Abstract
Background: Oxidative stress is implicated in the pathogenesis of asthma and chronic obstructive pulmonary disease (COPD). Analysis of the expired breath condensate (EBC) has been suggested to provide non-invasive inflammatory markers that reflect oxidative stress in the airways. Objective: The present study attempts to elucidate whether the hydrogen peroxide (H2O2) levels and pH values in EBC may be useful as biomarkers of the activity or severity of asthma and COPD. Methods: We measured the H2O2 levels and pH values using a derivatives of reactive oxygen metabolites exhalation test kit (Diacron) and a pH analyser, respectively, in EBC obtained using an EcoScreen from 29 patients with asthma, 33 with COPD, and 33 healthy individuals (all non-smokers). We then examined the relationships among oxidative stress and the asthma control test (ACT) or COPD assessment test (CAT) scores, pulmonary function, fractional exhaled nitric oxide (FeNO), and the extent of low attenuation areas on HRCT. Results: The H2O2 levels were elevated and pH was lower in both asthma (H2O2; 8.75 ± 0.88 μM, p < 0.01, pH; 7.14 ± 0.07, p < 0.05) and COPD (H2O2; 7.44 ± 0.89 μM, p < 0.01, pH; 6.87 ± 0.10, p < 0.01) compared with control subjects (H2O2; 3.42 ± 0.66 μM, pH; 7.35 ± 0.04). Neither the H2O2 levels nor pH correlated with the ACT scores and FeNO in asthma patients. Neither the H2O2 levels nor pH significantly correlated with the pulmonary function in asthma and COPD. However, the CAT scores significantly correlated with the H2O2 levels in patients with COPD (r = 0.52, p < 0.01). Conclusions: These findings suggest that oxidative stress is involved in the pathogenesis of asthma and COPD and that the H2O2 levels in EBC might reflect the health status in COPD.
Introduction
Airway inflammation in both asthma and COPD closely correlates with oxidative stress, which arises when the oxidant activity outweighs that of antioxidants (Citation1, 2). Oxidative stress induces lung tissue injury, bronchial hyperresponsiveness, damage and apoptosis of airway epithelial cells, proinflammatory effects, inactivation of antioxidants enzymes, and airflow obstruction, and increases mucus production (Citation1).
Hydrogen peroxide (H2O2) is a mediator of oxidative stress that plays an important role in asthma and COPD. Increased levels of H2O2 in exhaled breath condensate (EBC), which allows the collection of biological samples from the lower respiratory tract even from patients with advanced or severe disease, have been identified in cigarette smokers (Citation3), as well as in patients with COPD (Citation4) and asthma (Citation2). The pH of the EBC, which reflects oxidative stress in the airways, is also lower in patients with COPD and moderate asthma, and endogenous airway acidification is closely related to the underlying inflammatory process in asthma and COPD (Citation5). However, the mechanism of airway acidification might differ between asthma and COPD, and different manipulations of EBC samples for standardization might generate different findings (`.
Currently, the levels of H2O2 and the pH of EBC are considered to be useful biomarkers of oxidative stress for the management of asthma and COPD. However, the relationships among these biomarkers and disease activity, severity and phenotype are not fully understood. The present study examined the relevance of the H2O2 levels and pH of EBC as biomarkers for assessing the disease activity in asthmatic patient, and the disease severity, including the health status and emphysema, in patients with COPD.
Methods
Subjects
The present study enrolled 33 patients with stable COPD, 29 with asthma who had never smoked and who attended the outpatient clinic of Shinshu University Hospital, and 33 healthy volunteers who had never smoked from April 2011 to September 2012.
All COPD was smoking-related without any α1-antitrypsin deficiency, and these patients all had a smoking history of over 20 pack-years (20 cigarettes per day for 20 years) but had stopped smoking for over 1 year. We excluded current smokers with COPD because current smoking affects the EBC pH (Citation7). We diagnosed COPD based on a clinical history of exertional dyspnea and pulmonary function characterized by not fully reversible airflow obstruction. Not fully reversible airflow obstruction was defined as a FEV1/FVC < 70% after the inhalation of a β2-agonist and treatment with bronchodilators in accordance with Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Citation8).
