Abstract
Background: High-intensity noninvasive positive pressure ventilation (HI-NPPV) is an effective treatment option in patients with stable hypercapnic chronic obstructive pulmonary disease (COPD). However, the effect of HI-NPPV compared with spontaneous breathing (SB) on minute ventilation (MV) in patients receiving long-term treatment remains to be determined. This study compared MV during HI-NPPV and SB. In addition, the ability of intelligent volume assured pressure support (iVAPS) to increase MV to the same extent as HI-NPPV was determined. Methods: Daytime pneumotachographic measurements were performed during SB, HI-NPPV and iVAPS. Results: Twenty-seven stable hypercapnic COPD patients (mean FEV1 34 ± 15% predicted) who had been treated with HI-NPPV for a median of 22 months (interquartile range 8.5–84 months) were enrolled. Mean MV was 9.5 ± 1.7 L/min during SB and 12.1 ± 2.8 L/min during HI-NPPV, an increase of 2.5 L/min (95% CI [1.5–3.6] p < 0.001), or 26%. MV during iVAPS was 11.7 ± 3.6 L/min, an increase of 1.8 L/min (95%CI [0.7–3.0], p = 0.003) compared with SB. There was no difference in MV between HI-NPPV and iVAPS (p = 0.25). Conclusion: Long-term HI-NPPV increased MV by an average of 26% compared with SB in stable hypercapnic COPD patients. A similar increase in MV was observed during use of iVAPS.
Introduction
There is currently no consensus about the use of noninvasive positive pressure ventilation (NPPV) for the treatment of stable hypercapnic chronic obstructive pulmonary disease (COPD) (Citation1–4). Recent research suggests that NPPV application technique is a major determinant of the acceptance and effectiveness of this intervention (Citation5). High-intensity NPPV (HI-NPPV) has been shown to be superior to the more widely used low-intensity NPPV (LI-NPPV) with respect to improvements in blood gases, lung function, exercise-induced dyspnoea and health-related quality of life in stable hypercapnic COPD patients (Citation6,7). Furthermore, HI-NPPV can be successfully used for the long-term management of these patients (Citation8,9). However, initiation of HI-NPPV requires a longer titration period compared with LI-NPPV (Citation6). The goal of HI-NPPV is to achieve normocapnia or a significant reduction in arterial partial pressure of carbon dioxide (PaCO2); to date this has only been achieved by a stepwise increase in inspiratory positive airway pressure (IPAP) resulting in improvements in arterial blood gases (Citation6,Citation8). Therefore, strategies to facilitate the process of establishing HI-NPPV are likely to improve patient comfort and have economic benefits.
The overall goal of NPPV therapy for the treatment of chronic hypercapnic respiratory failure is to improve alveolar ventilation, which, in turn, improves minute ventilation (MV) (Citation10). However, there is no data on MV during HI-NPPV compared with spontaneous breathing (SB) in stable hypercapnic COPD patients receiving long-term HI-NPPV. Knowledge of the effects of a successful treatment strategy like HI-NPPV compared with SB might provide useful information about the use of NPPV with target volume in patients with COPD (Citation6,7,Citation9).
New NPPV techniques using pressure-controlled ventilation with target volumes are increasingly being used in patients with chronic respiratory failure (Citation11–13), and therefore might facilitate the initiation of NPPV because a predefined pressure range might avoid the requirement to titrate inspiratory positive airway pressure (IPAP). Intelligent volume assured pressure support (iVAPS) is a hybrid mode of NPPV combining pressure support ventilation with a target volume aiming to guarantee defined alveolar ventilation by adjusting the level of pressure support in predefined pressure ranges (Citation14–16). However, the effectiveness of this approach compared with HI-NPPV for the treatment of stable hypercapnic COPD remains to be determined.
The aim of the present study was to assess differences in MV between SB and long-term HI-NPPV in stable hypercapnic patients with COPD. In addition, the ability of intelligent volume assured pressure support (iVAPS) to increase MV to the same extent as HI-NPPV was determined.
