Abstract
Background and Objective: Angiopoietin-2 (Ang-2) is an important mediator of angiogenesis and has been implicated in many inflammatory diseases. COPD is characterized by systemic inflammation, which is enhanced during exacerbations and may be assessed by measuring serum C-reactive protein (CRP). The aim of the study was to evaluate serum CRP and Ang-2 levels on the first (D1) and seventh day (D7) of hospitalization due to a COPD exacerbation and to examine possible associations of CRP and Ang-2 levels and kinetics with the length of hospital stay and outcome. Methods: We conducted a prospective study and evaluated 90 patients admitted to the hospital with a diagnosis of an acute exacerbation of COPD. A venous blood sample was obtained from all patients on D1 and D7 of hospitalization, for the measurement of Ang-2 and CRP. Results: Serum Ang-2 levels were significantly higher on D1 compared to D7 during the course of COPD exacerbation (p < 0.001). Serum CRP levels were also significantly higher on D1 compared to D7 (p < 0.001). Serum Ang-2 presented a significant positive correlation with CRP levels both on D1 and D7 (r = 0.315 and r = 0.228, respectively). Patients with unfavorable outcome had significantly higher Ang-2 levels both on D1 (p = 0.04) and D7 (p = 0.01). Conclusions: Serum Ang-2 levels are elevated at the onset of COPD exacerbations and are positively associated with CRP levels. Ang-2 levels decrease during the course of COPD exacerbations in patients with favorable outcome. Serum Ang-2 may serve as a biomarker that could predict the outcome of a COPD exacerbation.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by persistent airflow limitation, which is progressive and not fully reversible with an enhanced inflammatory response of the airways and the lung parenchyma to noxious particles or gases (Citation1,2). It is increasingly recognized that in addition to an inflammatory process in the lung itself, systemic inflammation plays an important role in COPD (Citation1,Citation3–5).
Recent studies have provided evidence that inflammation exists in a mutually dependent association with angiogenesis (Citation6–8). Angiogenesis is a complex multiphase process, potentially involving a great number of growth factors, cytokines, chemokines, and numerous other mediators, but the specific role of each molecule has not been clearly defined (Citation9).
Vascular endothelial growth factor (VEGF) is considered to be the most important angiogenic agent that induces vascular endothelial cell proliferation, tubule formation and increases microvascular permeability (Citation10–12). Angiopoietins 1 and 2 (Ang-1, Ang-2) are members of a family of endothelial-derived vascular growth factors necessary for both normal and abnormal angiogenesis (Citation13). They are both ligands for the endothelial cell-specific Tie2 surface receptor and may act in a complimentary and coordinated manner along with VEGF in the airway microvascular process (Citation13). Ang-1 is known to promote sprouting and stabilizing of nascent vessels by promoting interactions between endothelial cells and surrounding support cells. In contrast, Ang-2 acts as a natural antagonist of Ang-1 that competes for Tie-2 receptor and reduces vascular integrity, leading subsequently to increased vascular permeability and mucosal edema (Citation14–17).
In a recent study serum Ang-2 levels were found significantly elevated during acute exacerbations of COPD, as compared to stable COPD (Citation18).
CRP is an acute-phase reactant produced by the liver. In the last two decades, increased serum CRP levels have been extensively evaluated as a marker of systemic inflammation in COPD exacerbations (Citation19,20). Current studies have reported that CRP alone is neither sensitive nor specific in predicting clinical severity or outcome of COPD exacerbations and speculate that utilizing combinations of biomarkers may prove more useful (Citation21–23).
Most COPD patients experience exacerbations during the natural course of their disease. Exacerbations are associated with a decline in lung function, deterioration of quality of life, increased frequency of hospital admissions and therefore socioeconomic costs and mortality (Citation24–26). We hypothesized that serum CRP and Ang-2 levels are elevated on hospital admission due to a COPD exacerbation. We also attempted to evaluate the kinetics of CRP and Ang-2 levels on day 1 (D1) and day 7 (D7) during the course of the exacerbation.
The aim of the present study was to evaluate serum CRP and Ang-2 levels on the first and seventh day of hospitalization due to a COPD exacerbation and to examine possible associations of CRP and Ang-2 levels and kinetics with length of hospital stay and outcome.