Patients with a history of asthma or asthmatic symptoms, such as coughing or wheezing at rest in a stable phase, as well as patients who had taken oral steroids or who had a respiratory tract infection or exacerbation during the preceding 3 months, were excluded from the COPD group. Among the 33 patients with stable COPD, 22 were treated with a long-acting anti-cholinergic agent (tiotropium), 6 with long-acting β2-agonists, 9 with salmeterol/fluticasone compounds (SFC), and 9 with oral theophylline.
Asthma was defined as a clinical history of intermittent wheezing, cough, chest tightness or dyspnea, documented diurnal variation in airflow obstruction and reversible airflow obstruction, either spontaneously or with treatment using an inhaled β2-agonist or inhaled corticosteroid, and bronchial hyperresponsiveness to methacholine. Asthma was diagnosed according to the guidelines of the Global Initiative for Asthma (GINA) (Citation9). Patients with complications involving other obvious respiratory disorders or severe cerebral-cardiovascular disturbances were excluded. None of the 29 patients with asthma had ever smoked.
Among these patients, 14 were treated with inhaled corticosteroids (ICS), 11 with SFC, 16 with anti-leukotriene receptor antagonists, six with long-acting β2-agonists and eight with oral theophylline. The dose of ICS was from 400 to 1000 μg of fluticasone propionate/day. Four patients with asthma were steroid-naïve. We invited the public participation of healthy volunteers who had never smoked and had no history or symptoms of respiratory disorders or allergic diseases.
Protocol and measurements
All patients were instructed to continue their usual medications, but to withhold short-acting β2-agonists for 12 h, slow-release theophylline, leukotriene antagonist and inhaled corticosteroid for 24 h, and long-acting β2-agonists, ICS/LABA and anti-cholinergic agents (tiotropium) for 48 h before the pulmonary function testing and EBC collection. The status of asthma control and of the health of the patients with COPD for 4 weeks prior to the study was assessed using the asthma control test (ACT) and the COPD assessment test (CAT), respectively.
We collected the EBC from all participants after they completed the pulmonary function test, and then measured the fractional exhaled nitric oxide (FeNO). The measurement of FeNO was done more than 30 minutes after pulmonary function test. All patients with COPD were examined by high-resolution computed tomography (HRCT) of the chest on a different day before the study, and the severity of emphysematous changes was evaluated using the Lung Vision software program (CYBERNET, Tokyo, Japan). This study was approved by the institutional Research Ethics Committee of Shinshu University School of Medicine, and all patients and volunteers provided written informed consent to participate.
Pulmonary function test
Spirometry proceeded, and the FRC and DLCO were measured using a Chestac-8800 instrument (Chest Co. Ltd., Tokyo, Japan). We measured the FRC using helium gas dilution and the DLCO using the single breath method. Tests were repeated two or three times to guarantee consistency. Japanese local reference data, developed by the Japanese Respiratory Society, were adopted for the predicted FEV1 and VC values, and the predicted values for the DLCO and lung volumes (FRC, RV and TLC) were determined using the formulas described by Nishida et al. (Citation10) and Boren et al. (Citation11), respectively.
Collection of EBC
The participants rinsed their mouths, positioned a nose clip and then breathed tidally with a normal frequency through the mouthpiece of an EcoScreen (Jaeger, Wurzburg, Germany) for 15 minutes. The expired breath condensates (1.0–1.5 mL) were transferred to siliconized plastic tubes for immediate H2O2 and pH measurement.
Measurement of H2O2 and pH
The EBC hydrogen peroxide concentration was quantified by means of a commercially available kit, i.e., d-ROMs test exhalation (Diacron International, Grosseto, Italy). This test is an application to the exhaled breath condensate of the d-ROMs test that normally is used to measure the total oxidant capacity of blood serum/plasma samples (Citation12). The d-ROMs test measures all the serum/plasma compounds/activities, mainly alkoxyl and hydroperoxyl radicals (deriving from iron-catalysed hydroperoxide breakdown) that are able to oxidise the N,N-diethylparaphenylendiamine (chromogenic substrate).
