Abstract
To assess risk factors related to the development of chronic obstructive pulmonary disease (COPD) including smoking and occupational exposure (OE) to dusts, gases or fumes, we performed a longitudinal 11-year follow-up postal survey. The original study population was a random population sample of 8000 inhabitants of Helsinki aged 20 to 69 years in 1996. Participants of the first postal questionnaire were invited to this follow-up survey in 2007 with 4302 (78%) answers obtained. Cumulative incidence of COPD in 11 years was 3.43% corresponding to an incidence rate of 3.17/1000/year after exclusion of those with self-reported physician-diagnosed COPD and ever COPD in 1996. Smoking and age, but not gender, were associated with incident COPD. Reported family history of COPD increased the cumulative incidence to 8.55% vs 3.04% among those without a family history (p < 0.001). In multivariate analysis, significant independent risk factors for incident COPD were: current smoking in 1996 (OR 4.40 [95% CI 2.89–6.71]), age over 50 (OR 3.42 [95% CI 2.22–5.26]), family history of COPD (OR 2.08 [1.27–3.43]), ever asthma (OR 2.28 [1.35–3.86]), and self-reported OE (OR 2.14 [1.50–3.05]). Occupational exposure to dusts, gases or fumes, assessed both based on self-reported exposure and a job exposure matrix using reported professions, was an independent risk factor for incident COPD. Smoking and OE together yielded an additive effect on incidence of COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic usually progressive respiratory disease characterized by persistent airways obstruction and limited response to pharmacological treatments. The burden of COPD is growing worldwide despite the fact of being a preventable disease (Citation1). Substantial underdiagnosis is reported from several countries (Citation2–4), while early diagnosis and recognition of causal factors would give an opportunity for intervention.
Smoking is the dominant cause of COPD. Other recognized risk factors include genetic predisposition, severe childhood infections, history of asthma, low socioeconomic status, exposure to biomass fuels, outdoor air pollution, and occupational exposures (Citation1,Citation5–8). Different occupational exposures have been independently associated with COPD (Citation8). In cross-sectional studies occupational exposure (OE) to vapors, gases, dusts or fumes together with smoking have yielded an additive effect (Citation9,10). Recently, the harmful effects of OE to dust, fumes or gases were found to be associated with an increased risk for incident COPD in a longitudinal study (Citation11). Continued occupational exposures may also contribute to the progression of COPD (Citation12).
Our aim was to estimate incidence rate of physician-diagnosed COPD and to assess the concurrent effects of smoking and occupational exposures to dusts, gases or fumes as risk factors for incident COPD in a longitudinal 11-year follow-up of a population based cohort in Helsinki, Finland.
Material and methods
Study population
This is a part of a large epidemiological FinEsS study (Finland-Estonia-Sweden), which has been conducted from 1996 onwards. The original study population consisted of random subjects obtained from the national population registers with a sampling protocol designed to correspond to the general population. The original study population in Helsinki, Finland, consisted of 8,000 subjects aged 20–69 years described in detail earlier (Citation13). In 1996, 6062 (76%) subjects participated to the first postal survey. A follow-up study was performed in 2007, when 5,484 subjects could be traced out of the original 6,062 participants in 1996, with 4,302 complete answers obtained corresponding to a response rate of 78%. After excluding those with physician-diagnosed COPD or ever COPD in 1996, the population at risk consisted of 4,080 subjects. Further exclusions of those with longstanding cough and dyspnea at baseline were done when calculating adjusted incidence of COPD, with a population at risk of 3,911 subjects. The study population is described in . This study was approved by the Ethical Committee of Helsinki University Central Hospital.
