Abstract
Background: Chronic bronchitis in COPD has been associated with an increased exacerbation rate, more hospitalizations, and an accelerated decline in lung function. The clinical characteristics of patients with advanced emphysema and chronic bronchitis have not been well described. Methods: Patients randomized to medical therapy in the National Emphysema Treatment Trial were grouped based on their reports of cough and phlegm on the St. George's Respiratory Questionnaire(SGRQ) at baseline: chronic bronchitis(CB+) and no chronic bronchitis(CB–). The patients were similarly categorized into severe chronic bronchitis(SCB+) or no severe chronic bronchitis (SCB–) based on the above definition plus report of chest trouble. Kaplan-Meier survival analysis was used to determine the relationships between chronic bronchitis and severe chronic bronchitis and survival and time to hospitalization. Lung function and SGRQ scores over time were compared between groups. Results: The CB+(N = 234; 38%) and CB- groups(N = 376; 62%) had similar survival (median 60.8 versus 65.7 months, p = 0.19) and time to hospitalization (median 26.9 versus 24.9 months, p = 0.84). The SCB+ group(N = 74; 12%) had worse survival (median 47.7 versus 65.7 months, p = 0.02) and shorter time to hospitalization (median 18.5 versus 26.7 months, p = 0.02) than the SCB- group (N = 536; 88%). Mortality and hospitalization rates were not increased when chest trouble was analyzed by itself. The CB+ and CB–groups had similar lung function and SGRQ scores over time. The SCB+ and SCB–groups had similar lung function over time, but the SCB+ group had significantly worse SGRQ scores. Conclusions: Severe chronic bronchitis is associated with worse survival, shorter time to hospitalization, and worse health-related quality of life.
Background
Chronic bronchitis (CB) is seen in 18–45% of patients with COPD and is associated with an increased exacerbation rate, worse health-related quality of life, and accelerated lung function decline (Citation1–4). It was commonly believed that CB was not a significant pathologic finding in patients with emphysema. However, we now increasingly recognize the presence of CB and airway disease even in those with a predominantly emphysematous phenotype. We have shown that small airway remodeling is present to varying degrees in patients with advanced emphysema (Citation5), and that a greater degree of goblet cell hyperplasia is present in patients who underwent lung volume reduction surgery (LVRS) compared to those with lesser degrees of airflow obstruction (Citation6). A large lung pathology study also found that the number of small airways occluded by mucus plugs increased as COPD disease severity worsened (Citation7).
Mucus hypersecretion may also have an effect on mortality. Chronic bronchitis has been associated with an increase in all-cause mortality independently from the degree of airflow obstruction (Citation8,9), and the degree of small airway mucus occlusion has been related to mortality after LVRS (Citation10). However, as the clinical phenomenon of CB may be a manifestation of large airway pathology, its presence and sequelae in those with advanced emphysema are unclear.
We analyzed the effects of CB on outcomes in the medically treated patients in the National Emphysema Treatment Trial (NETT). Specifically, we analyzed long-term survival, time to first hospitalization rate, lung function, health-related quality of life, and clinical profiles of medically treated patients with and without CB in the NETT. We hypothesized that those with CB would have worse health-related quality of life, a greater decline in lung function, and increased mortality and hospitalizations.
Methods
Enrollment criteria for NETT have been previously described (Citation11). Briefly, inclusion criteria for the trial included a physical exam consistent with emphysema, radiographic evidence on HRCT scan of bilateral emphysema, FEV1 ≤ 45% predicted, but ≥ 15% of predicted if age 70 or older, TLC ≥ 100% predicted, RV ≥ 150% predicted, PCO2 ≤ 60 mmHg, PO2 ≥ 45 mmHg on room air, and a post-rehabilitation 6-minute walk distance >140 meters. Patients also needed to be stable on ≤20 mg prednisone/day, nonsmoking for 4 months, and have a body mass index (BMI) ≤ 31.1 kg/m2 in men and ≤ 32.3 kg/m2 in women. All patients underwent 6–10 weeks of pulmonary rehabilitation, then were randomized to either LVRS or continued medical therapy per ATS guidelines. All patients provided written informed consent, and the study was approved by the institutional review board at each center. Baseline measurements of pulmonary function, 6-minute walk distance, and health-related quality of life obtained from the St. George's Respiratory Questionnaire (SGRQ) were obtained after completing pulmonary rehabilitation but prior to randomization.
As the classic definition of CB was not assessed at baseline, we divided patients in the medical arm into two groups based on the presence or absence of CB, using the answers given to the cough and sputum questions on the SGRQ administered after pulmonary rehabilitation and before randomization. If the patient answered “most days of the week” or “several days of the week” to both questions regarding cough and sputum, then the patient was placed in the chronic bronchitis group (CB+). If the patient answered “a few days a month,” “only with chest infections,” or “not at all” to either of the two questions regarding cough or sputum, then the patient was placed in the no chronic bronchitis group (CB−).
In addition, the patients were divided into a severe chronic bronchitis group (SCB+) or no severe chronic bronchitis group (SCB−) based on the above definition plus the answer to a question on chest trouble in the SGRQ. The patients were asked “Over the last year, in an average week, how many good days (with little chest trouble) have you had?” If the patient answered “no good days” or “1 or 2 good days” and was in the CB+ group, the patient was placed in the SCB+ group. If the patient was in the CB+ group and answered “3 or 4 days,” “nearly every day is good,” or “every day is good” or if the patient was in the CB− group, the patient was placed in the SCB-group.
Patients were followed for clinical outcomes through 31 December 2003. Up to 31 December 2002, patients were queried about hospitalization since the prior visit during periodic telephone visits (3 to 5 per year) and clinic visits (2 in the first year of follow-up, annually thereafter). Between 1 January 2003 and 31 December 2003, patients completed a single interim history interview.