Methods
Patients
We conducted a prospective study and recruited 90 patients with a diagnosis of acute exacerbation of COPD between November 2008 and October 2010. To be eligible for the study, patients had to be admitted on the basis of clinical history, physical examination and chest radiography, and to meet post-bronchodilator spirometric criteria (FEV1/FVC ratio <70%) for COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines. COPD exacerbations were defined as a change in baseline dyspnoea, cough or sputum production that was greater than normal day-to-day variation and sufficient to warrant a change in treatment (Citation27).
All patients were clinically stable, with no evidence of acute exacerbation for at least 8 weeks prior to enrolment. Patients were excluded from the study if they had asthma or cystic fibrosis, a history of malignancy or coronary heart disease or if infiltrates on chest radiography were present at hospital admission. Eventually, the study cohort comprised of 90 patients with a diagnosis of COPD exacerbation.
All patients were treated according to the GOLD guidelines immediately after admission to hospital. Subjects provided information on their smoking status and were classified as current smokers or ex-smokers (those who had stopped smoking at least 1 year before entering the study). All patients or the next of kin gave informed consent for blood sampling and approval for collection. The local ethics committee approved the study.
Lung function
Spirometry was performed by trained lung function technicians. Forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio were measured using the Master Screen Body system (Viasys Healthcare, Jaeger, Hoechberg, Germany) according to the ATS guidelines.
Blood sampling
A venous blood sample was obtained from all patients on day 1 (D1) and day 7 (D7) of hospitalization, for the measurement of Ang-2 and CRP. Blood samples on D1 were collected within the first hour of admission (either in the Emergency or in the Respiratory Medicine Department) and before the administration of systemic corticosteroids. Measurement of arterial blood gases was performed on admission day (D1) as well. All samples were collected in evacuated blood collection tubes and centrifuged (×1500 g for 20 min) at room temperature within 60 minutes of collection. The supernatants were divided into aliquots, and stored at −80°C until analysed.
Measurements of serum Ang 2 and CRP
Circulating levels of Ang-2 were assessed by ELISA according to manufacturer's instructions (R&D Systems, Minneapolis, MN). The minimum detectable level of Ang-2 ranged from 1.20–21.3 pg/ml. Circulating levels of CRP were measured using an immunoturbumetric method with a commercially available kit (Dade Behring, Deerfield, IL, USA). Normal values for CRP were < 0.30 mg/dL. The intra and inter-assay variability for CRP were assessed according to the manufacturer's instructions and were within acceptable CV%. All samples were measured in duplicate. The intra and inter-assay variability for Ang-2 were 6% and 9%, respectively.
Statistical analysis
Results are presented as mean ± SD. Baseline characteristics of the patients were compared using the t-test for continuous variables and the chi-square test for categorical variables. Correlations between serum Ang-2 and CRP levels and FEV1/FVC, FEV1, PO2, PCO2 and WBC were performed using Spearman's correlation. The paired t-test was used to determine whether Ang-2 and CRP levels on D1 differed from those on D7.
The independent samples t-test was used to determine whether Ang-2 and CRP levels differed on D1 and D7 between patients with favorable and unfavorable outcome. To analyze the relationship among different variables and Ang-2 levels on hospital admission, a multiple linear regression model including age, PaO2, PaCO2, WBC counts, CRP, hemoglobin, total protein levels, albumin levels and FEV1 percentage of predicted was used. p-values < 0.05 were deemed to indicate statistical significance. All statistical analyses were performed using SPSS software, ver. 17.0 (SSPS Inc., Chicago, IL, USA).
Results
Baseline characteristics of the study population are presented in . Ninety-eight percent of patients were receiving medical treatment for COPD on hospital admission, including long-acting inhaled β2-agonists (92%), long acting anticholinergics (78%), inhaled steroids (87%), oral steroids (7%), theophylline (20%) and home oxygen (10%).