Table 1. Descriptive statistics of the study population
Questionnaire
The questionnaire was a self-administered postal questionnaire (Citation13) with two reminders. It was originally developed for the first Swedish OLIN (Obstructive lung disease in Northern Sweden) survey (Citation14) in 1986 from a revised version (Citation15) of the British Medical Research Council questionnaire (Citation16). In addition to the questions relating to diagnoses and symptoms of respiratory diseases, the subjects were asked in what profession they had worked for the longest duration and the length of that work engagement. At the follow-up examination in 2007 the reported job titles were classified according to the International Standard Classification of Occupations (ISCO-88) as recommended nationally by Statistics Finland (Citation17,18).
Each ISCO-88 job category was separately weighted for biological dusts, mineral dusts and gases/fumes based on these individual components (Citation19,20) as presented by Mehta et al. (Citation12). High level of exposure was assessed according to Mehta et al. (Citation11). Low levels of exposures were assessed as probable or commonly known low exposure to biological dusts, mineral dusts, gases or fumes, because data on low exposures are lacking in earlier literature Self-reported OE to dusts, gases or fumes was assessed with the following question: “In your present or past work environment, is there or has there been lots of dust, gases or fumes?”
Definitions
Ever COPD: “Have you ever had COPD?”
Ever asthma: “Have you ever had asthma?”
Ever allergic rhinoconjunctivitis: “Have you ever had hay fever or allergic rhinitis or conjunctivitis?”
Physician-diagnosed COPD: “Have you been diagnosed as having COPD by a doctor?”
Physician-diagnosed asthma: “Have you been diagnosed as having asthma by a doctor?”
Longstanding cough: “Have you had longstanding cough during the last years?”
Sputum production: “Do you usually have phlegm when coughing, or do you have phlegm which is difficult to bring up?”
Chronic productive cough refers to those who report having had sputum production on most days during periods of at least 3 months in at least 2 successive years.
Shortness of breath (SOB): “Have you had asthma symptoms (intermittent breathlessness) or attacks of breathlessness?”
Past year wheeze: “Have you had wheezing or whistling in the chest at any time in the last 12 months?”
Recurrent wheeze: “Do you usually have wheezing or whistling in your chest when breathing?”
Dyspnea: “Do you have dyspnea when walking on flat ground with age peers?”
Current smoking refers to those currently smoking any quantity of cigarettes, pipe or cigars and those that have quit during the preceding 12 months.
Ex-smokers are those who have quitted smoking at least one year previously.
Ever smokers refer to current smokers and ex-smokers.
Cumulative incidence of COPD was calculated by excluding from the population at risk those with either physician-diagnosed COPD or ever COPD in 1996, and calculating new cases of physician-diagnosed COPD in 2007.
Statistical analyses
Statistical analyses were performed with the Statistical Package for Social Sciences (IBM SPSS for Windows version 21.0, IBM SPSS, Chigaco, IL, USA). The chi-square test was used for bivariate comparisons. Determinants for incident cases of COPD were calculated by multiple logistic regression, with gender, age group, smoking history, history of asthma or allergic rhinoconjunctivitis, family history of COPD, asthma and allergic rhinoconjunctivitis, and occupational exposure to dusts, gases or fumes as independent variables.
Unadjusted odds ratios (OR) were calculated with only the studied categorical variable in the model. Adjusted OR included age as a continuous variable and gender in each of the models in addition to the studied categorical variable reported. Multiple logistic regression analysis was used because there was no information of time of events. A p-value < 0.05 was definied as statistically significant in all analyses.
Results
New physician-diagnosed COPD occurred in 140 subjects, with a cumulative 11-year incidence of 3.43%, corresponding to an incidence rate of 3.17/1000/year. The cumulative incidence of COPD was 3.58% and incidence rate 3.29/1000/year among men, 3.40% and 3.09/1000/year among women, respectively, without any significant gender difference. The cumulative 11-year incidence based on 116 COPD cases, was 2.97%, corresponding to 2.70 cases/1000/year (men 2.99/1000/year; women 2.49/1000/year, p = 0.34) when those reporting longstanding cough and dyspnea at baseline were excluded. An increase in cumulative incidence by age was found from 1.06% among those aged 31–40 to 6.27% among those 61–70 years of age. Cumulative incidence of COPD was 8.55% among those with a family history of COPD, while it was 3.04% among those without a family history (p < 0.001). Family history of COPD increased the OR for incident COPD to 2.35 (95% CI 1.45–3.81) in the multiple regression model adjusted for age, sex and smoking history.
