Abstract
Progressive decline in lung function has been widely accepted as the hallmark of chronic obstructive pulmonary disease (COPD); however, recent evidence indicates that the rate of decline measured as decline in forced expiratory volume in one second (FEV1) is higher in mild to moderate COPD than in severe COPD. Usually changes in FEV1 are measured in ml that is “absolute”; however, changes can also be measured “relative” as a percentage of the actual FEV1. We hypothesize that relative measurements could be more appropriate than absolute measurements for describing changes in lung function. We analyzed data from 3,218 relatively healthy heavy smokers who participated in the Danish Lung Cancer Screening Trial. The influences of age, sex, height, body mass index, smoking, and severity of airflow limitation on FEV1 were analyzed in mixed effects models. In absolute terms those with the best lung function consistently showed the steepest decline, whereas in relative terms most fast decliners are found among those with low lung function. Measuring changes in relative terms implied statistically significant acceleration of decline with advancing age, smoking (pack-years) and severity of airflow limitation. Relative measurements may lead to a better understanding of changes in lung function. Smoking and severity of airflow limitation speed up the loss of lung function, and this emphasizes the importance of abstaining from smoking the sooner the better. Measuring changes in relative terms could have important implications for the interpretation of results from clinical trials where FEV1 is the primary outcome. DLCST; www.ClinicalTrials.org, registration number: NCT00496977.
Introduction
Ever since the landmark study by Fletcher and Peto and co-workers in the 1970s (Citation1,2) it has been widely accepted that chronic obstructive pulmonary disease (COPD) is characterized by an accelerated decline in lung function.
By merits of their design and inclusion criteria lung cancer screening trials offer an opportunity for investigating the longitudinal change in forced expiratory volume in 1 second (FEV1) in populations with high risk of having or developing COPD. Measuring changes in relative terms could have important implications for the interpretation of results from clinical trials where FEV1 is the primary outcome and in predicting future “fast decliners.”
The accelerated decline seen in COPD where the decline is initially slow and increases, when lung function decreases is known as “the horse-racing effect” (Citation3). This theory makes a great deal of sense, because as stated by Fletcher and Peto (Citation1,2) “In a race between fast and slow horses . . . one would expect to find the faster horses out in front.” Translated into pulmonary physiology this means that those with the lowest lung function should have the fastest decline, however, recent evidence from large clinical studies of smokers and patients with COPD indicates that the rate of decline is steepest/faster in smokers with mild to moderate COPD and slower in severe COPD (Citation4–7).
Several hypotheses have been put forward as an explanation for this counter-intuitive finding: 1) Selection bias that is selective dropout (or death) of those with low lung function that continue a fast decline; 2) Initial fast decline (horse-racing) and later when lung function is reduced, decline loses speed and levels off (but they are still in front); 3) Those with low lung function may have had a low lung function early in life (lead) because they (due to early onset of smoking) never reached the normal maximum at about 25 years. However, data in support of these hypotheses are remarkably scarce or non-existing (Citation7).
We used lung function data from the Danish Lung Cancer Screening Trial (DLCST) to characterize longitudinal decline in FEV1 and factors influencing this decline. We kept in mind the intuitive “horse-racing effect” proposed by Fletcher and Peto, and analysed changes in FEV1 in both relative (i.e.% decline per year) and absolute terms (i.e. ml decline per year). Our hypothesis was that relative changes could be more appropriate for describing lung function decline and would support the “horse-racing” concept.
Methods
Study population
The study population participated in the DLCST, which is a 5-year trial investigating the effect of screening on lung cancer mortality. Individuals volunteered to the trial in response to advertisement in local free newspapers. From October 2004 to March 2006, 4,104 participants were randomized to either annual screening with low dose CT or no screening (control group). Participants were 50–70 years of age without lung cancer related symptoms. Inclusion criteria were: current or ex-smoker with minimum 20 pack-years, and FEV1 of at least 30% of predicted normal at baseline. Ex-smokers had to have quit after the age of 50 years and less than 10 years before inclusion.