Unfortunately the d-ROMs test cannot be performed on exhaled breath condensate because this fluid does not contain iron that is necessary, according to the so-called Fenton's reaction, to catalyse EBC hydrogen peroxide breakdown to hydroxyl radical that can oxidise N,N-diethylparaphenylendiamine, for which colour change is measured photometrically. Therefore in the d-ROMs test exhalation 1 mL of EBC reacts with a buffered water solution containing iron sulphate as catalyst and N,N-diethylparaphenylendiamine as chromogenic substrate; the EBC hydrogen peroxide undergoes to the Fenton's reaction thus generating hydroxyl radicals that in turn oxidises N,N-diethylparaphenylendiamine to its pink-coloured radical cation.
The reaction is monitored photometrically at 505 nm and the absorbance change is proportional to the amount of generated radical cation that is proportional to the amount of hydroxyl radicals and hence to the concentration of initial EBC hydrogen peroxide. A preliminary clinical trial validated d-ROMs exhalation test in patients suffering from COPD (Citation13). The pH of the EBC was measured using a pH analyzer (Horiba, Tokyo, Japan) that had been calibrated with buffers at pH 7 and pH 4.
Measurement of the FeNO
The levels of FeNO were evaluated using a personal device (NIOX MINO; Aerocrine AB; Smidesvägen, Sweden) with identical mouth flow rates and pressure settings for each participant. The function of the device based on electrochemical principles was in line with the published procedures for FeNO measurements (Citation14). The average of two successive recordings taken at 2-minute intervals was recorded.
Evaluation of emphysema on chest HRCT
A helical CT scanner (Somato Sensation 16, Siemens, Munich, Germany) was used for HRCT scanning at full inspiration (at the TLC level) with 1 mm collimation under conditions of 120 kVp, 200 mA and a pitch of 1.0. Low attenuation areas were evaluated and calculated using Digital Imaging and Communication in Medicine (DICOM) files and a windows-compatible software program (Lung Vision, Cybernet). The extent of low attenuation areas was assessed as the ratio (%) of lung voxels with X-ray attenuation values below –960 Hounsfield units (HU) (% low attenuation areas, LAA%). Emphysema was scored in the bilateral upper, middle and lower lung fields as described by Goddard et al. (Citation15). The software program can also calculate the ratio of the volume occupied by the LAA for total lung volume, which is expressed as the low attenuation volume (LAV%).
Statistical analysis
The values are shown as the means ± SEM. The comparison among the three groups was performed with the Kruskall–Wallis test for non-normally distributed data confirmed by Kolmogorov–Smirnov test, and then the variables on each two groups were compared using the non-parametric Mann–Whitney U-test. Simple correlations between variables were examined by calculating Spearman's rank correlation coefficient. The data were statistically analysed using a Windows-compatible software package (Stat Flex ver. 5.0, Artech Ltd., Osaka, Japan). The p-values of <0.05 were considered to be significant in all statistical analyses.
Results
shows the characteristics of the participants and the results of the pulmonary function tests, the FeNO, ACT score, CAT score, total scores of emphysema and the LAV%. The age significantly differed among the three groups, and the population of females in the group with COPD was lower. Twelve of the patients with asthma had an airflow limitation defined as FEV1/FVC < 70%. The average ACT score was 21.4 ± 0.8 (range, 9–25), and 8 patients had total control (ACT score 25), whereas 14 and 7 patients had good (ACT score 20–24) and poor (ACT score < 20) control, respectively. The severity of COPD was staged according to the GOLD criteria as mild in 10 patients, moderate in 14 patients, severe in 8 and very severe in 1.