Chronic respiratory symptoms were common among those with incident COPD. The prevalence of all respiratory symptoms was at least double already in 1996 among those with incident COPD in 2007 compared to all others (). The prevalence of respiratory symptoms remained the same 11 years later among those who didn`t develop COPD, whereas prevalence of respiratory symptoms increased among subjects with incident COPD in 2007 (). In univariate analysis, chronic respiratory symptoms in 1996 were strongly associated with incident COPD in 2007; chronic productive cough yielded an OR 4.84 (95% CI 3.33–7.03) and recurrent wheeze an OR 4.86 (95% CI 3.04–7.75). In multivariate models adjusted for age, sex and smoking history, chronic productive cough (OR 3.34 (95% CI 2.45–4.96)), waking up with difficulties of breathing (OR 3.08 (2.16–4.40)), and recurrent wheeze (OR 2.83 (1.70-4.71)) yielded the highest ORs for incident COPD ().
Figure 1. Adjusted odds ratios (OR) between 2007 and 1996 for symptoms suggestive of obstructive airways diseases assessed with a multiple logistic regression model adjusted for categorized age decade, gender, and smoking history.
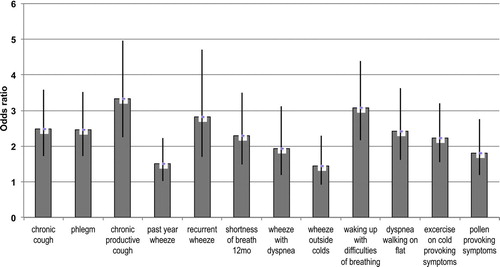
Table 2. Prevalence of respiratory symptoms in subjects with and without incident COPD in 2007 and the odds ratios for respiratory symptoms reported in 1996 predicting incident COPD in 2007
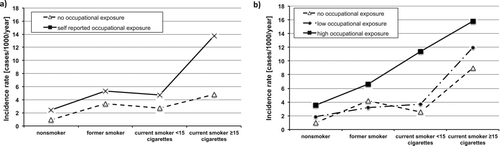
Incidence of COPD was strongly associated with smoking; new COPD occurred in 2.45% of non-smokers and in 6.52% of smokers (p < 0.001). A similar difference was found for self-reported OE to dusts, gases or fumes; new COPD developed in 2.63% of non-exposed and in 5.90% of exposed subjects (p < 0.001). Combination of smoking and OE resulted in an additive pattern: the annual incidence rate of COPD was 1.88/1000 among non-exposed non-smokers, 3.87/1000 among exposed non-smokers, 4.68/1000 among non-exposed smokers, and 9.03/1000 among exposed smokers. Incidence of COPD increased with increasing amount of cigarettes per day, with a higher incidence rate among those exposed to dusts, gases or fumes at work compared with those non-exposed in all smoking categories (). A similar trend of increasing incidence of COPD with increasing amount of cigarettes per day and increasing OE was found when exposure was assessed according to a job-exposure matrix and classified to none, low and high cumulative exposure to occupational dust, gases or fumes ().
Figure 2. Incidence rate of COPD (cases/1000/year) by different smoking categories and by a) self-reported OE to dust, gases or fumes, and b) cumulative exposure categories (none, low, high) according job-exposure matrix.
In multivariate analysis including also family history and self-reported history of asthma and allergic rhinoconjunctivitis, OE to dusts, gases or fumes doubled the risk for incident COPD (). An increased risk of similar magnitude was related with having a family history of COPD or ever asthma. Current smoking yielded an OR 4.40 (95% CI 2.89–6.71), and former smoking an OR 1.93 (95% CI 1.14–3.28) for incident COPD. In the multivariate model including gender, smoking history, age and occupational exposure assessed by job-exposure matrix, low cumulative exposure yielded an OR 1.38 (95% CI 0.91–2.10), and high cumulative exposure an OR 1.98 (95% CI 1.08–3.64) for incident COPD compared to the non-exposed.