Exclusion criteria were: body weight >130 kg or previous treatment for any kind of cancer within 5 years, tuberculosis within 2 years or any serious illness with life expectancy <10 years. In the screening arm 2,052 volunteers were scanned annually for 5 years (2004–2010). In addition, for all participants spirometry was performed, smoking habits were recorded and carbon monoxide level in exhaled breath was measured annually. Detailed description of the study design and study population has been published previously (Citation8).
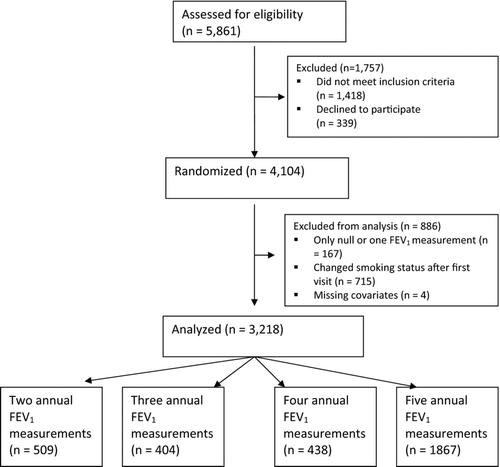
In the current analysis we chose to exclude participants who changed smoking habits after first visit or did not have a minimum of two FEV1 measurements. Participants in the current analysis are described in flowchart . The DLCST (www.ClinicalTrials.org, registration number: NCT00496977) was approved by the Ethical Committee of Copenhagen County (identification no. H-KA-02045, supplementary protocol 20148) and funded in full by the Danish Ministry of Interior and Health. Approval of data management in the trial was obtained from the Danish Data Protection Agency. All participants provided written informed consent before randomization.
Lung function testing
Spirometry was performed annually for 5 years. Data were collected at a single institution by professionally trained and experienced hospital-based pulmonary function technicians, equipment calibration was performed daily and checked prior to each test, and the flow sensor was cleaned daily, according to manufacturer's recommendations. The spirometry was performed according to recommendations by the European Respiratory Society (Citation9) using a computerized system (Spirotrac IV software; Fleich Pneumotach model 6800, Vitalograph, Buckingham, UK). No bronchodilatation was applied. Measurements included FEV1 and forced vital capacity (FVC) and their ratio (FEV1/FVC). FEV1 and FVC were expressed in absolute values and as percent of predicted values according to European reference equations (Citation10), and airflow limitation (AFL) was defined as FEV1/FVC < 70%. The severity of AFL was classified by FEV1 in percent of predicted (FEV1%) according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (Citation11). Participants were categorized as having mild AFL (GOLD I) when FEV1% predicted was ≥80%, and moderate to severe AFL (GOLD II-III) when FEV1% was >30% and <80% (Citation11).
Statistical analysis
The influences of age, sex, height, BMI, smoking and AFL on FEV1 and FEV1 decline were analyzed in an absolute and a log-transformed multiple regression model. A more detailed description of the statistical model is reported in the appendices. No transformation of the outcome was applied in the “absolute” model (abs-model), whereas the logarithm of FEV1 was outcome in the log-model. The reference group was former smoking men with no AFL and other characteristics close to the average of the cohort (i.e. height 1.75 m, body mass index (BMI) 25 kg/m2 and 35 pack-years), and the time point corresponding to age 60 years was used for cross sectional comparisons. Interactions between age and other explanatory variables (sex, BMI, smoking and AFL) were included in the model as indications of the influences of these variables on rate of decline of FEV1. We were not interested in immediate effects of changes in smoking habits (i.e. cessation or relapse), and therefore, we eliminated FEV1 data of individuals who changed their smoking habits during the study, so that only data before change in smoking status were used for analysis (Citation12). With the aim of picking up any non-linearity, we included squared age (age2) as explanatory variable in the models.