Table 1. The characteristics, pulmonary function, and fractional exhaled nitric oxide in healthy volunteers (Control) and patients with asthma and COPD, the ACT scores in asthmatic, and the CAT scores and emphysema scores in COPD patients
The average CAT score among patients with COPD was 12.8 ± 1.5 (range, 0–32). The CAT score significantly correlated with the FEV1 (% of predicted value) (r = –0.50, p < 0.01), DLCO (% of predicted value) (r = –0.49, p < 0.01), and LAV% (r = 0.56, p < 0.01). The concentrations of FeNO were significantly higher in asthmatic patients than in healthy volunteers who had never smoked (control) and in patients with COPD, and were significantly higher in COPD patients than in the control group. The FeNO values and ACT scores were not significantly correlated (r = –0.01, p = 0.61). The severity of emphysema on chest HRCT was graded as 0, 1, 2 and 3 in 3, 18, 8 and 2 patients, respectively. The mean LAV% was 14.3 ± 2.2% (range, 0.6–47.9%) and significantly correlated with FEV1 (% of predicted value) (r = –0.49, p < 0.01), DLCO (% of predicted value) (r = –0.51, p < 0.01).
H2O2 and pH in EBC
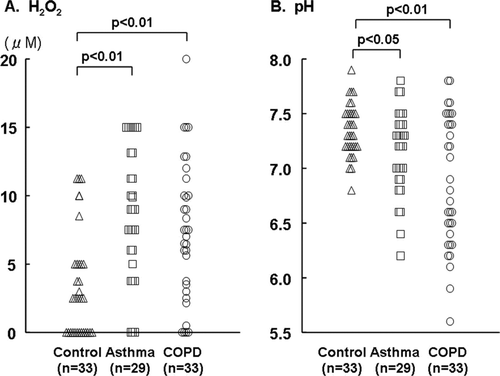
The mean concentrations of H2O2 in the EBC were significantly elevated in patients with asthma and COPD compared with the control group (8.75 ± 0.88 and 7.44 ± 0.89 vs. 3.42 ± 0.66 μM) (). However, these values did not significantly differ between the patients with asthma and COPD. The mean pH levels were significantly lower in the patients with asthma and COPD than in the controls (7.14±0.07 vs 6.87 ± 0.10 vs. 7.35 ± 0.04; ). The H2O2 levels did not correlate with the pH of the EBC (r = –0.01, p = 0.91).
Relationships between the H2O2 concentration or pH in the EBC and the ACT score, FeNO or pulmonary function in asthmatic patients
Neither the H2O2 concentration nor the pH correlated with the ACT scores (). The levels of H2O2 did not significantly correlate with the FEV1 (% of predicted value) or FeNO, and the pH did not significantly correlate with the FEV1 (% of predicted value) or FeNO.
Table 2. Simple regression analysis using Spearman's rank correlation coefficient in asthma and COPD
Relationships between the H2O2 concentration or pH in the EBC and the CAT score, FeNO, pulmonary function, or LAA% in COPD patients
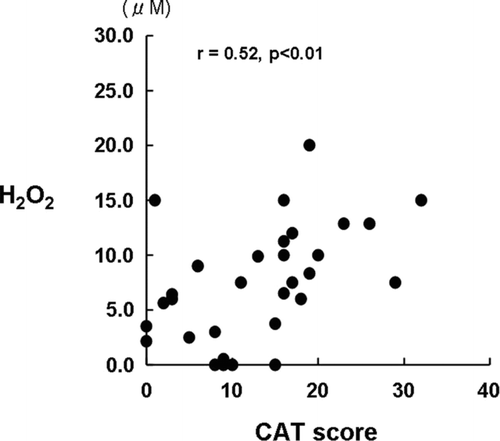
We found that the CAT score significantly, correlated with the H2O2 oncentration (), but not with the pH of the EBC. Furthermore, significant correlations were not identified between the H2O2 concentration and the FEV1 (% of predicted value), RV (% of predicted value), DLCO (% of predicted value), FeNO or LAV% or between the pH and FEV1, RV, DLCO, FeNO or LAV%.
