with, Genuair versus HandiHaler after 2 weeks’ daily practice with placebo-containing devices (Citation33).
However, in contrast with the previous study, patients inhaled both aclidinium and placebo through Genuair, and both tiotropium and placebo via HandiHaler in the present study. Patients were not asked to consider perceived efficacy when indicating their preferred device and preference for Genuair was maintained regardless of whether it was used to deliver placebo or aclidinium.
Finally, aclidinium was generally well tolerated over 6 weeks in this study, with a similar safety profile to tiotropium. Our findings are consistent with previous Phase III studies in terms of similar incidences of AEs and serious AEs in patients treated with aclidinium or placebo (Citation24, 25). The potential for anticholinergic AEs, such as dry mouth, constipation, urinary retention, and cardiovascular events, is a risk associated with LAMAs.
Aclidinium, however, has been shown to undergo more rapid hydrolysis than tiotropium in human plasma (Citation34, 35) and could, therefore, be considered to have the potential for fewer systemic side effects than tiotropium. Tiotropium has been associated with an increased risk of all-cause and cardiovascular-related mortality when administered via soft mist inhaler (Citation36–38) but not when administered via HandiHaler (Citation39).
In this 6-week study, the incidence of dry mouth, constipation, and other anticholinergic AEs (<1.5% for any event) was somewhat lower than has been observed in a pooled analysis of tiotropium safety data from 26 Phase III and IV studies (Citation39), possibly as a result of the short trial duration and patients’ previous exposure to tiotropium. The incidence of anticholinergic AEs was similarly low in the aclidinium treatment group, consistent with findings from other Phase III studies (Citation24, 25).
Conclusions
These findings suggest that in patients with moderate-to-severe COPD, aclidinium 400 μg BID provides significant 24-hour bronchodilation compared with placebo from day 1 and over 6 weeks of treatment. At 6 weeks, the bronchodilatory effects of aclidinium and tiotropium are generally comparable. Aclidinium provides consistently numerically greater improvements in COPD symptoms, including early-morning and nighttime symptoms, than tiotropium and is well tolerated, with a similar safety profile. Patients in this study preferred Genuair over HandiHaler for the administration of their inhaled medications.
Declaration of Interest Statement
Jutta Beier has received consulting fees, speaker's fees, and travel expenses from Almirall. Anne-Marie Kirsten has received research grants from Almirall. Rosa Segarra, Ferran Chuecos, and Esther Garcia Gill are employees of Almirall and have stock/stock options in Almirall. Cynthia Caracta is an employee of Forest Research Institute. Robert Mró declares that he has no conflicts of interest to disclose.
Genuair® is the registered trademark of Almirall S.A., Barcelona, Spain for use within the European Union, Iceland, and Norway, and within the United States as PressairTM.
The authors alone are responsible for the content and writing of the paper.
Acknowledgment
This study was supported by Almirall, S.A., Barcelona, Spain, and Forest Laboratories, Inc., New York, NY, USA. Editorial support, funded by Almirall, S.A., was provided by Lynsey Stevenson of Complete Medical Communications.
References
- Postma DS, KoÎter GH, vd Mark TW, Reig RP, Sluiter HJ. The effects of oral slow-release terbutaline on the circadian variation in spirometry and arterial blood gas levels in patients with chronic airflow obstruction. Chest 1985; 87:653–657.
- Calverley PM, Lee A, Towse L, van Noord J, Witek TJ, Kelsen S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 2003; 58:855–860.
- van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, Cornelissen PJ. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest 2006; 129:509–517.
- Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, Ostinelli J. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J 2011; 37:264–272.
- Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin 2009; 25:2043–2048.
- Agusti A, Hedner J, Marin JM, BarbÈ F, Cazzola M, Rennard S. Nighttime symptoms: a forgotten dimension of COPD. Eur Respir Rev 2011; 20:183–194.
- http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html (accessed February 2013).
- Donohue JF, van Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ, Jr., Kesten S, Towse L. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002; 122:47–55.
- http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002211/human_med_001571.jsp&mid=WC0b01ac058001d124 (accessed March 2013).
- =Search.DrugDetails (accessed March 2013).
- Fuhr R, Magnussen H, Sarem K, Llovera AR, Kirsten AM, FalquÈs M, Caracta CF, Garcia Gil E. Efficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest 2012; 141:745–752.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. www.goldcopd.com 2010.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Murray L, Houle C, Stolar M, Hsieh R, Jones PW, Monz BU, Ramachandran S, Goldman M, Sethi S, Leidy NK. Quantifying the severity of respiratory symptoms of chronic obstructive pulmonary disease (COPD): performance properties of the EXAcerbations of Chronic pulmonary disease Tool - Respiratory Symptoms (E-RS) in 3 randomized controlled trials. Am J Resp Crit Care Med 2012; 185:A1515.