Vital status, ascertained as of 30 September 2008, was determined by reports from the clinical centers (obtained through 31 December 2003) and review of the Social Security Administration's September 2008 Death Master File (Citation12,13). Time to death and time to first hospitalization from all causes were measured from the day of randomization. Patients who were known to have received non-NETT LVRS or lung transplant after randomization in the NETT (reports obtained through 31 December 2003) were censored as of the date of surgery.
Lung function and SGRQ scores were assessed at baseline and at 6-, 12-, 24-, and 36-month follow-up visits.
Statistics
Data analysis was performed using JMP 8.0, SAS 9.2 (Cary, NC), and Stata (College Station, TX). Continuous variables are reported as mean ± SD, except where otherwise noted. A p value of <0.05 was considered statistically significant. Unpaired t-test was used to compare baseline and follow-up data between the CB+ and CB− groups as well as the SCB+ and SCB- groups separately. Kaplan–Meier survival analysis was used to determine differences between the CB+ and CB− groups in survival and time to hospitalization and to determine differences between the SCB+ and SCB− groups in these outcomes.
We also used Kaplan–Meier survival analysis to check for differences between those reporting chest trouble but not chronic bronchitis (CT+) and those reporting neither chest trouble nor chronic bronchitis (CT−) in these outcomes. Cox proportional hazard regression was used to model time to the outcomes of death, and separately, first hospitalization in relation to SCB status and a set of 7 candidate covariates; stepwise backwards selection (p(removal) = 0.05) was used to assess the independent contribution of SCB to each outcome, using the smallest set of significant covariates.
Results
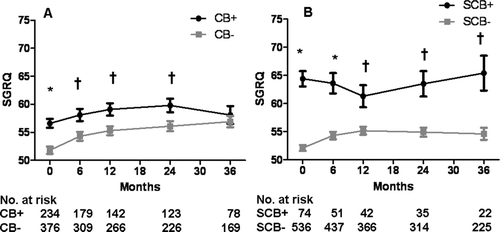
Baseline patient characteristics of the 610 patients included in our analysis are summarized in . There were 234 patients (38% of entire cohort) in the CB+ group and 376 in the CB–group. The CB+ group consisted of more males (70.9% vs. 59.8%, p = 0.006) compared to the CB- group. Racial distribution, smoking history, body mass index, presence of upper lobe predominant emphysema, presence of low exercise capacity subgroup, lung function, gas exchange, and 6-minute walk distance were similar between groups, with the exception of a higher FVC in the CB+ group (69.2 ± 15.8 vs. 66.0 ± 14.7% predicted, p = 0.01). SGRQ total score was greater in the CB+ group (56.6 ± 12.5 vs. 51.8 ± 12.4, p < 0.0001). There were 74 patients in the SCB+ group (12% of entire cohort) and 536 in the SCB− group. The groups were similar in age, gender, race, smoking history, lung function, presence of upper lobe predominant emphysema and low exercise capacity, and lung function. SGRQ total score was also significantly higher in the SCB+ group compared to the SCB− group (65.1 ± 9.3 vs. 52.1 ± 12.3, p < 0.0001).
Table 1. Baseline characteristics of 610 participants randomized to medical treatment in the National Emphysema Treatment Trial, by chronic bronchitis status and by severe chronic bronchitis status
Survival and time to first hospitalization were not different between the CB+ and CB− groups (median survival 60.8 months vs. 65.7 months, p = 0.19; median time to first hospitalization 26.9 months vs. 24.9 months, p = 0.84). However, compared to the SCB–group, survival was lower in the SCB+ group (median 47.7 months vs. 65.7 months, p = 0.02) and time to first hospitalization was shorter (median 18.5 months vs. 26.7 months, p = 0.02). See and and . Cox regression analysis showed that risk of death independently increased with age and SCB+ status, but was reduced with greater FEV1% predicted and 6-minute walk distance (). Similarly, the risk of hospitalization increased with SCB+ status, but was reduced with greater TLC percent predicted, male gender, and greater FEV1% predicted (). When those reporting cough and phlegm most or several days per week were excluded, there were no significant differences in mortality or time to first hospitalization between those reporting chest trouble most days per week (CT+) and those reporting chest trouble less frequently (CT−); see .
Figure 1. Mortality in A) the chronic bronchitis versus no chronic bronchitis groups and B) the severe chronic bronchitis versus no severe chronic bronchitis groups.
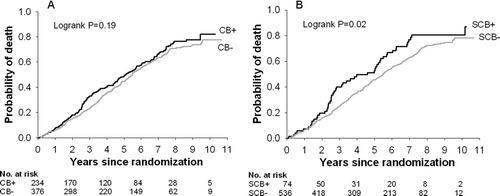
Figure 2. Time to first hospitalization in A) the chronic bronchitis versus no chronic bronchitis groups and B) the severe chronic bronchitis versus no severe chronic bronchitis groups.
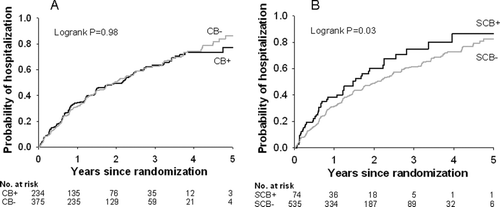
Figure 3. A) Time to death and B) time to hospitalization in those reporting chest trouble most days per week and not reporting cough and phlegm most or several days per week (CT+) versus those with little or no chest trouble and not reporting cough and phlegm most or several days per week (CT−). Those reporting cough and phlegm most or several days per week are excluded from both the CT+ and CT− groups.