Methods
This open-label prospective study was conducted at the “Albert-Ludwigs University Hospital Freiburg, Germany. The protocol was approved by Ethics Committee at the Albert-Ludwigs University, Freiburg, Germany (approval number 276/10) and was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. Written, informed consent was obtained from all patients.
Patients
Consecutive patients with chronic hypercapnic respiratory failure due to COPD who had been receiving NPPV for at least 2 months were enrolled. All patients were receiving appropriate medical treatment and long-term oxygen therapy, where appropriate, according to the GOLD guidelines (Citation17). Patients were excluded from the study if they had acute on chronic respiratory failure (pH < 7.35), or signs of acute exacerbation (with at least two of the following: increasing cough, purulent sputum, elevated leukocytes or C-reactive protein > 5 mg/L, pulmonary infiltrates on chest X-ray, need for antibiotic treatment).
Measurements
Lung function parameters (Masterlab-Compact® Labor, Jaeger, Hochberg, Germany) were assessed in accordance with international guidelines (Citation18). Capillary blood gases were assessed during NPPV plus supplemental oxygen (AVL OMNI, Roche Diagnostics GmbH, Graz, Austria). Pneumotachographic measurements (RSS 100 Research PneumoSeries, Hans Rudolph, Inc., Shawnee, KS, USA) were made using a flow sensor connected to a mouthpiece during SB or between the ventilatory mask and the exhalation port (Silentflow 2, Weinmann, Hamburg, Germany) during NPPV (). Pneumotachographic data were calculated by using RSS for Windows® Software (KORR Medical Technologies Inc. Salt Lake City, Utah, USA). Expiratory tidal volume was used for the determination of MV. Leakage was defined as percentage difference between inspiratory and expiratory tidal volume. MV, respiratory rate (RR) and leakage were calculated by averaging data from the 10-minute testing period in each setting. Patients used a nose clip during SB to avoid breathing through the nose. Patients used their own non-vented ventilatory masks. In case of nasal masks patients were asked to keep their mouth closed for the duration of testing.
Study design
Assessments were performed during a routine inpatient control visit for NPPV. Lung function parameters and ventilator settings were assessed at admission. Nocturnal blood gases during HI-NPPV were assessed overnight at 1:00 a.m. regardless the underlying sleep stage. Supplemental oxygen was applied during HI-NPPV as needed. HI-NPPV was performed at least 6 hours at night before entering the study. The three pneumotachographic measurements lasting 10 minutes each were performed during SB, HI-NPPV and iVAPS, with the patient in a supine position. Measurements were not performed in a random order. Between each setting patients were breathing spontaneously for 5 minutes. The primary endpoint of the study was the difference in MV during SB versus HI-NPPV.
Noninvasive positive pressure ventilation
All patients were established on effective HI-NPPV prior to the study, which has been described in more detail previously (Citation9). Ventilator settings and the ventilator were not changed for pneumotachographic measurements during HI-NPPV. For iVAPS, the Stellar 150® (ResMed Inc., Sydney, Australia) was used. iVAPS has a variable backup rate, which is intended to maximize the patient's opportunity to spontaneously trigger the ventilator. When spontaneous triggering ceases the set respiratory rate is adopted. It will adjust quickest (typically within 4–5 breaths) when ventilation is below the target ventilation. During spontaneous triggered ventilation the device will reduce the backup rate to two-thirds of the set respiratory rate. A more detailed description of iVAPS can be found elsewhere (Citation19).
Alveolar ventilation was defined as MV minus anatomical dead space ventilation. The anatomical dead space was calculated by the ventilator's software using
patient height (cm) as follows: . This formula has been adapted by the ventilator company from published data (Citation20). MV needed for the calculation of alveolar target volume was assessed during ventilation with the Stellar 150® using settings from HI-NPPV. Expiratory positive airway pressure (EPAP) and respiratory rate (RR) during iVAPS were the same as those during HI-NPPV, whereas the range of inspiratory pressure support was set between 10 and 30 mbar. In patients receiving an EPAP >5 mbar, maximum pressure support was 35 mbar minus EPAP because maximal IPAP using the Stellar 150® is limited to 35 mbar. Trigger sensitivity during iVAPS was set at “medium.”