Discussion
We confirmed that the H2O2 levels were significantly elevated and the values of pH were significantly lower in the EBC from patients with asthma and COPD compared with those in healthy individuals who had never smoked. The increased H2O2 production in the airways indicates enhanced oxidative stress, and implies the involvement of this process in the airway inflammation associated with both asthma and COPD.
The new knowledge is that we have been able to prove a significant correlation between CAT score and oxidative stress in the airway of COPD patients, whereas any correlation between asthma symptoms represented by ACT score and the airway oxidative stress was not found in patients with asthma in this study. In the management of stable COPD according with the revised edition of GOLD guideline in 2011 (Citation8), two-dimensional evaluations by current symptoms and a risk of exacerbation were recommended, and mMRC scale and CAT were specified in current symptom evaluation. In this study, we have shown that the oxidative stress in the airways contributes to current symptoms of COPD because a significant correlation between the oxidative stress and CAT score was found, and the H2O2 levels in EBC is a useful bio-marker for the management of COPD, which is the first knowledge.
Significant correlations between the concentration of H2O2 in the EBC and the eosinophil counts in induced sputum, disease severity (Citation16), smoking habits (Citation17) and the presence of symptoms (Citation18) in unstable asthma patients have been reported. The ACT score calculated from the answers to five simple questions is a simple tool commonly used to assess asthma control (Citation19).
However, no significant correlations between the oxidative stress and ACT scores or FeNO, a biomarker of airway inflammation in asthma (Citation20, 21), were observed in the present study. Of note, most of our patients with asthma were treated with ICS or ICS/LABA. When the samples were collected, current therapies were shortly withheld for 24–48 hours; however, bronchodilators are unlikely to have an effect on oxidative stress, whereas the effect of ICS on oxidative stress in COPD patients is limited (Citation22, 23).
Therefore, we could not obtain a significant correlation between the FeNO and ACT or pulmonary functions. A meta-analysis of the exhaled H2O2 levels in EBC revealed that there were significantly lower levels in patients with asthma who were treated with ICS than in those who were not, and in patients with well-controlled asthma compared with those who had uncontrolled asthma (Citation2). Our patients with asthma were relatively well-controlled, and only nine patients were poorly controlled and symptomatic. The different background of the patients may have been responsible for the different results obtained in the present study compared to previous studies.
As same as the asthma patients, most patients with COPD were already treated with LABA, ICS/LABA or LAMA. Although these agents may affect airway inflammation and modified the biomarkers, which may be resulted in a wide distribution and overlap, a significant correlation between H2O2 in EBC and CAT score, mainly representing symptoms, was obtained. To our knowledge, this is the first report that the H2O2 levels in the EBC correlated with the health status represented by the CAT score in COPD patients. The CAT is an 8-item questionnaire designed to assess and quantify the impact of COPD symptoms on the patient's health status, and is closely associated with findings from the St George's Respiratory Questionnaire (SGRQ) (Citation24).
In this study, the CAT score significantly correlated with the pulmonary function test and LAV%. It has been reported that the CAT score is elevated in frequent exacerbators and further increases at exacerbation, and can provide a reliable score of exacerbation severity (Citation25). COPD exacerbation is closely associated with an increase in airway inflammation, and further increases in H2O2 in the EBC were demonstrated at such exacerbation in a previous study (Citation4). It has been also demonstrated that the H2O2 in EBC is significantly correlated with the neutrophil numbers in induced sputum and dyspnea score (Citation26).
The ECLIPSE study revealed that significant associations were observed between the relative neutrophil counts in sputum and the health status measured using the St George Respiratory Questionnaire (Citation27). It is well known that the severity of COPD is determined not only by the pulmonary function or emphysema scores, but that the health status is also an important determinant of the severity of COPD. These findings suggest that the H2O2 levels in EBC may represent increased airway inflammation and can be a useful biomarker to assess the symptomatic severity of COPD.