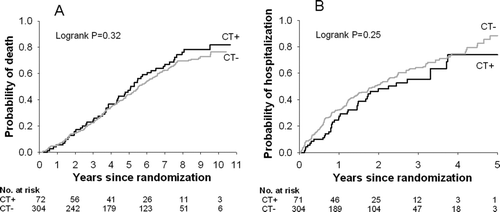
Table 2. Time from randomization to death and time from randomization to first hospitalization, by chronic bronchitis status and severe chronic bronchitis status for 610 patients randomized to medical treatment in the National Emphysema Treatment Trial
Table 3. Multivariate models of time to death and first hospitalization
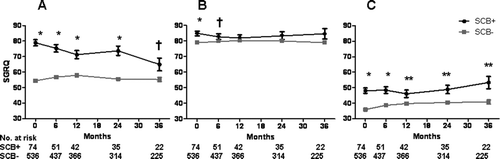
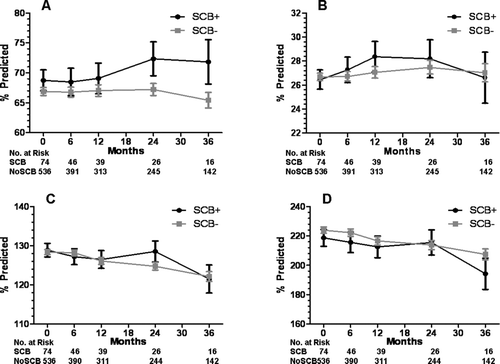
Overall, there were significant differences in FVC between the CB+ and CB− groups at baseline and at the 6- and 36-month follow-up timepoints, but otherwise FEV1 and lung volumes were the same (). There were no significant differences in lung function at baseline or follow-up between the SCB+ and SCB− groups ().Compared to the CB–group, SGRQ total score was elevated in the CB+ group at baseline and 6-, 12-, and 24-months follow-up (). Compared to the SCB− group, SGRQ total score was significantly higher in the SCB+ group at baseline () and during all follow-up timepoints. When divided into the three components of symptoms, activity, and impact, SGRQ scores in the SCB+ group were greater at all time points for symptoms, greater at baseline and 6 months only for activity, and greater at all time points for impact ().
Figure 4. Lung function at baseline and during follow-up for the CB+ and CB− groups. A) forced vital capacity, B) forced expiratory volume in 1 second, C) total lung capacity, and D) residual volume. Data presented as mean ± SE. *p < 0.05,**p < 0.01,†p < 0.005.
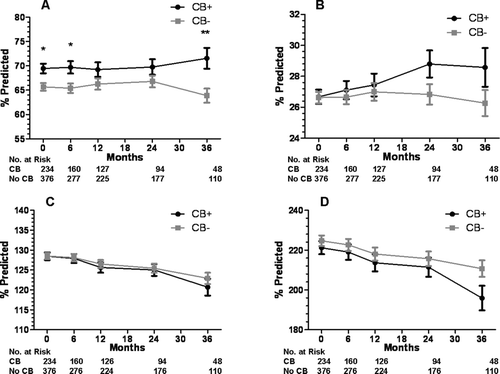
Figure 5. Lung function at baseline and during follow-up for the SCB+ and SCB− groups. A) forced vital capacity, B) forced expiratory volume in 1 second, C) total lung capacity, and D) residual volume. Data presented as mean ± SE.
Discussion
We showed that, in a group of patients with advanced emphysema and severe airflow obstruction, severe chronic bronchitis was associated with increased mortality and shorter time to hospitalization. The triad of cough, phlegm, and chest trouble that makes up our definition of severe chronic bronchitis distinguished those with shorter time to death and shorter time to first hospitalization. These outcomes were independent of any differences in lung function during follow-up as well as chest trouble when analyzed alone. We also showed that those with chronic bronchitis had worse health-related quality of life (HRQoL), that HRQoL was significantly worse in those with severe chronic bronchitis, and that these differences were durable over time. To our knowledge, this is the first study of the effects of chronic bronchitis using this novel definition on outcomes in a large well characterized group of advanced emphysema patients.
Chronic bronchitis has been associated with several clinical sequelae in COPD. A multicenter cross-sectional study of 433 COPD patients found that chronic cough and phlegm production were associated with a greater number of exacerbations (Citation3). In the Genetic Epidemiology Study of COPD (COPDGene), a greater exacerbation frequency was found in those with chronic bronchitis in the year prior to enrollment and during longitudinal follow-up (Citation4, Citation14).
In addition, there have been multiple studies analyzing the role of chronic bronchitis on the development of airflow obstruction or lung function decline with conflicting results (Citation2, Citation8, Citation15, 16). Pelkonen et al. show that chronic bronchitis was associated with the development of COPD in 1,711 men followed for 30 years (Citation8). However, a retrospective analysis of the Copenhagen City Heart Study did not show that smokers with respiratory symptoms were at any greater risk for the development of airflow obstruction than smokers without respiratory symptoms (Citation16).
Chronic bronchitis has also been related to mortality in previous studies. The underlying reason is unclear but, as chronic bronchitis is caused by heightened lung inflammation, there may be greater systemic inflammation as a result, which can cause accelerated atherosclerotic heart disease, osteoporosis, and other metabolic abnormalities. The Tucson Epidemiologic Survey of Airway Obstructive Disease followed 1,412 adults with chronic bronchitis and no airflow obstruction and found that those with chronic bronchitis had higher levels of serum IL-8 and C-reactive protein at enrollment, which was associated with a higher all-cause mortality in those under the age of 50 (Citation9). Pelkonen et al. found that chronic bronchitis conferred a hazard ratio of 1.3 on all cause mortality, even after adjusting for lung function (Citation8). In addition, the major causes of death in this study were cardiac or respiratory disease. Therefore, we hypothesize that the observed increase in mortality and hospitalizations seen in our population may be related to greater systemic inflammation in those with severe chronic bronchitis. Whether this is true remains to be proven.