Statistical analysis
The study was designed to show a difference in MV between SB and HI-NPPV of 1.6 L/min, assuming a standard deviation of 1.6 L/min according to previous findings from unpublished data. Using these assumptions, 13 patients were required to show a difference with a paired t-test at a two-sided significance level of 0.05 with a power of 0.90. A subgroup analysis based on body mass index (BMI) was planned to investigate differences between non-obese COPD patients (BMI ≤ 30 kg/m2) and obese COPD patients (BMI > 30 kg/m2). Therefore, the target was to enrol 13 non-obese and 13 obese patients.
Differences in demographic data, lung function parameters and ventilator settings for HI-NPPV, duration of HI-NPPV prior to the study and blood gases during nocturnal HI-NPPV between obese and non-obese patients were analysed using the unpaired t-test. For the comparison of blood gases and lung function parameters measured before and after initiation of HI-NPPV, the paired t-test was used. Differences were calculated with 95% confidence intervals (CI). The Wilcoxon signed rank test was used for data with a non-normal distribution.
For comparison of MV during SB, HI-NPPV and iVAPS a linear regression model for repeated measures was used including breathing setting (SB, HI-NPPV and iVAPS), BMI (≤ 30 kg/m2, > 30 kg/m2), and the interaction between breathing settings and BMI. Differences between breathing settings were estimated with 95% CI for the whole study population, and the obese and non-obese subgroups. In addition, interactions between breathing setting and BMI, and between respiratory rate and leakage were analysed.
Results
Twenty-seven patients were included. Demographic data and lung function parameters at baseline are shown in . Measurements of MV, RR and leakage during SB and HI-NPPV were completed in all 27 patients, but data during iVAPS were only available for 21 patients.
Table 1. Demographic data and lung function parameters for all patients and the two subgroups
All patients were established on HI-NPPV prior to the study. Home mechanical ventilation was applied using Breas VIVO 30® (n = 9) or VIVO 40® (n = 12), (Breas Medical AB, Molnlycke, Sweden), Weinmann VentimotionTM (n = 1), Ventimotion 2TM (n = 4) (Weinmann Geraete für Medizin GmbH + Co. KG, Hamburg, Germany), or Airox SmartairTM (n = 1) (Airox, Covidien, Hampshire, United Kingdom). Ventilation parameters and capillary blood gases during nocturnal HI-NPPV are reported in . Patients had been receiving effective treatment with HI-NPPV for a median of 22 (interquartile range 8.5–84) months, with significant improvements in lung function and blood gases ().
Table 2. Ventilator settings for HI-NPPV, duration of HI-NPPV prior to the study and blood gases during nocturnal HI-NPPV for all patients and the two subgroups
Table 3. Blood gases and lung function parameters before and after initiation of HI-NPPV (n = 27)
Mean MV increased by 2.5 L/min (95% CI 1.5–3.6, p < 0.001), or 26%, during HI-NPPV compared with SB. MV during iVAPS increased by 1.8 L/min (95% CI 0.7-3.0, p = 0.003) compared with SB. There was no significant difference in MV during HI-NPPV and iVAPS () ().
Figure 2. Pneumotachographic measurements comparing SB, HI-NPPV and iVAPS. Data are mean ± SD. HI-NPPV = high intensity non-invasive positive pressure ventilation, iVAPS = intelligent volume assured pressure support, SB = spontaneous breathing. Comparison between SB, HI-NPPV and iVAPS was made using a linear regression model for repeated measures.