Results
The number of participants in the DLCST was 4,104 and information from 3,218 (78%) of these subjects was included in the analyses presented in this article (). The characteristics of the whole population are described elsewhere (Citation8) and the characteristics of the participants included in the current analysis are shown in .
Table 1. Characteristics of the study population
The majority was men (55%), 25% were former smokers, and at inclusion age ranged from 50 to 70 years with an average of 58 years. In , participants are divided in four subgroups according to smoking habits (current or former) and AFL (present or absent), and for all subgroups the correlation between FEV1 and decline in FEV1 (∆FEV1) was positive in absolute terms (ml) and negative in relative terms (%). This means that in absolute terms decline increased with increasing FEV1 (anti-horse-racing effect) whereas in relative terms the opposite was true, that is decline increased with decreasing FEV1 (horse-racing effect).
shows the results of the two multivariable models. The residual variation is 161 ml in absolute terms and 6.5% in relative terms. In both models all explanatory variables have statistically significant influences on level of FEV1; however, the models differ with regard to the influences of explanatory variables on rate of decline of FEV1. Thus, sex and height have significant influences on decline in the absolute model, but no influence in the relative model, where on the other hand both BMI and current smoking significantly increase the annual decline. Furthermore, in GOLD grade II + III the decline of FEV1 is significantly steeper in relative terms only. For both models the effects of various covariates on the level and rate of decline of FEV1 are illustrated in .
Figure 2. The influences of various factors on decline in lung function. Figures in upper row (A-C) are results from the absolute model and figures (D-F) are based on the relative model.
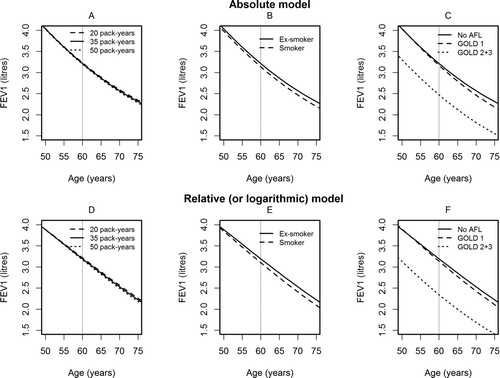
Table 2. Results of the mixed effects models showing the effect of explanatory variables on level of FEV1 at age 60 and the annual rate of change (slope) in relation to a reference group of former smoking men with no air flow obstruction at entry to the trial
Squared age (age2) was highly significant (p < 0.001) in both models () showing that decline is curvilinear. In the absolute model the coefficient was positive whereas in the relative model the coefficient was negative. Because participants lose lung function (slope is negative) a positive coefficient implies a negative acceleration or decelerating effect of age on rate of decline of FEV1, whereas a negative coefficient indicates that loss of lung function accelerates with advancing age. Furthermore, in the absolute model squared age has no significant interactions with other covariates, and in the relative model squared age interacts significantly with both smoking history (pack-years) and severity of AFL. Both interactions are negative indicating that decline accelerates with accumulation of pack-years (−0.00088% per pack-year, p = 0.021) and worsening of AFL (GOLD I: −0.020%, p = 0.119; GOLD II+III: −0.034%, p = 0.011).
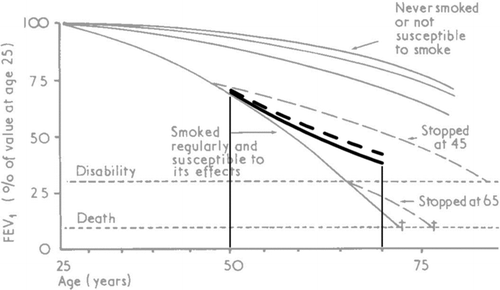
In we have shown the predictions of the relative model beyond the limits of our data superimposed on the well-known Fletcher-Peto diagram (Citation2). By backward extrapolation we predicted FEV1 for a heavy smoker from age 25 to 75 years (solid line). The dashed line from age 50 to 75 shows the estimated change in lung function assuming the subject quits smoking at age 50.