Not all studies, however, have found a significant association between chronic bronchitis and mortality. The National Health and Nutrition Epidemiologic Survey III found that mortality was dictated by severity of airflow obstruction and not respiratory symptoms alone (Citation17). The Copenhagen City Heart Study found an increase in mortality in those with chronic mucus hypersecretion in women but not in men (Citation18). Another study of 884 men found an odds ratio of 1.65 for chronic phlegm for mortality but this did not reach statistical significance (95% CI 0.95–2.89)(19). These negative results may be related to differences in study population, the definition of chronic bronchitis, or both.
Regardless, in our cohort those with chronic cough, phlegm, and chest trouble had a greater all-cause mortality and a shorter time to hospitalization. This provides greater insight into the natural history of advanced emphysema and severe airflow obstruction. The majority of the hospitalizations are presumed to be related to COPD but as the data on causes of hospitalization were not available, this is a matter of speculation. The causes of death are also unknown.
Although these findings are consistent with other studies in different cohorts, some limitations are worthy of mention. We acknowledge that the definitions of chronic bronchitis and severe chronic bronchitis used in this analysis are innovative. Whereas the classic definition for chronic bronchitis includes chronic cough and phlegm production for 3 months a year for 2 years based on the ATS DLD-78 questionnaire (Citation20), we used surrogate questions regarding cough and phlegm from the SGRQ, which has been used in some recent analyses as a new definition of chronic bronchitis. How this definition compares to the classic definition remains to be determined.
To our knowledge, we are the first to define severe chronic bronchitis with the addition of a question on chest trouble. It was assumed that the symptom of chest trouble, while nonspecific, was related to CB, given that there are separate questions regarding shortness of breath, wheezing, and episodic worsening of symptoms on the SGRQ. One could question whether frequent chest trouble alone was the most important determinant of mortality and hospitalization in this cohort, but the triad of these SGRQ questions captures something more than either component captures. Just as patients reporting frequent cough and frequent phlegm had similar time to death and time to hospitalization as those reporting infrequent cough or infrequent phlegm ( and ), patients reporting frequent chest trouble had similar time to death and time to hospitalization as those reporting infrequent or no chest trouble once those reporting frequent cough and phlegm were excluded (). Despite the novelty of these definitions, it is evident that outcomes are worse in this highly symptomatic subset of patients with severe chronic bronchitis.
In addition, the differences in HRQoL between groups are interesting and hypothesis-generating. We found that HRQoL was worse at baseline but not after 2 years of follow-up in the chronic bronchitis group, and that HRQoL was significantly worse at all time points in the severe chronic bronchitis group. It can be argued that the increase in SGRQ score seen in those with severe chronic bronchitis is a result of use of the SGRQ questions regarding cough and phlegm to define chronic bronchitis. However, when the SGRQ was broken down into its three components, the symptom subscore was greater at all timepoints (as expected), as was the impact subscore.
Second, the overall differences in SGRQ scores in those with severe chronic bronchitis compared to those without it are very large and are consistent during follow-up. The exact differences are difficult to quantitate, but observed differences in overall and component SGRQ scores suggest that those with severe chronic bronchitis indeed have a worse HRQoL compared to those without severe chronic bronchitis. It is not known how these differences in HRQoL relate to the differences in mortality, and whether it is part of the cause or an effect of the increased hospitalizations is unclear.
Contrary to our hypothesis, FVC was greater at some timepoints in those with chronic bronchitis, and there was a trend toward a greater FEV1 as well. In addition, there was no difference in DLCO between these groups, which is contrary to the expected higher DLCO in chronic bronchitics. This may be a virtue of the predominance of an emphysema phenotype on lung function testing in all patients, regardless of the presence or absence of chronic bronchitis. We also found no significant differences in lung function between those with severe chronic bronchitis and those without. Therefore, the differences in mortality and hospitalizations in those with severe chronic bronchitis cannot be explained by differences in lung function.
These results contrast with other studies that show an acceleration of lung function decline in those with cough and phlegm (Citation2, Citation8, Citation9, Citation19). There are several possible explanations. Perhaps cough and phlegm have no effect on lung function in those with GOLD stage IV disease or in those with an emphysema phenotype. There may have been a “healthy survivor” effect, where sicker individuals who had a significant decline in lung function passed away or were lost to follow-up. Finally, the study may not have been powered adequately to detect significant differences between groups.
In summary, in those with advanced emphysema, chronic bronchitis is a common phenomenon as is severe chronic bronchitis. Severe chronic bronchitis has a significant impact on all cause mortality and hospitalization rate without any significant effect on lung function decline. These effects were not seen when chest trouble was analyzed separately. In addition, severe chronic bronchitis is associated with a worse HRQoL that is sustained over time. The mechanism of the effects of severe chronic bronchitis on outcomes in advanced emphysema patients should be the subject of further investigation.
Acknowledgments
Members of the NETT Research Group
Office of the Chair of the Steering Committee, University of Pennsylvania, Philadelphia, PA: Alfred P. Fishman, MD (Chair); Betsy Ann Bozzarello; Ameena Al-Amin.