Table 3. Multiple logistic regression model* for risk factors for incident COPD in the population
The effects of smoking and occupational exposures on crude incidence rate and risk for incident physician-diagnosed COPD are further elaborated in . In the whole population at risk, ever smokers had an OR 4.50 (95% CI 2.92–6.93) for incident COPD in the adjusted model controlling for age and gender, with OR 3.83 (95% CI 2.43–6.04) when further excluding those with longstanding cough and dyspnea at baseline. Self-reported exposure to OE yielded correspondingly OR 2.42 (95% CI 1.71–3.41) and OR 2.33 (95% CI 1.60–3.40), respectively.
Table 4. Crude incidence rate and risk of incident physician-diagnosed chronic obstructive pulmonary disease (COPD) in relation to cigarette smoking and occupational exposure to dusts, gases or fumes
Discussion
An overall incidence of COPD of 3.17/1000/year was found in this 11-year follow-up of a population-based cohort. Occupational exposure (OE) to dusts, gases or fumes was found to predispose to incident COPD assessed both by self-reported exposure and by job-exposure matrix. Furthermore, incidence of COPD increased with increasing combined occupational exposure to dusts, gases or fumes and smoking. Although there are several studies on prevalence of COPD associated with OE to dusts, gases or fumes, only few studies have assessed the incidence of COPD associated with these exposures. The present results suggest for an additive effect of smoking and exposure to occupational inhalant exposures on the development of COPD.
Although tobacco smoking is the most important risk factor for COPD, it is a multifactorial disease comprising also genetic factors, occupational and environmental exposures (1,6,7). Based on earlier literature, 15–20% of COPD is estimated to be work-related (Citation21–23). The combined additional risk from smoking and occupational exposure to vapors, gas, dust or fumes for prevalence of COPD has been previously found to be additive when COPD was diagnosed according to GOLD stage II or greater (Citation24).
Another recent study on the prevalence of COPD also found a higher than additive risk for COPD or emphysema by joint exposure to smoking and occupational factors (Citation10). An interaction between smoking and occupational exposure to VGDF was suggested to be the base on this finding, thus being simultaneously exposed to VGDF and smoking would harm the lungs more than would be expected for the addition of each effect separately. In the study of Mehta et al. (Citation11) on the incidence of COPD, a patient material with similar size as in the present population report was studied. They diagnosed COPD by spirometry using both the GOLD criterion of FEV1/FVC <0.7 and FEV1/FVC <80% of predicted value, and found increased incidence rates of COPD associated with high levels of occupational exposures. No synergy between smoking and occupational exposures was seen, but they concluded that the joint effect of smoking and occupational exposure would be more than additive.
In the present study, COPD is assessed by self-reported physician-diagnosed COPD. Its prevalence was 3.7% in the original study cohort in 1996 (Citation13). In the Finnish National Guidelines, spirometry is required for the diagnosis of COPD. Therefore, although lung function data were not available for this study, the reported physician-diagnoses of COPD are probably based on spirometric results. However, grading of severity of COPD is not possible based on this self-reported questionnaire data. The incident COPD cases were highly symptomatic already in 1996, with a clear difference in levels of symptoms compared to all others. All respiratory symptoms increased significantly by 2007 among those with incident COPD, but remained at the same level among others, confirming that the incident cases were most likely true cases despite lacking spirometry data.