We found that EBC pH levels from patients with asthma and COPD were significantly lower compared with those in healthy individuals, and the patients with COPD showed further significant lower values of pH, which suggests the presence of oxidative stress in the airway especially in COPD. However, the lower values of pH did not show any correlations with asthma control status, COPD health status, pulmonary functions and FeNO. The pH of EBC has been demonstrated to be lower in patients with moderate-to-severe or uncontrolled asthma, but not in those with stable, steroid-treated and controlled asthma (Citation5, Citation28, Citation29).
One explanation for no correlations with disease activity, severity and health status might be due to modification of airway inflammation by the anti-inflammatory treatment, and another reason might be that most were well-controlled, and only seven had ACT scores below 20. The other reason might be that EBC samples were not de-aerated with CO2-free gas before measurements. The pH of EBC is profoundly affected by the CO2 content, and de-aeration has been suggested as a means of eliminating this effect (Citation6). However, even after de-aeration, an unpredictable amount of CO2 remaining in samples might still result in some bias in the pH values. It has been demonstrated that the pH after de-aeration with CO2 in patients with exacerbated COPD results in no difference from that in healthy individuals (Citation30). Further studies on de-aeration are therefore needed to determine its impact on the pH.
There were some limitations in this study. COPD patients were ex-smokers, whereas healthy subjects and patients with asthma were never smokers. It was demonstrated that H2O2 concentration may be influenced by smoking history (Citation31). On the other hand, it was also demonstrated that no correlation was found between expired H2O2 and daily cigarette consumption or cumulative cigarette consumption in current smokers or ex-smokers with COPD (Citation32). Concerning EBC pH, a significant decrease in stable COPD compared with ex-smokers without COPD matched for pack-year (Citation7). It is controversial. Therefore, we cannot exclude a persistent effect of previous smoking habit on oxidative stress independent of COPD. Furthermore, there was the relevant different in age among the three groups.
It was demonstrated that the H2O2 concentration in EBC was influenced by age, and the mean H2O2 concentration was higher in non-smokers above 40 years, and it positively correlated with age in never smoked subjects (Citation33). However, in this study, we can not demonstrate any correlation between the concentration of H2O2 n EBC and age among never smokers. Therefore, the differences among the three groups were not due to age differences.
In conclusion, oxidative stress is implicated in the pathogenesis of asthma and COPD, and the H2O2 level in the EBC might serve as a useful inflammatory biomarker to assess the health status and disease activity of COPD patients. However, in asthmatic patients, oxidative stress did not closely reflect the asthma control.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lin JL, Thomas PS. Current perspectives of oxidative stress and its measurement in chronic obstructive pulmonary disease. COPD 2010; 7:291–306.
- Teng Y, Sun P, Zhang J, Hydrogen peroxide in exhaled breath condensate in patients with asthma: a promising biomarker? Chest 2011; 140:108–116.
- Guatura SB, Martinez JA, Santos Bueno PC, Increased exhalation of hydrogen peroxide in healthy subjects following cigarette consumption. Sao Paulo Med J 2000; 118:93–98.
- Dekhujzen PN, Aben KK, Dekker I, Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 154:813–816.
- Kostikas K, Papatheodorou G, Ganas K, pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med 2002; 165:1364–1370.
- Lin J-L, Bonnichsen MH, Thomas PS. Standardization of exhaled breath condensate: effects of different de-aeration protocols on pH and H2O2 concentrations. J Breath Res 2011 Mar; 5(1): 011001. doi: 10.1088/1752-7155/5/1/011001. Epub 2011 Jan 11.
- Papaioannou AI, Loukides S, Minas M, Exhaled breath condensate pH as a biomarker of COPD severity in ex-smokers. Respir Res 2011; 22:12–67.
- National Institute of Health, National Heart, Lung and Blood Institute. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management an Prevention of Chronic Obstructive Pulmonary Disease. 2011; Available at www.goldcopd.com.
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Workshop Report. Update 2006. http://www.ginasthma.com.
- Nishida O, Kambe M, Sewake N, Pulmonary function in healthy subjects and its prediction: 5. Pulmonary diffusing capacity in adults. Jpn J Clin Pathol 1976; 24:941–947.