Clinical centers
Baylor College of Medicine, Houston, TX: Marcia Katz, MD (Principal Investigator); Carolyn Wheeler, RN, BSN (Principal Clinic Coordinator); Elaine Baker, RRT, RPFT; Peter Barnard, PhD, RPFT; Phil Cagle, MD; James Carter, MD; Sophia Chatziioannou, MD; Karla Conejo-Gonzales; Kimberly Dubose, RRT; John Haddad, MD; David Hicks, RRT, RPFT; Neal Kleiman, MD; Mary Milburn-Barnes, CRTT; Chinh Nguyen, RPFT; Michael Reardon, MD; Joseph Reeves-Viets, MD; Steven Sax, MD; Amir Sharafkhaneh, MD; Owen Wilson, PhD; Christine Young PT; Rafael Espada, MD (Principal Investigator 1996–2002); Rose Butanda (1999–2001); Minnie Ellisor (2002); Pamela Fox, MD (1999–2001); Katherine Hale, MD (1998–2000); Everett Hood, RPFT (1998 B 2000); Amy Jahn (1998–2000); Satish Jhingran, MD (1998–2001); Karen King, RPFT (1998–1999); Charles Miller III, PhD (1996–1999); Imran Nizami, MD (Co-Principal Investigator, 2000–2001); Todd Officer (1998–2000); Jeannie Ricketts (1998–2000); Joe Rodarte, MD (Co-Principal Investigator 1996-2000); Robert Teague, MD (Co-Principal Investigator 1999-2000); Kedren Williams (1998–1999).
Brigham and Women's Hospital, Boston, MA: John Reilly, MD (Principal Investigator); David Sugarbaker, MD (Co-Principal Investigator); Carol Fanning, RRT (Principal Clinic Coordinator); Simon Body, MD; Sabine Duffy, MD; Vladmir Formanek, MD; Anne Fuhlbrigge, MD; Philip Hartigan, MD; Sarah Hooper, EP; Andetta Hunsaker, MD; Francine Jacobson, MD; Marilyn Moy, MD; Susan Peterson, RRT; Roger Russell, MD; Diane Saunders; Scott Swanson, MD (Co-Principal Investigator, 1996–2001).
Cedars-Sinai Medical Center, Los Angeles, CA: Rob McKenna, MD (Principal Investigator); Zab Mohsenifar, MD (Co-Principal Investigator); Carol Geaga, RN (Principal Clinic Coordinator); Manmohan Biring, MD; Susan Clark, RN, MN; Jennifer Cutler, MD; Robert Frantz, MD; Peter Julien, MD; Michael Lewis, MD; Jennifer Minkoff-Rau, MSW; Valentina Yegyan, BS, CPFT; Milton Joyner, BA (1996–2002).
Cleveland Clinic Foundation, Cleveland, OH: Malcolm DeCamp, MD (Principal Investigator); James Stoller, MD (Co-Principal Investigator); Yvonne Meli, RN,C (Principal Clinic Coordinator); John Apostolakis, MD; Darryl Atwell, MD; Jeffrey Chapman, MD; Pierre DeVilliers, MD; Raed Dweik, MD; Erik Kraenzler, MD; Rosemary Lann, LISW; Nancy Kurokawa, RRT, CPFT; Scott Marlow, RRT; Kevin McCarthy, RCPT; Priscilla McCreight, RRT, CPFT; Atul Mehta, MD; Moulay Meziane, MD; Omar Minai, MD; Mindi Steiger, RRT; Kenneth White, RPFT; Janet Maurer, MD (Principal Investigator, 1996–2001); Terri Durr, RN (2000–2001); Charles Hearn, DO (1998–2001); Susan Lubell, PA-C (1999–2000); Peter O'Donovan, MD (1998–2003); Robert Schilz, DO (1998–2002).
Columbia University, New York, NY in consortium with Long Island Jewish Medical Center, New Hyde Park, NY: Mark Ginsburg, MD (Principal Investigator); Byron Thomashow, MD (Co-Principal Investigator); Patricia Jellen, MSN, RN (Principal Clinic Coordinator); John Austin, MD; Matthew Bartels, MD; Yahya Berkmen, MD; Patricia Berkoski, MS, RRT (Site coordinator, LIJ); Frances Brogan, MSN, RN; Amy Chong, BS, CRT; Glenda DeMercado, BSN; Angela DiMango, MD; Sandy Do, MS, PT; Bessie Kachulis, MD; Arfa Khan, MD; Berend Mets, MD; Mitchell O = Shea, BS, RT, CPFT; Gregory Pearson, MD; Leonard Rossoff, MD; Steven Scharf, MD, PhD (Co-Principal Investigator, 1998–2002); Maria Shiau, MD; Paul Simonelli, MD; Kim Stavrolakes, MS, PT; Donna Tsang, BS; Denise Vilotijevic, MS, PT; Chun Yip, MD; Mike Mantinaos, MD (1998–2001); Kerri McKeon, BS, RRT, RN (1998–1999); Jacqueline Pfeffer, MPH, PT (1997–2002).
Duke University Medical Center, Durham, NC: Neil MacIntyre, MD (Principal Investigator); R. Duane Davis, MD (Co-Principal Investigator); John Howe, RN (Principal Clinic Coordinator); R. Edward Coleman, MD; Rebecca Crouch, RPT; Dora Greene; Katherine Grichnik, MD; David Harpole, Jr., MD; Abby Krichman, RRT; Brian Lawlor, RRT; Holman McAdams, MD; John Plankeel, MD; Susan Rinaldo-Gallo, MED; Sheila Shearer, RRT; Jeanne Smith, ACSW; Mark Stafford-Smith, MD; Victor Tapson, MD; Mark Steele, MD (1998–1999); Jennifer Norten, MD (1998–1999).