Figure 3. Effect of smoking on decline in lung function. A subject who began smoking at age 25 and continued smoking the same amount until age 70 (solid line). The dashed line from age 50 to 70 shows the estimated change in lung function assuming the subject quitted smoking at age 50 that is no further smoking-related increase in slope after age 50. In grey the Fletcher-Peto diagram (2).
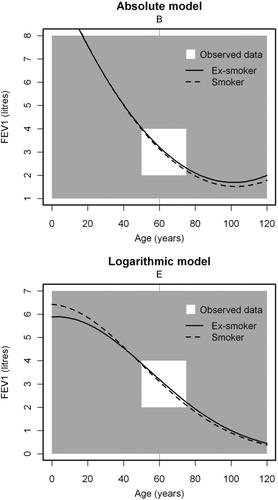
displays the fundamental mathematical differences in the two models by backward and forward extrapolation beyond the observed data to an age range from 0–120 years (assuming height unchanged).
Discussion
Measuring changes in FEV1 in absolute terms (ml) is a natural first approach, and this was the choice of Fletcher and Peto when they analyzed the results of their landmark study. They made several important observations some of which conflicted with the orthodoxy of that time. They observed a continuous and smooth decline of FEV1 over an individual's life and a tendency of FEV1 decline to accelerate with advancing age (Citation2). Furthermore, they saw that AFL progresses slowly at the beginning and that loss of function markedly increases when the degree of AFL gets more severe, and they called this a horse-racing effect (Citation1). Consequently, based on these concepts and both backward and forward extrapolation of their data, they constructed the famous diagram that is shown in the background of .
However, recent large clinical studies of patients with COPD challenge the well-known diagram and the horse-racing concept behind it (Citation4–6). In a recent review Tantucci and Modina (Citation7) analyzed spirometric data of COPD patients included in the placebo arms of clinical trials published after 1990, and they consistently found that rate of decline decreases with increasing severity of COPD. The only exception is a recent analysis of data from the Lung Health Study, that showed a significant horse racing effect (Citation13).
However, in this analysis participants were stratified according to baseline FEV1, and the observed horse-racing effect is probably due to “prebronchodilator FEV1/FVC less than 0.70 and prebronchodilator FEV1 between 55 and 90% predicted” as an inclusion criterion, and regression towards the mean. We consistently saw an anti horse-racing effect in absolute terms (), and recently, this was also observed in the large Dutch/Belgian lung cancer screening trial (NELSON) (Citation14). Because these studies cover a broad range of lung functions from normal to very severe AFL, anti-horse-racing in absolute terms seems to be a general phenomenon which contradicts the hypothetical existence of “a phase with fast horse-racing” mentioned in the introduction. These counter-intuitive findings speak against the conventional way of analyzing decline in absolute terms.
In nature the size of changes is usually related (and often proportional) to the starting point, and changes are seldom absolute and independent of the starting point. For instance, calculations of relative changes are used daily when calculating concentrations of drugs based on knowledge of half-life (T½). Therefore, measuring changes in relative terms is an obvious alternative to the current orthodoxy, and we believe this new concept leads to more intuitive results and a better understanding of changes in lung function. The consequences of a relative concept are discussed in more detail in the following paragraphs.
First, it appears from that when change in FEV1 is measured in relative terms (∆FEV1 in%), the correlation coefficients are consistently negative that is lower lung function relates to steeper decline. In other words, the relative model shows a consistent horse-racing effect. In fact, applying the relative model to recent clinical studies that reported anti-horse-racing converts this phenomenon into horse racing. Thus, in ECLIPSE (Citation4) for GOLD grade II, III and IV the average FEV1 was 1,750, 1,130 and 720 ml (personal communication), decline was 35 ml, 33 ml and 25 ml per year, and the percentage decline was 35/1750 = 2.0%, 33/1130 = 2.9% and 25/720 = 3.5%, respectively.