Symptom-based questionnaires have yielded a high sensitivity, 80–88%, in identifying COPD, with a specificity between 25% and 72% (Citation25,26). Our data support these earlier findings encouraging evaluation of patients with chronic respiratory symptoms in order to better identify COPD-cases. Undiagnosed COPD associates with impaired health-related quality of life and activities of daily living (Citation27). Earlier Barr et al. assessed the validity of self-reported COPD among nurses finding 78% confirmation rate based on review of medical records (Citation28). They also confirmed that questionnaire-based COPD research should focus on minimizing false positive rate to improve accuracy. In well functioning healthcare systems having nationally accepted diagnostic criteria and treatment guidelines, we believe that the physician-diagnoses of obstructive airways diseases reflect true changes of disease prevalence and incidence, since they also include physician assessment of the differential diagnostics and thus contribute towards minimizing the false positive rate.
From previous studies, the overall rates of incidence of COPD vary widely depending on the method of diagnosis, patient characteristics and study design (Citation29). In Sweden, the neighbor country of Finland, where the socioeconomic level and smoking habits are similar to ours, the reported incidence rates (IR) for physician-diagnosed COPD are almost similar to the present study (Citation30). However, in another Swedish studies using spirometry-based definition on GOLD criteria, the cumulative incidence of COPD was greater, up to 11% in 7 years (Citation31). The greater incidence rate in the latter study could be explained by differences in age groups: the youngest were 46 years of age in the beginning of the follow up, while the lower age limit in our study was 20 years.
Furthermore, self-reporting of COPD might cause underestimation of COPD compared to spirometric studies, since COPD is commonly underdiagnosed, and previously undetected cases may be found when performing a spirometry (Citation2–4). However, a strictly spirometry-based definition of COPD also fails to take into account the differential diagnosis of asthma. Since the study of Lindberg et al. (Citation31) is from 1986–1996 and present data from 1996–2007, the decreasing prevalence of smoking may have contributed to the lower incidence in our study. From other studies in Finland, the prevalence of irreversible airflow limitation consistent with COPD has been reported to have remained at the same level comparing population surveys from 1978–1980 to 2000–2001 (Citation32).
In the Netherlands, a registry-based assessment of incidence of COPD found an overall IR of physician-diagnosed COPD of 2.92/1000 PY (person years) (95% CI 2.78–3.06), higher in men than women. This is very close to our IR despite large differences in study design and sampling (Citation33). In the Copenhagen City Heart Study (Denmark) crude incidence rate for GOLD defined COPD in subjects over 25 years was higher at 7.98/1000 PY (Citation34). The use of GOLD criteria, longer duration of follow-up and greater smoking prevalence in Denmark compared to Finland may explain the difference. Incidence rates for 10 year follow-up were not reported, but increasing amount of cases were found during extended follow-up (Citation34).
Studies on combined exposure of smoking and OE to dusts, gases or fumes are difficult to compare, since the methods differ. This holds for comparison of the present results with those of the study of Mehta et al. (Citation11), where the combined effect of smoking and exposure was assessed by spirometric screening of population, and the result was a higher incidence rate than in the present study. Assessing COPD by questioning on physician- diagnosed COPD probably results in finding clinically more severe ones and missing those with less symptoms and a less severe disease, while spirometric screening may also find the mild and asymptomatic cases. The advantage of using data on physician-diagnosed COPD rather than spirometric screening, is that differential diagnoses such as asthma have been taken into account. This further improves the accuracy and reduces false positives.
Family history of COPD as a risk factor to increased incidence of COPD might depend on exposure to environmental tobacco smoke at home in addition to genetic factors. According to Eisner et al. (Citation35) environmental tobacco smoke exposure and personal smoking might act synergistically to increase the risk of COPD. In Helsinki, we have previously found both occupational background in manual and non-manual work in industry and prior asthma diagnosis to significantly increase the risk of airflow limitation consistent with COPD (Citation4).