Mayo Foundation, Rochester, MN: James Utz, MD (Principal Investigator); Claude Deschamps, MD (Co-Principal Investigator); Kathy Mieras, CCRP (Principal Clinic Coordinator); Martin Abel, MD; Mark Allen, MD; Deb Andrist, RN; Gregory Aughenbaugh, MD; Sharon Bendel, RN; Eric Edell, MD; Marlene Edgar; Bonnie Edwards; Beth Elliot, MD; James Garrett, RRT; Delmar Gillespie, MD; Judd Gurney, MD; Boleyn Hammel; Karen Hanson, RRT; Lori Hanson, RRT; Gordon Harms, MD; June Hart; Thomas Hartman, MD; Robert Hyatt, MD; Eric Jensen, MD; Nicole Jenson, RRT; Sanjay Kalra, MD; Philip Karsell, MD; Jennifer Lamb; David Midthun, MD; Carl Mottram, RRT; Stephen Swensen, MD; Anne-Marie Sykes, MD; Karen Taylor; Norman Torres, MD; Rolf Hubmayr, MD (1998–2000); Daniel Miller, MD (1999–2002); Sara Bartling, RN (1998–2000); Kris Bradt (1998–2002).
National Jewish Medical and Research Center, Denver, CO: Barry Make, MD (Principal Investigator); Marvin Pomerantz, MD (Co-Principal Investigator); Mary Gilmartin, RN, RRT (Principal Clinic Coordinator); Joyce Canterbury; Martin Carlos; Phyllis Dibbern, PT; Enrique Fernandez, MD; Lisa Geyman, MSPT; Connie Hudson; David Lynch, MD; John Newell, MD; Robert Quaife, MD; Jennifer Propst, RN; Cynthia Raymond, MS; Jane Whalen-Price, PT; Kathy Winner, OTR; Martin Zamora, MD; Reuben Cherniack, MD (Principal Investigator, 1997–2000).
Ohio State University, Columbus, OH: Philip Diaz, MD (Principal Investigator); Patrick Ross, MD (Co-Principal Investigator); Tina Bees (Principal Clinic Coordinator); Jan Drake; Charles Emery, PhD; Mark Gerhardt, MD, PhD; Mark King, MD; David Rittinger; Mahasti Rittinger.
Saint Louis University, Saint Louis, MO: Keith Naunheim, MD (Principal Investigator); Robert Gerber, MD (Co-Principal Investigator); Joan Osterloh, RN, MSN (Principal Clinic Coordinator); Susan Borosh; Willard Chamberlain, DO; Sally Frese; Alan Hibbit; Mary Ellen Kleinhenz, MD; Gregg Ruppel; Cary Stolar, MD; Janice Willey; Francisco Alvarez, MD (Co-Principal Investigator, 1999-2002); Cesar Keller, MD (Co-Principal Investigator, 1996–2000).
Temple University, Philadelphia, PA: Gerard Criner, MD (Principal Investigator); Satoshi Furukawa, MD (Co-Principal Investigator); Anne Marie Kuzma, RN, MSN (Principal Clinic Coordinator); Roger Barnette, MD; Neil Brister, MD; Kevin Carney, RN, CCTC; Wissam Chatila, MD; Francis Cordova, MD; Gilbert D'Alonzo, DO; Michael Keresztury, MD; Karen Kirch; Chul Kwak, MD; Kathy Lautensack, RN, BSN; Madelina Lorenzon, CPFT; Ubaldo Martin, MD; Peter Rising, MS; Scott Schartel, MD; John Travaline, MD; Gwendolyn Vance, RN, CCTC; Phillip Boiselle, MD (1997–2000); Gerald O'Brien, MD (1997–2000).
University of California, San Diego, San Diego, CA: Andrew Ries, MD, MPH (Principal Investigator); Robert Kaplan, PhD (Co-Principal Investigator); Catherine Ramirez, BS, RCP (Principal Clinic Coordinator); David Frankville, MD; Paul Friedman, MD; James Harrell, MD; Jeffery Johnson; David Kapelanski, MD; David Kupferberg, MD, MPH; Catherine Larsen, MPH; Trina Limberg, RRT; Michael Magliocca, RN, CNP; Frank J. Papatheofanis, MD, PhD; Dawn Sassi-Dambron, RN; Melissa Weeks.
University of Maryland at Baltimore, Baltimore, MD in consortium with Johns Hopkins Hospital, Baltimore, MD: Mark Krasna, MD (Principal Investigator); Henry Fessler, MD (Co-Principal Investigator); Iris Moskowitz (Principal Clinic Coordinator); Timothy Gilbert, MD; Jonathan Orens, MD; Steven Scharf, MD, PhD; David Shade; Stanley Siegelman, MD; Kenneth Silver, MD; Clarence Weir; Charles White, MD.
University of Michigan, Ann Arbor, MI: Fernando Martinez, MD (Principal Investigator); Mark Iannettoni, MD (Co-Principal Investigator); Catherine Meldrum, BSN, RN, CCRN (Principal Clinic Coordinator); William Bria, MD; Kelly Campbell; Paul Christensen, MD; Kevin Flaherty, MD; Steven Gay, MD; Paramjit Gill, RN; Paul Kazanjian, MD; Ella Kazerooni, MD; Vivian Knieper; Tammy Ojo, MD; Lewis Poole; Leslie Quint, MD; Paul Rysso; Thomas Sisson, MD; Mercedes True; Brian Woodcock, MD; Lori Zaremba, RN.
University of Pennsylvania, Philadelphia, PA: Larry Kaiser, MD (Principal Investigator); John Hansen-Flaschen, MD (Co-Principal Investigator); Mary Louise Dempsey, BSN, RN (Principal Clinic Coordinator); Abass Alavi, MD; Theresa Alcorn, Selim Arcasoy, MD; Judith Aronchick, MD; Stanley Aukberg, MD; Bryan Benedict, RRT; Susan Craemer, BS, RRT, CPFT; Ron Daniele, MD; Jeffrey Edelman, MD; Warren Gefter, MD; Laura Kotler-Klein, MSS; Robert Kotloff, MD; David Lipson, MD; Wallace Miller, Jr., MD; Richard O = Connell, RPFT; Staci Opelman, MSW; Harold Palevsky, MD; William Russell, RPFT; Heather Sheaffer, MSW; Rodney Simcox, BSRT, RRT; Susanne Snedeker, RRT, CPFT; Jennifer Stone-Wynne, MSW; Gregory Tino, MD; Peter Wahl; James Walter, RPFT; Patricia Ward; David Zisman, MD; James Mendez, MSN, CRNP (1997–2001); Angela Wurster, MSN, CRNP (1997–1999).