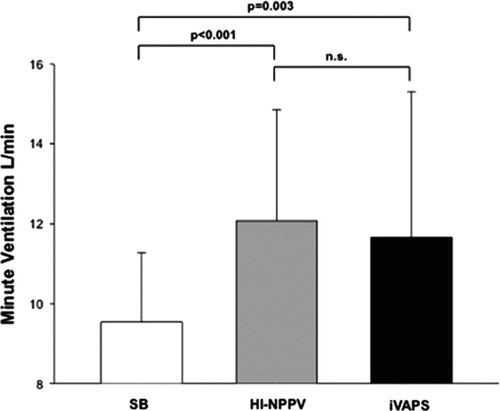
Table 4. Pneumotachographic measurements comparing SB (n = 27), HI-NPPV (n = 27) and iVAPS (n = 21) for all patients and for the two subgroups
RR was similar during HI-NPPV and SB, and during iVAPS and SB. However, RR was higher during HI-NPPV compared with iVAPS (). Leakage occurred during both modes of NPPV without any difference between groups ().
Subgroup analyses
Subgroup analysis revealed no differences between non-obese and obese COPD patients for age, pack-years, blood gases and ventilation parameters except for EPAP, which tended to be higher in obese COPD patients (Tables and ). However, non-obese COPD patients had greater impairment of lung function ().
MV increased during HI-NPPV compared with SB in both obese and non-obese COPD patients (). Increases in MV during iVAPS compared with SB only occurred in obese COPD patients (). The test for an interactive effect between breathing setting and BMI with regard to MV showed a p value of 0.033. RR tended to be lower in non-obese patients during iVAPS compared to HI-NPPV, whereas no difference was found in obese patients ().
Discussion
The results of this study showed that use of HI-NPPV for the long-term treatment of patients with stable hypercapnic COPD increased MV by a mean of 2.5 L/min (26%) compared with SB. In addition, a similar increase in MV can be achieved by using iVAPS instead of HI-NPPV.
NPPV has been shown to improve tidal volume by 35% compared to SB in stable hypercapnic COPD patients using an IPAP of 17 mbar (Citation21). However, there is clear evidence that higher levels of IPAP are needed to improve blood gases, health-related quality of life, lung function parameters and treatment compliance in COPD patients over the long term (Citation6,7). Recent data have shown that HI-NPPV improves MV by 58% compared to SB in stable hypercapnic COPD patients (Citation22), but patients were naïve to NPPV and the aim of the study was to assess physiological changes during HI-NPPV. Therefore, IPAP was set to the maximum tolerated level (mean 28 mbar) because the pressure level needed for effective ventilation over a long-term period was unknown (Citation22). In contrast, patients in the present study had been receiving HI-NPPV for a mean of 45 months, and PaCO2 could be significantly reduced using a mean IPAP of 25 mbar. This effective ventilation strategy increased MV by 26% on average compared to SB.
MV was similar between HI-NPPV and iVAPS, but higher compared to SB. Therefore, the present study shows that, at least during daytime, iVAPS is capable of providing effective ventilation and improving MV in COPD patients to a similar extent as HI-NPPV. The effectiveness of iVAPS in this setting might help to simplify the establishment of effective long-term NPPV in stable hypercapnic COPD patients because it uses a predefined inspiratory pressure range that does not require titration of IPAP over several days. The clinical importance of this finding could be quite significant because, even in experienced centers, several days are needed for the initiation of HI-NPPV (Citation6). However, further studies are needed to assess if effective ventilation can be established more easily, more quickly and, therefore, more cost-effectively by measuring MV during SB and then using a 26% higher MV for iVAPS with a wide pressure range. Hereby, MV during spontaneous breathing should be assessed by using a mouthpiece as it was done in the present study. This might be important due to the fact that it has been shown in healthy individuals that using a mouthpiece per se results in an increase of MV compared to breathing without a mouthpiece (Citation23).
The results of this study showed that HI-NPPV improves MV in both obese and non-obese COPD patients. However, iVAPS increased MV only in obese patients with COPD. It is possible that non-obese COPD patients in the present study were more hyperinflated than obese patients as a result of their greater total lung capacity. It has been suggested that COPD patients with hyperinflation have greater difficulty triggering the ventilator compared to those who are not hyperinflated (Citation24,25). Patients are supposed to breathe spontaneously during iVAPS and therefore triggering the ventilator is of importance. This might explain why iVAPS did not improve MV in non-obese COPD patients. Additional evidence for this hypothesis is provided by the fact that RR tended to be slightly lower during iVAPS compared with HI-NPPV in non-obese COPD patients whereas there was no difference in obese COPD patients.