Family history of obstructive airways diseases was an independent risk factor for COPD in a cross-sectional study in Northern Finland (Citation36). It is possible that some yet unidentified genetic predispositions and environmental exposures exist that explain the role of family history as a risk factor for COPD. Furthermore, family history of COPD is also likely to be associated with common socioeconomic factors in addition to a shared environment and cultural factors e.g. related to attitudes toward smoking. In multivariate analyses, ever asthma was independently associated with an increased risk for incident COPD. Considerable overlap between COPD and asthma exist and prior asthma is a known risk factor for incident COPD. In Finland, reimbursement regulations for inhaler medication may have favored diagnosis of asthma in patients with COPD, since proven reversibility entitles patients to partial reimbursement for asthma medications from the National Healthcare System, whereas COPD diagnosis is reimbursed only at severe state of the disease. In 2006 these criteria have been amended to extend reimbursement to more patients with moderate COPD on predefined clinical criteria, thus potentially diminishing the mislabeling of COPD as asthma based on partial reversibility.
Our study also has some potential limitations. The OE to dusts, gases or fumes was not asked in the first study phase in 1996, thus comparisons of the reported past exposure between 1996 and 2007 are not available. On the other hand, the question was formulated accordingly to include current and past exposures. Exact characterization and objective measurements of OE to dusts, gases or fumes exposure are lacking. However, the professions of the subjects were classified according to an internationally accepted classification, and the possible cumulative exposure based on this classification was assessed using a job exposure matrix. This classification was made at the follow-up phase of the study, corresponding to the time of questionnaire on OE.
In conclusion, the cumulative incidence of physician-diagnosed COPD in Helsinki, Finland, was found to be 3.17/1000/year or 3.4% in 11 years, an incidence rate very similar reported from the other Nordic countries. The present findings indicate that, in addition to smoking, the incidence of COPD was significantly influenced by environmental factors. Combined exposure to smoking and occupational exposure to dusts, gases or fumes caused an additive increase of incidence. The results indicate the importance of smoking cessation and prevention of airborne occupational exposures.
Declaration of Interest Statement
The authors have declared no conflicts. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The study was funded by the Research Foundation of the Pulmonary Diseases in Finland (PP) and Jalmari and Rauha Ahokas Foundation (AK). The Finess study has been funded by the Special Governmental Subsidy for Health Sciences Research (Helsinki University Central Hospital project codes TYH1235, TYH2303, TYH4251). Research Unit for Pulmonary Diseases is warmly thanked for undertaking the questionnaire studies in 1996 and 2007.
References
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370:765–773.
- Jordan RE, Lam KB, Cheng KK, Case finding for chronic obstructive pulmonary disease: a model for optimizing a targeted approach. Thorax 2010; 6:492–498.
- Hill K, Goldstein RS, Guyatt GH, Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ 2010; 7:673–678.
- Kainu A, Rouhos A, Sovijärvi A, Lindqvist A, Sarna S, Lundbäck B. COPD in Helsinki, Finland –socioeconomic status based on occupation has an important impact on prevalence. Scand J Pub Health 2013; 41:570–578.
- Shaheen SO, Barker DJ, Holgate ST. Do lower respiratory tract infections in early childhood cause chronic obstructive pulmonary disease? Am J Respir Crit Care 1995; 151:1649–51.
- Viegi G, Pistelli F, Sherill DL, Definition, epidemiology and natural history of COPD. Eur Respir J 2007; 30:993–1013.
- Bakke PS, Rönmark E, Eagan T, ERS Task Force Report. Recommendations for epidemiological studies on COPD. Eur Respir J 2011; 38:1261–1277.
- Eisner MD, Anthonisen N, Coultas D, An Official American Thoracic Society Public Policy Statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 182:693–718.
- Trupin L, Earnest G, San Pedro M, The occupational burden of chronic obstructive pulmonary disease. Eur Respir J 2003; 22:462–469.
- Blanc PD, Menezes AMB, Plana E, Occupational exposures and COPD: an ecological analysis of international data. Eur Respir J 2009; 33:298–304.
- Mehta AJ, Miedinger D, Keidel D, Occupational exposure to dusts, gases and fumes and incidence of chronic obstructive pulmonary disease in the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults. Am J Respir Crit Care Med 2012 Jun 15; 185(12):1292–1300.