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD (Principal Investigator); James Luketich, MD (Co-Principal Investigator); Colleen Witt, MS (Principal Clinic Coordinator); Gerald Ayres; Michael Donahoe, MD; Carl Fuhrman, MD; Robert Hoffman, MD; Joan Lacomis, MD; Joan Sexton; William Slivka; Diane Strollo, MD; Erin Sullivan, MD; Tomeka Simon; Catherine Wrona, RN, BSN; Gerene Bauldoff, RN, MSN (1997–2000); Manuel Brown, MD (1997–2002); Elisabeth George, RN, MSN (Principal Clinic Coordinator 1997–2001); Robert Keenan, MD (Co-Principal Investigator 1997–2000); Theodore Kopp, MS (1997–1999); Laurie Silfies (1997–2001).
University of Washington, Seattle, WA: Joshua Benditt, MD (Principal Investigator), Douglas Wood, MD (Co-Principal Investigator); Margaret Snyder, MN (Principal Clinic Coordinator); Kymberley Anable; Nancy Battaglia; Louie Boitano; Andrew Bowdle, MD; Leighton Chan, MD; Cindy Chwalik; Bruce Culver, MD; Thurman Gillespy, MD; David Godwin, MD; Jeanne Hoffman; Andra Ibrahim, MD; Diane Lockhart; Stephen Marglin, MD; Kenneth Martay, MD; Patricia McDowell; Donald Oxorn, MD; Liz Roessler; Michelle Toshima; Susan Golden (1998–2000).
Other participants
Agency for Healthcare Research and Quality, Rockville, MD: Lynn Bosco, MD, MPH; Yen-Pin Chiang, PhD; Carolyn Clancy, MD; Harry Handelsman, DO.
Centers for Medicare and Medicaid Services, Baltimore, MD: Steven M Berkowitz, PhD; Tanisha Carino, PhD; Joe Chin, MD; JoAnna Baldwin; Karen McVearry; Anthony Norris; Sarah Shirey; Claudette Sikora Steven Sheingold, PhD (1997–2004).
Coordinating Center, The Johns Hopkins University, Baltimore, MD: Steven Piantadosi, MD, PhD (Principal Investigator); James Tonascia, PhD (Co-Principal Investigator); Patricia Belt; Amanda Blackford, ScM; Karen Collins; Betty Collison; Ryan Colvin, MPH; John Dodge; Michele Donithan, MHS; Vera Edmonds; Gregory L. Foster, MA; Julie Fuller; Judith Harle; Rosetta Jackson; Shing Lee, ScM; Charlene Levine; Hope Livingston; Jill Meinert; Jennifer Meyers; Deborah Nowakowski; Kapreena Owens; Shangqian Qi, MD; Michael Smith; Brett Simon, MD; Paul Smith; Alice Sternberg, ScM; Mark Van Natta, MHS; Laura Wilson, ScM; Robert Wise, MD.
Cost Effectiveness Subcommittee: Robert M. Kaplan, PhD (Chair); J. Sanford Schwartz, MD (Co-Chair); Yen-Pin Chiang, PhD; Marianne C. Fahs, PhD; A. Mark Fendrick, MD; Alan J. Moskowitz, MD; Dev Pathak, PhD; Scott Ramsey, MD, PhD; Steven Sheingold, PhD; A. Laurie Shroyer, PhD; Judith Wagner, PhD; Roger Yusen, MD.
Cost Effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle, WA: Scott Ramsey, MD, PhD (Principal Investigator); Ruth Etzioni, PhD; Sean Sullivan, PhD; Douglas Wood, MD; Thomas Schroeder, MA; Karma Kreizenbeck; Kristin Berry, MS; Nadia Howlader, MS.
CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City, IA: Eric Hoffman, PhD (Principal Investigator); Janice Cook-Granroth, BS; Angela Delsing, RT; Junfeng Guo, PhD; Geoffrey McLennan, MD; Brian Mullan, MD; Chris Piker, BS; Joseph Reinhardt, PhD; Blake Wood; Jered Sieren, RTR; William Stanford, MD.
Data and Safety Monitoring Board: John A. Waldhausen, MD (Chair); Gordon Bernard, MD; David DeMets, PhD; Mark Ferguson, MD; Eddie Hoover, MD; Robert Levine, MD; Donald Mahler, MD; A. John McSweeny, PhD; Jeanine Wiener-Kronish, MD; O. Dale Williams, PhD; Magdy Younes, MD.
Marketing Center, Temple University, Philadelphia, PA: Gerard Criner, MD (Principal Investigator); Charles Soltoff, MBA.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Gail Weinmann, MD (Project Officer); Joanne Deshler (Contracting Officer); Dean Follmann, PhD; James Kiley, PhD; Margaret Wu, PhD (1996–2001).
Other acknowledgments
Arthur Gelb, MD, Lakewood Regional Medical Center, Lakewood, CA.
Declaration of Interest Statement
VK has nothing to disclose in relationship to this manuscript but has participated in clinical trials sponsored by Boehringer Ingelheim, Glaxo-Smith-Kline, Medimmune, and Roche pharmaceuticals. VK is supported by NHLBI K23HL094696-03.