This study has several limitations. Firstly, measurements comparing SB and HI-NPPV were available in all patients, but measurements during iVAPS were only available in 21 patients due to technical issues. Nevertheless, the study was powered to show a difference between SB and HI-NPPV, and the comparison with iVAPS was a secondary outcome parameter. Secondly, pneumotachographic measurements were performed during the daytime and therefore conclusions about the increase in MV cannot be extrapolated to night-time ventilation. In addition, measurement of expired tidal volume could have been underestimated during HI-NPPV and iVAPS due to mask leak. Finally, HI-NPPV and iVAPS are based on different respiratory rate strategies since HI-NPPV aims to achieve controlled ventilation by using high breathing frequency rates whereas iVAPS was designed to maximize the patient's opportunity to spontaneously trigger the ventilator. Nevertheless, both approaches were associated with good increases in MV but RR might be important in the subgroup of COPD patients without obesity as discussed above.
In conclusion, long-term use of HI-NPPV in stable hypercapnic COPD patients increased MV by an average of 26% compared with SB. A similar increase in MV was achieved using iVAPS. Therefore, iVAPS might help to facilitate the initiation process of noninvasive ventilation in these patients.
Declaration of Interest Statement
The authors have reported the following conflicts of interest: Emelie Ekkernkamp: Travel funding for national and international research congresses by ResMed and Vivisol. Open research grant from ResMed. Hans-Joachim Kabitz: Speeking fees from Heinen und Löwenstein. Travel funding for national and international research congresses by Breas, Heinen und Löwenstein, Vivisol and Weinmann. Open research grant from Breas. David J Walker: Travel funding for national and international research congresses by Vivisol. Claudia Schmoor: no conflicts of interest. Jan H Storre: Speaking fees from Heinen und Löwenstein, Werner und Müller Medizintechnik, Respironics, VitalAire, Breas, Radiometer and SenTec. Travel funding for national and international research congresses from Breas, Respironics, SenTec, Vivisol, Weinmann and Werner und Müller Medizintechnik. Open research grant from Breas, Respironics and ResMed. Wolfram Windisch: Speaking fees from Dräger Medical, Heinen und Löwenstein, VitalAire, Respironics, Weinmann, ResMed, Linde Healthcare, Siare and Maquet. Open research grant from Respironics and Breas. Michael Dreher: Speaking fees from VitalAire, ResMed, Drager Medical, Linde Healthcare, Weinmann and Respironics. Travel funding for national and international research congresses from Respironics, Vivisol, Weinmann and ResMed. Open Research grant from ResMed.
The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The study was supported by an unrestricted research grant from ResMed Ltd., Sydney. The study devices were provided by ResMed GmbH& Co KG, Martinsried, Germany. The authors state that the sponsor had no input into the study design, results or interpretation, or manuscript preparation.
English language editing assistance was provided by Nicola Ryan. This assistance was funded by Resmed.
References
- Rossi A. Noninvasive ventilation has not been shown to be ineffective in stable COPD. Am J Respir Crit Care Med 2000 Mar; 161(3 Pt 1):688–689.
- Hill NS. Noninvasive ventilation in chronic obstructive pulmonary disease. Clin Chest Med 2000 Dec; 21(4):783–797.
- Wijkstra PJ, Lacasse Y, Guyatt GH, Casanova C, Gay PC, Meecham Jones J, A meta-analysis of nocturnal noninvasive positive pressure ventilation in patients with stable COPD. Chest 2003 Jul; 124(1):337–343.
- Elliott MW. Domiciliary non-invasive ventilation in stable COPD? Thorax 2009 Jul; 64(7):553–556.
- Schönhofer B. Non-invasive positive pressure ventilation in patients with stable hypercapnic COPD: light at the end of the tunnel? Thorax 2010 Sep; 65(9):765–767.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010 Apr; 65(4):303–308.