Table 1. Demographic characteristics of the study participants
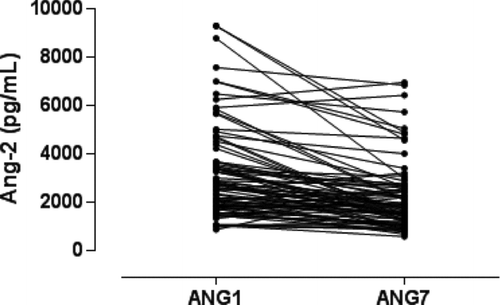
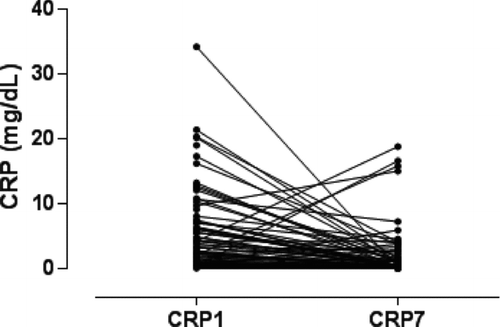
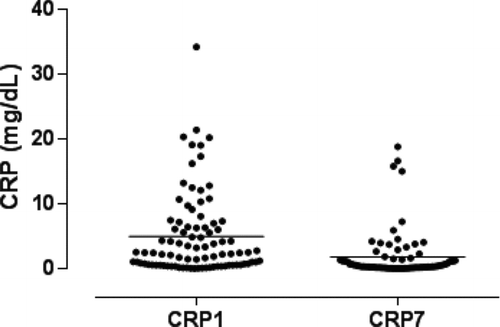
Antibiotic treatment and systemic steroids were prescribed for all patients (mean dose, 37.5 mg of prednisolone/day). The mean length of hospital stay was 10.9 ± 12 days. Serum Ang-2 was significantly higher on D1 compared to D7 during the course of COPD exacerbation (3164.3 ± 1902.8 pg/mL vs 2126.5 ± 1379.5,p < 0.001) ( and ). Serum CRP was significantly higher on D1 compared to D7 during the course of COPD exacerbation (4.9 ± 6.3 mg/dL vs 1.7 ± 3.6, p < 0.001) ( and ).
Figure 1a. Serum Angiopoietin-2 on days 1 (ANG1) and 7 (ANG7) - scatterplot (horizontal lines represent mean values).
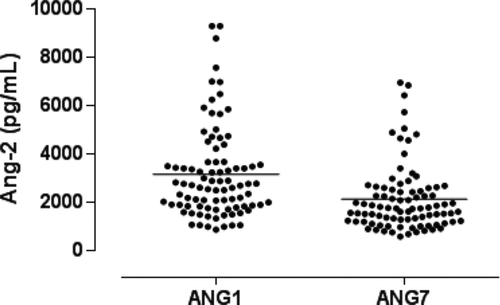
Figure 2a. Serum C-reactive protein (CRP) on days 1 (CRP1) and 7 (CRP7) - scatterplot (horizontal lines represent mean values).
Relationship of serum Ang-2 and CRP levels
Serum Ang-2 levels showed a significant positive correlation with CRP levels both on D1 (r = 0.315, p = 0.03) and on D7 (r = 0.228, p = 0.042).
Relationship of serum Ang-2 and CRP levels with lung function, arterial blood gases and laboratory findings
No significant correlation was detected between serum Ang-2 or CRP levels either on D1 or D7 with the length of hospital stay, FEV1/FVC, FEV1, PO2, PCO2, WBC. Ang-2 levels on hospital admission were not affected by long-term oxygen therapy (p = NS) or long-term oral steroid use (p = NS).
In our multivariate linear regression, none of the parameters entered in the model was a significant predictor of Ang-2 levels on admission.
Favorable vs. unfavorable outcome
Unfavorable outcome was defined as death or as need for endotracheal intubation and mechanical ventilation during hospitalization. Eleven out of 90 patients had an unfavorable outcome. Patients with unfavorable outcome had significantly higher Ang-2 levels both on D1 (p = 0.04) and D7 (p = 0.01) ().
Table 2. Comparison of serum Ang-2 and CRP levels between patients with favorable and unfavorable outcome
Regarding CRP, patients with unfavorable outcome had comparable CRP levels on D1 with those with favorable outcome but significantly higher on D7 (p < 0.01).