- Harber P, Tashkin DP, Simmons M, Effect of occupational exposures on decline of lung function in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:994–1000.
- Pallasaho P, Lundbäck B, Läspä SL, Increasing prevalence of asthma but not of chronic bronchitis in Finland? Report from the FinEsS-Helsinki study. Respir Med 1999; 93:798–809.
- Lundbäck B, Nyström L, Rosenhall L, Obstructive lung disease in northern Sweden: respiratory symptoms assessed in a postal survey. Eur Respir J 1991; 4:257–266.
- Mikaelsson B, Stjernberg N, Wiman LG. Prevalence of bronchial asthma and chronic bronchitis in an industrial community in northern Sweden. Scand J Soc Med 1982; 10:11–16.
- Medical Research Council`s committee on the aetiology of chronic bronchitis –standardized questionnaires on respiratory symptoms. BMJ 1960; ii:1665.
- International Labour Office. International Standard Classification of Occupations: ISCO-88. Geneva: International Labour Organization; 1990.
- Statistics Finland. Ammattiluokitus 2010, Tilastokeskus, 2011, Käsikirjoja 14, Edita Publishing Oy, Finland.
- Sunyer J, Kogevinas M, Kromhout H, Pulmonary ventilatory defects and occupational exposures in a population-based study in Spain; Spanish group of the European Community Respiratory Health Survey. Am J Respir Crit Care Med 1998; 157:512–517.
- Matheson MC, Venke G, Raven J, Biological dust exposure in the work-place is a risk factor for chronic obstructive pulmonary disease. Thorax 2005; 60:645–651.
- Balmes J, Becklake M, Blanc P, American Thoracic Society statement: occupational contribution to the burden of airway disease. Am J Respir Crit Care Med 2003; 167:787–797.
- Blanc PD, Toren K. Occupation in chronic obstructive pulmonary disease and chronic bronchitis: an update. Int J Tuberc Lung Dis 2007 Mar; 11(3):251–257.
- Darby AC, Waterhouse JC, Stevens V, Chronic obstructive pulmonary disease among residents of an historically industrialised area. Thorax 2012 Oct; 67(10):901–907.
- Blanc PD, Iribarren C, Trupin L, Occupational exposures and the risk of COPD: dusty trades revisited. Thorax 2009 Jan; 64(1):6–12.
- Price DB, Tinkelman DG, Halbert RJ Symptom-based questionnaire for identifying COPD in smokers. Respiration 2006; 73:285–295.
- Mintz ML, Yawn BP, Mannino DM Prevalence of airway obstruction assessed by lung function questionnaire. Mayo Clin Proc 2011; 86(5):375–381.
- Miravitlles M, Soriano JB, Garcia-Rio F , Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64:863–868.
- Barr RG, Herbstman J, Speizer FE Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol 2002; 155:965–971.
- Rycroft CE, Heyes A, Lanza L, Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J COPD 2012; 7:457–494.
- Nihlén U, Nyberg P, Montnémery P, Influence of family history and smoking habits on the incidence of self-reported physician's diagnosis of COPD. Respir Med 2004; 98:263–270.
- Lindberg A, Eriksson B, Larsson L-G, Seven-year cumulative incidence of COPD in an age-stratified general population sample. Chest 2006; 129:879–885.
- Vasankari TM, Impivaara O, Heliövaara M, No increase in the prevalence of COPD in two decades. Eur Respir J 2010; 36:766–773.
- Afonso ASM, Verhamme KMC, Sturkenboom MCJM, Brusselle GGO. COPD in the general population: Prevalence, incidence and survival. Resp Med 2011; 105:1872–1884.
- Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax 2006; 61:935–939.
- Eisner MD, Balmes J, Katz P, Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health 2005; 47:1–8.
- Kotaniemi J-T, Sovijärvi A, Lundbäck B. Chronic obstructive pulmonary disease in Finland: prevalence and risk factors. J COPD 2005; 3:331–339.