- Boren HG, Kory RC, Syner LC, The veterans administration-army cooperative study of pulmonary function. 2. The lung volume and its subdivisions in normal men. Am J Med 1966; 41:96–101.
- Bodini A, Peroni D, Loiacono A, Exhaled nitric oxide daily evaluation is effective in monitoring exposure to relevant allergens in asthmatic children. Chest 2007; 132:1520–1525.
- Alberti A, Bolognini L, Macciantelli D, The radical cation of N,N-diethyl-para-phenylendiamine: a possible indicator of oxidative stress in biological samples. Res Chem Intermed 2000; 26:253–267.
- Melillo G, Iorio EL, Giuliano F, Oxidative stress in patients with chronic obstructive pulmonary disease: validation of a new photometric test (exhalation test) for the measurement of hydrogen peroxide in exhaled breath condensate. Rass Patol App Resp 2007; 22:98–104.
- Goddard PR, Nicholson EM, Laszlo G, Computed tomography in pulmonary emphysema. Clin Radiol 1982; 33:379–387.
- Loukides S, Bouros D, Papatheodorou G, The relationship among hydrogen peroxide in expired breath condensate, airway inflammation, and asthma severity. Chest 2002; 121:338–346.
- Ganas K, Loukides S, Papatheodorou G, Total nitrite/nitrate in expired breath condensate of patients with asthma. Respir Med 2001; 95:649–654.
- Horvath I, Donnelly LE, Kiss A, Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am J Respir Crit Care Med 1998; 158:1042–1046.
- Nathan RA, Sorkness CA, Kosinski M, Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004; 113: 59-65.
- Kharitonov S, Alving K, Barnes PJ: Exhaled and nasal nitric oxide measurements: recommendations. The European Respiratory Society Task Force. Eur Respir J 1997; 10:1683–1693.
- Montuschi P, Mondino C, Koch P, Effects of Montelukast treatment and withdrawal on fractional exhaled nitric oxide and lung function in children with asthma. Chest 2007; 132:1876–1881.
- Petsky HL, Cates CJ, Li A, Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev 2009; 4:CD006340: doi: 10.1002/14651858.
- Montuschi P, Barnes PJ. New perspectives in pharmacological treatment of mild persistent asthma. Drug Discov Today 2011; 16:1084–1091.
- Jones PW, Harding G, Berry P, Development and first validation of the COPD assessment test. Eur Respir J 2009; 34:648–654.
- Mackay AJ, Donaldson GC, Patel AR, Utility of the COPD assessment test (CAT) to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med 2012; 185:1218-24.
- Kostikas K, Papatheodorou G, Psathakis K, Oxidative stress in expired breath condensate of patients with COPD. Chest 2003; 124:1373–1380.
- Singh D, Edwards L, Tal-Singer R, Rennard S. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res 2010; 11:77.
- Ojoo JC, Mulrennan SA, Kastelik JA, Exhaled breath condensate pH and exhaled nitric oxide in allergic asthma and in cystic fibrosis. Thorax 2005; 60:22–26.
- Carraro S, Folesani G, Corradi M, Acid-base equilibrium in exhaled breath condensate of allergic asthmatic children. Allergy 2005; 60:476–481.
- Antus B, Barta I, Kullmann T, Assessment of exhaled breath condensate pH in exacerbations of asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 182:1492–1497.
- Van Beurden WJ, Dekhuijzen PN, Harf GA, Variability of exhaled hydrogen peroxide in stable COPD patients and matched healthy controls. Respiration 2002; 69:211–216.
- Nowak D, Kasielski M, Pietras T, Cigarette smoking does not increase hydrogen peroxide levels in expired breath condensate of patients with stable COPD. Monaldi Arch Chest Dis 1998; 53:268–273.
- Nowak D, Kalucka S, Bialasiewicz P, Exhalation of H2O2 and thiobarbituric acid reactive substances (TBARS) by healthy subjects. Free Radic Biol Med 2001; 30:178–186.