Discussion
The main findings of our study were as follows: serum Ang-2 and CRP levels were significantly higher on D1 compared to D7 during the course of a COPD exacerbation; serum Ang-2 levels were positively correlated with CRP levels on D1 and D7; patients with unfavorable outcome had significantly higher serum Ang-2 levels on D1 and D7 compared to patients with a favorable outcome.
Serum biomarkers have been extensively evaluated in the assessment of systemic inflammation and possible prediction of outcomes in COPD. Additionally, these biomarkers can be measured readily and repeatedly and may serve as useful tools for monitoring through changes in their levels. Although serum CRP is not specific for COPD exacerbations, increased serum CRP levels have been detected in COPD exacerbations and decreased substantially thereafter (Citation19,20). Our finding that CRP levels were significantly higher on D1 compared to D7 during the course of COPD exacerbations was somehow expected and in accordance to previous studies.
Data derived from previous studies suggest that angiogenesis is associated with numerous inflammatory conditions, such as atherosclerosis, arthritis, retinopathy, and tumor growth (Citation28). Valipour et al. gave evidence that circulating levels of VEGF and markers of systemic inflammation are up-regulated in patients with acute exacerbated COPD and decrease after recovery from the exacerbation (Citation29). However, this study did not evaluate the time kinetics of these markers during the course of an exacerbation.
Furthermore, Y Ji Cho et al., recently reported that Ang-2 levels were elevated during acute exacerbations of COPD as compared with stable COPD, and that they decreased significantly after clinical recovery from the acute exacerbation (Citation18). We also found that serum Ang-2 levels were significantly higher on D1 compared to D7 during the course of COPD exacerbations which is in accordance with the previous findings.
More interestingly, serum Ang-2 levels were positively correlated with CRP levels both on D1 and D7 in our study, supporting the idea that inflammation exists in a mutually dependent association with angiogenesis. However, it cannot be excluded that serum Ang-2 may act as an inflammatory mediator reflecting systemic inflammation more specifically due to originating from the lung compared to CRP. This positive correlation has been reported in a previous study (Citation18), but at a specific time point (onset of exacerbation) and further emphasizes the potential value of serum Ang-2 as a biomarker in COPD.
Moreover, this is the first study to our knowledge that evaluates the kinetics of serum Ang-2 during a COPD exacerbation. Our data provide evidence that Ang-2 levels decrease during the course of a COPD exacerbation within a few days.
Clearly, there is the need for a plasma biomarker in COPD that will not only aid in distinguishing stable disease from exacerbation, but might also have prognostic value.
Patients with unfavorable outcome had significantly higher serum Ang-2 levels on D1 and D7 compared to patients with favorable outcome. This indicates that serum Ang-2 levels could serve as a predictor of the outcome of a COPD exacerbation indicating a prognostic value for this biomarker. Moreover, the better prognostic performance of Ang-2 compared to CRP on D1 supports a clinical utility for this biomarker.
The present study has several limitations. First, the sample size is rather small that precludes from extracting safe conclusions about the usefulness of serum Ang-2 as a biomarker in COPD exacerbation. Notably, the prognostic significance of elevated serum Ang-2 levels on D1 requires further examination since the number of patients with unfavorable outcome was small. Second, the study was not designed to compare CRP and Ang-2 as biomarkers. However, we measured CRP because it is a biomarker that has been extensively evaluated and was shown to have a good predictive value. Third, COPD patients were treated with steroids that are known to markedly suppress CRP (Citation29). Moreover, the vast majority of our COPD patients were receiving ICS prior to the exacerbation and accordingly it was not possible to assess an effect of such treatment to blood vessel permeability and serum Ang-2 levels. Definitely, more studies are needed to define the exact role of Ang-2 in COPD exacerbations.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Conclusions
Serum Ang-2 levels are elevated at the onset of COPD exacerbations and are positively correlated with CRP levels. Ang-2 levels decrease during the course of COPD exacerbations in patients with favorable outcome. COPD exacerbations are characterized by elevated Ang-2 levels that decrease in parallel with CRP levels during the course of the exacerbation. Serum Ang-2 levels may serve as predictors of the outcome of a COPD exacerbation.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Revised 2011. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html (accessed on 15 March 2012).