MKH has participated in advisory boards for Boehringer Ingelheim, Pfizer, GlaxoSmithKline, Genentech, Novartis, and Medimmune; participated on speaker's bureaus for Boehringer Ingelheim, Pfizer, GlaxoSmithKline, Grifols therapeutics, and the National Association for Continuing Education, and WebMD; has consulted for Novartis and United Biosource Corporation; and has received royalties from UpToDate and ePocrates.
GW has received grants from the NHLBI to perform quantitative image analysis and have been a paid consultant for MedImmune and Spiration, and his spouse is an employee of Merck Research Laboratories.
AS has no disclosures.
Over the last three years, BJM has participated in advisory boards, speaker bureaus, consultations and multi-center clinical trials with funding from the National Heart Lung and Blood Institute, Abbott, Astellas, AstraZeneca, Boerhinger-Ingelheim, Coviden, Dey, Forest, GlaxoSmithKline, Merck, MedImmune, NABI, Novartis, Pfizer, Respironics, Sepracor, Sequal and Talecris.
FJM has served on advisory boards relating to COPD-related topics for GlaxoSmithKline, MedImmune, AstraZeneca, Merck, Pearl Therapeutics, Novartis, United BioSource Corporation, Forest Laboratories, and Almirall; he has consulted for Actelion Pharmaceuticals, Boehringer Ingelheim, Nycomed, Forest Laboratories, F. Hoffmann-La Roche; Bayer, Merck/Schering-Plough, Health Learning Systems, Talecris Biotherapeutics, Comgenex, fb Communications, BoomComm, and Actelion; has served on speaker's bureaus for GlaxoSmithKline, National Association for Continuing Education, Med-Ed, Potomac Center for Medical Education, Pfizer, Boehringer Ingelheim, Merck/Schering-Plough, Vox Medica, American Lung Association, WebMD, ePocrates, AstraZeneca, France Foundation, CME Incite, and Altana/Nycomed; his institution has received funds from Boehringer Ingelheim for a clinical trial; has received royalties from Associates in Medical Marketing and Castle Connolly; has developed educational materials for the France Foundation, HIT Global, and ePocrates; has served on steering committees for clinical trials supported by GlaxoSmithKline, Nycomed, Forest Laboratories, and Actelion.
GJC has served on Advisory Committees for Dey, Boehringer Ingelheim, Ikaria, Uptake Medical, PortAero and Pulmonx, Inc. All of these sums are less than $2,500. GJC has received research grants from: Boehringer Ingelheim, Forest, Actelion, Glaxo-Smith-Kline, Advanta, Daiichi Asubio, Pfizer, Roche and Novartis Pharmaceuticals, Emphasys Medical, Inc., and Aeris Therapeutics. All research grant monies are deposited and controlled by Temple University.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS); and the Agency for Healthcare Research and Quality (AHRQ).
This article is subject to the NIH Public Access Policy (http://publicaccess.nih.gov/policy.htm; Notice Number: NOT-OD-08-033).
The authors alone are responsible for the content and writing of the paper.
References
- Sherman CB, Xu X, Speizer FE, Ferris BG Jr, Weiss ST, Dockery DW. Longitudinal lung function decline in subjects with respiratory symptoms. Am Rev Respir Dis 1992; 146:855–859.
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Amer J Respir Crit Care Med 1996; 153(5):1530–1535.
- Burgel PR, Nesme-Meyer P, Chanez P, Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 2009; 135(4):975–982.
- Kim V, Han MK, Vance GB, The Chronic Bronchitic Phenotype of COPD: An analysis of the COPDGene study. Chest 2011; 140(3):626–633.
- Kim V, Criner GJ, Abdallah HY, Gaughan JP, Furukawa S, Solomides CC. Small airway morphometry and improvement in pulmonary function after lung volume reduction surgery. Amer J Respir Crit Care Med 2005; 171(1):40–47.
- Kim V, Kelemen SE, Abuel-Haija M Small airway mucous metaplasia and inflammation in chronic obstructive pulmonary disease. J Chron Obstruct Pulmon Dis 2008; 5(6):329–338.
- Hogg JC, Chu F, Utokaparch S The nature of small-airway obstruction in chronic obstructive pulmonary disease. New Engl J Med 2004; 350(26):2645–2653.
- Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest 2006; 130(4):1129–1137.
- Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 2009; 64(10):894–900.
- Hogg JC, Chu FS, Tan WC, Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Amer J Respir Crit Care Med 2007; 176(5):454–459.
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung volume–reduction surgery with medical therapy for severe emphysema. New Engl J Med 2003; 348(21):2059–2073.
- Social Security Administration's Death Master File (full file). National Technical Information Service, 2002 (NTIS order no. SUB-5251), Springfield, VA, 2002.
- Social Security Administration's Death Master File (monthly updates file). National Technical Information Service, 2008 (NTIS order no. SUB-5446), Springfield, VA, 2008.
- Kim V, Make BJ, Reagan EA Chronic bronchitis predicts greater frequency and severity of COPD exacerbations in moderate to severe COPD (abstract) (abstract). Am J Respir Crit Care Med 2012; 185:A3735.
- Fletcher CM, Peto R, Tinker C Speizer FE. The Natural History of Chronic Bronchitis and Emphysema. Oxford UK: Oxford University Press; 1976.
- Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Amer J Respir Crit Care Med 2002; 166(3):329–332.
- Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003; 58(5):388–393.
- Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Relation of ventilatory impairment and of chronic mucus hypersecretion to mortality from obstructive lung disease and from all causes. Thorax 1990; 45(8):579–585.
- Tockman MS, Comstock GW. Respiratory risk factors and mortality: longitudinal studies in Washington County, Maryland. Amer Rev Respir Dis 1989; 140(3 Pt 2):S56–563.
- Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Amer Rev Respir Dis 1978; 118(6 Pt 2):1–120.