- Dreher M, Ekkernkamp E, Walterspacher S, Walker D, Schmoor C, Storre JH, Noninvasive ventilation in COPD: impact of inspiratory pressure levels on sleep quality. Chest 2011 Oct; 140(4):939–945.
- Windisch W, Kostić S, Dreher M, Virchow JC Jr, Sorichter S. Outcome of patients with stable COPD receiving controlled noninvasive positive pressure ventilation aimed at a maximal reduction of Pa(CO2). Chest 2005 Aug; 128(2):657–662.
- Windisch W, Haenel M, Storre JH, Dreher M. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int J Med Sci 2009; 6(2):72–76.
- Bertella E, Vitacca M. Pressure Support Ventilation. In: Esquinas AM, editor. Noninvasive Mechanical Ventilation (Internet). Berlin, Heidelberg: Springer Berlin Heidelberg; 2010 (cited 2013 Mar 8). p. 21–26. Available from: http://link.springer.com/10.1007/978-3-642-11365-9_4
- Storre JH, Seuthe B, Fiechter R, Milioglou S, Dreher M, Sorichter S, Average volume-assured pressure support in obesity hypoventilation: A randomized crossover trial. Chest 2006 Sep; 130(3):815–821.
- Crisafulli E, Manni G, Kidonias M, Trianni L, Clini EM. Subjective sleep quality during average volume assured pressure support (AVAPS) ventilation in patients with hypercapnic COPD: a physiological pilot study. Lung 2009 Oct; 187(5):299–305.
- Janssens J-P, Metzger M, Sforza E. Impact of volume targeting on efficacy of bi-level non-invasive ventilation and sleep in obesity-hypoventilation. Respir Med 2009 Feb; 103(2):165–172.
- Jaye J, Chatwin M, Dayer M, Morrell MJ, Simonds AK. Autotitrating versus standard noninvasive ventilation: a randomised crossover trial. Eur Respir J 2009 Mar; 33(3):566–571.
- Oscroft NS, Ali M, Gulati A, Davies MG, Quinnell TG, Shneerson JM, A randomised crossover trial comparing volume assured and pressure preset noninvasive ventilation in stable hypercapnic COPD. COPD 2010 Dec; 7(6):398–403.
- Battisti A, Tassaux D, Bassin D, Jolliet P. Automatic adjustment of noninvasive pressure support with a bilevel home ventilator in patients with acute respiratory failure: a feasibility study. Intensive Care Med 2007 Apr; 33(4):632–638.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007 Sep 15; 176(6):532–555.
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Standardisation of the measurement of lung volumes. Eur Respir J 2005 Sep; 26(3):511–522.
- ResMed. Stellar 150 Clinical Guide. 2010.
- Hart MC, Orzalesi MM, Cook CD. Relation between anatomic respiratory dead space and body size and lung volume. J Appl Physiol 1963 Jan 5; 18(3):519–522.
- Navalesi P, Costa R, Ceriana P, Carlucci A, Prinianakis G, Antonelli M, Non-invasive ventilation in chronic obstructive pulmonary disease patients: helmet versus facial mask. Intens Care Med 2007 Jan; 33(1):74–81.
- Lukácsovits J, Carlucci A, Hill N, Ceriana P, Pisani L, Schreiber A, Physiological changes during low- and high-intensity noninvasive ventilation. Eur Respir J 2012 Apr; 39(4):869–875.
- Askanazi J, Silverberg PA, Foster RJ, Hyman AI, Milic-Emili J, Kinna JM. Effects of respiratory apparatus on breathing pattern. J Appl Physiol 1980 Apr; 48(4):577–580.
- Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intens Care Med 2006 Oct; 32(10):1515–1522.
- Hoffman RA, Ershowsky P, Krieger BP. Determination of auto-PEEP during spontaneous and controlled ventilation by monitoring changes in end-expiratory thoracic gas volume. Chest 1989 Sep; 96(3):613–616.