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Brusasco V, Burge PS, Calverley PM, Celli BR, Jones PW, Mahler DA, Make B, Miravitlles M, Page CP, Palange P, Parr D, Pistolesi M, Rennard SI, Rutten-van Mˆlken MP, Stockley R, Sullivan SD, Wedzicha JA, Wouters EF. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008; 31:416–469.
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005; 2:111–124.
- http://www.spiriva.com/ (accessed March 2013).
- Lasseter KC, Dilzer S, Jansat JM, Garcia Gil E, Caracta C, Ortiz S. Safety and pharmacokinetics of multiple doses of aclidinium bromide administered twice daily in healthy volunteers. Pulm Pharmacol Ther 2012; 25:193–199.
- Mocarski M, Caracta C, Tourkodimitris S, Park G, Garcia Gil E, Setyawan J. Nighttime symptoms of COPD in a clinical trial population: prevalence and impact. Am J Resp Crit Care Med 2011; 183, abs A1495.
- Martin RJ, Bartelson BL, Smith P, Hudgel DW, Lewis D, Pohl G, Koker P, Souhrada JF. Effect of ipratropium bromide treatment on oxygen saturation and sleep quality in COPD. Chest 1999; 115:1338–1345.
- McNicholas WT, Calverley PM, Lee A, Edwards JC. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J 2004; 23:825–831.
- Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1993; 148:1030–1036.
- Ryan S, Doherty LS, Rock C, Nolan GM, McNicholas WT. Effects of salmeterol on sleeping oxygen saturation in chronic obstructive pulmonary disease. Respiration 2010; 79:475–481.
- Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, de Miquel G, Segarra R, Caracta C, Garcia Gil E. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: The ATTAIN study. Eur Respir J 2012; 40:830–836.
- Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF, ACCORD I study investigators. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012; 9:90–101.
- Agusti A, Jones PW, Bateman ED, Singh D, Lamarca R, de Miquel G, Caracta C, Garcia Gil E. Improvement in symptoms and rescue medication use with aclidinium bromide in patients with chronic obstructive pulmonary disease: results from ATTAIN. Eur Respir J 2011; 38:149.
- Kerwin E, Rennard S, Gelb AF, Rekeda L, Garcia Gil E, Caracta C. ACCORD COPD I: improvements in nighttime symptoms and rescue medication use in COPD with twice-daily aclidinium bromide. Poster presented at the European Respiratory Society Annual Congress, Amsterdam, The Netherlands, 24–28 September, 2011.
- http://www.exactproinitiative.com/default.php (accessed April 2013).
- Toy EL, Beaulieu NU, McHale JM, Welland TR, Plauschinat CA, Swensen A, Duh MS. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med 2011; 105:435–441.
- Chrystyn H, Small M, Milligan G, Higgins V, Garcia Gil E, Karakurum C. Impact of patient satisfaction with their maintenance inhaler on treatment compliance and health outcomes in chronic obstructive pulmonary disease: an analysis of real-world clinical practice in Europe. Abstract presented at the British Thoracic Society Winter Meeting, London, UK, 5–7 December 2012.
- Lareau SC, Yawn BP. Improving adherence with inhaler therapy in COPD. Int J Chron Obstruct Pulmon Dis 2010; 5:401–406.
- Restrepo RD, Alvarez MT, Wittnebel LD, Sorenson H, Wettstein R, Vines DL, Sikkema-Ortiz J, Gardner DD, Wilkins RL. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis 2008; 3:371–384.
- van der Palen J, Ginko T, Kroker A, van der Valk P, Goosens M, Padulles L, Seoane B, Rekeda L, Garcia-Gil E. Preference, satisfaction and critical errors with Genuair® and HandiHaler® in patients with COPD. Eur Respir J 2012; 40 (Suppl 56): 389s.
- Gavald‡ A, Sentellas S, AlbertÌ J, Miralpeix M, AntÛn F, Salv‡ M, Beleta J. Rapid plasma hydrolysis of aclidinium bromide, a novel long-acting anticholinergic drug. Poster presented at the European Respiratory Society Annual Congress, Berlin, Germany, 4–8 October, 2008.
- Sentellas S, Ramos I, AlbertÌ J, Salv‡ M, AntÛn F, Miralpeix M, Beleta J, Gavald‡ A. Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci 2010; 39:283–290.
- Dong YH, Lin HH, Shau WY, Wu YC, Chang CH, Lai MS. Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trials. Thorax 2013; 68:48–56.
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 7:CD009285.
- Singh S, Loke YK, Enright PL, Furberg CD. Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trials. BMJ 2011; 342:d3215.
- Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest 2006; 130:1695–1703.