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004 Aug; 364(9434):613–620.
- Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis 2011; 6:413–421.
- Agusti AG, Noguera A, Sauleda J, Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003 Feb; 21(2):347–360.
- Gan WQ, Man SFP, Senthilselvan A, Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004 Jul; 59(7):574–580.
- Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006 Dec; 27(12):552–558.
- Jackson JR, Seed MP, Kircher CH, The codependence of angiogenesis and chronic inflammation. FASEB J. 1997 May; 11(6):457–465.
- Dvorak HF, Brown LF, Detmar M, Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995 May; 146(5):1029–1039.
- Zanini A, Chetta A, Imperatori AS, The role of the bronchial microvasculature in the airway remodelling in asthma and COPD. Respir Res 2010 Sep; 29(11):132.
- Walters EH, Soltani A, Reid DW, Vascular remodelling in asthma. Curr Opin Allergy Clin Immunol 2008 Feb; 8(1):39–43.
- Papaioannou AI, Kostikas K, Kollia P, Clinical implications for vascular endothelial growth factor in the lung: friend or foe? Respir Res 2006 Oct; 17(7):128.
- Goldie RG, Pedersen KE. Mechanisms of increased airway microvascular permeability: role in airway inflammation and obstruction. Clin Exp Pharmacol Physiol 1995 Jun-Jul; 22(6-7): 387–396.
- Makinde T, Murphy RF, Agrawal DK. Immunomodulatory role of vascular endothelial growth factor and angiopoietin-1 in airway remodeling. Curr Mol Med 2006 Dec; 6(8):831–841.
- Maisonpierre PC, Suri C, Jones PF, Angiopoietin-2, a natural antagonist for Tie-2 that disrupts in vivo angiogenesis. Science 1997 Jul; 277(5322):55–60.
- Suri C, Jones PF, Patan S, Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996 Dec; 87(7):1153–1155.
- Knox AJ, Stocks J, Sutcliffe A. Angiogenesis and vascular endothelial growth factor in COPD. Thorax 2005 Feb; 60(2):88–89.
- Gale NW, Thurston G, Hackett SF, Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell 2002 Sep; 3(3):411–423.
- Cho YJ, Ma JE, Yun EY, Serum angiopoietin-2 levels are elevated during acute exacerbations of COPD. Respirology 2011 Feb; 16(2):284–290.
- Hurst JR, Donaldson GC, Perera WR, Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006 Oct; 174(8):867–874.
- Muller B, Tamm M. Biomarkers in acute exacerbation of chronic obstructive pulmonary disease: among the blind, the one-eyed is king. Am J Respir Crit Care Med 2006 Oct; 174(8):848–849.
- Patel AR, Hurst JR, Wedzicha JA. The potential value of biomarkers in diagnosis and staging of COPD and exacerbations. Semin Respir Crit Care Med 2010 Jun; 31(3):267–275.
- Antonescu-Turcu AL, Tomic R. C-reactive protein and copeptin: prognostic predictors in chronic obstructive pulmonary disease exacerbations. Curr Opin Pulm Med 2009 Mar; 15(2):120–125.
- Ruiz-González A, Lacasta D, Ibarz M, C-reactive protein and other predictors of poor outcome in patients hospitalized with exacerbations of chronic obstructive pulmonary disease. Respirology 2008 Nov; 13(7):1028–1033.
- Donaldson GC, Seemungal TAR, Bhowmik A, Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002 Oct; 57(10):847–852.
- Seemungal TAR, Donaldson GC, Paul EA, Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998 May; 157(5 Pt 1):1418–1422.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005 Nov; 60(11):925–931.
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000 May; 117(5 Suppl 2):398–401.
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995 Jan; 1(1):27–31.
- Valipour A, Schreder M, Wolzt M, Circulating vascular endothelial growth factor and systemic inflammatory markers in patients with stable and exacerbated chronic obstructive pulmonary disease. Clin Sci (Lond) 2008 Oct; 115(7):225–232.
- Muller B, Peri G, Doni A, High circulating levels of the IL-1 type II decoy receptor in critically ill patients with sepsis: association of high decoy receptor levels with glucocorticoid administration. J Leukoc Biol 2002 Oct; 72(4):643–649.