Second, the models are quite similar in predicting level of lung function. The conventional absolute model and the alternative relative model are compared in . For instance, the estimates for FEV1 of the reference group differ by 19 ml only. On the other hand, because the models measure changes in lung function differently (ml vs.%), there are important differences in the prediction of changes in lung function:
In the relative model sex and height have no influence on rate of decline, which simplifies the model.
In the relative model both current smoking, mild (GOLD I) and moderate-to severe AFL (GOLD II+III) significantly increase the rate of decline which is reassuring from a clinical point of view.
The relative model is more sensitive to changes in subjects with low lung function, where the absolute model tends to underestimate the effect of treatment (Citation15).
In the relative model fast decliners have low lung function, whereas in the absolute model fast decliners have normal or near normal lung function.
Third, when comparing various models the ability to predict future events is essential, and a study by Wang et al. (Citation16) seems to indicate that relative changes are superior to absolute measurements in this regard. They analyzed spirometric data from 3,724 workers who performed at least two tests between 1973 and 2003, and they showed that persons with abnormal short term (1–5 years) declines in FEV1 were more likely to show excessive long-term (10–30 years) declines. Interestingly, excessive long term declines were more precisely predicted when basing it on percentage changes than on absolute changes in short-term declines in FEV1.
Due to large numbers from testing several thousand subjects annually for 5 years, we are able to show beyond any doubt that loss of lung function is not strictly linear. In fact decline in FEV1 decelerates in absolute terms ( p < 0.001) and accelerates in relative terms ( p < 0.001). Furthermore, the acceleration of the relative model interacts significantly with both smoking (pack-years) and severity of AFL indicating a potentiating effect of these covariates on the decline of FEV1. Histological studies in which progression of COPD was associated with the accumulation of inflammatory and repair or remodelling processes that thickens the walls of the airways even in subjects who stopped smoking many years ago (Citation17) could to some extend explain the observed accelerated decline.
The relative model combines accelerated and exponential decline in a sigmoid curve ( and ): initially when lung function is normal (or near normal) the decline accelerates as reported by Fletcher and Peto (Citation1,2), and later when lung function gets low the decline loses speed and levels off as observed in more recent clinical trials (Citation4–7). Thus, the relative model offers a unifying theory that fits results from both old and more recent studies. However, it should be noticed that even though curves look similar their interpretation may differ.
Thus, Fletcher and Peto saw the accelerated decline in the left, upper part of the sigmoid curve in as a horse-racing effect in absolute terms, whereas according to the relative model the accelerated rate of decline is a cumulative effect of aging, smoking and the development of AFL. Fletcher and Peto did not include information about pack-years in their analyses which may serve as an excuse for their possible misinterpretation of the data. Likewise, the right, lower part of the curve in is interpreted as anti-horse-racing or deceleration in the absolute model, and in contrast, as exponential decay and horse-racing in the relative model.
Finally, the sigmoid curve of the relative model in comprehends even rather extreme horse-racing recognized by many clinicians and originally described by Burrows in 1981 (Citation18) as pathway C: “there are cases in which the FEV1 has been observed to be normal and essentially stable throughout middle-age, then after age 50 to decline rapidly to the point of severe disability. This has even been observed after a patient has stopped smoking.”
Patients with COPD are usually heavy smokers, who started smoking at their teenage years. FEV1 is believed to reach its maximal value at about 25 years of age followed by a steady annual physiologic decline of 25 ml/year and 29 ml/year for females and males, respectively (Citation9). Nevertheless, we observed a remarkable loss of lung function. The mean decline of FEV1 was more than 80 ml/year, which is approximately three times as much as the expected normal decline. The Dutch lung cancer screening trial showed comparable loss (Citation14).
Some of this rapid decline in FEV1 might be explained by a waning enthusiasm from the participants when performing the spirometry from first visit to the end of the study, despite the encouragement from professionally trained and experienced hospital-based pulmonary function technicians. Mathematically this jump from a low normal decline to a rather rapid decline is difficult (if not impossible) to grasp in the context of the absolute model whereas in the relative model it is simply a consequence of the accelerating effect of age above 50 years and a smoking history of more than 20 pack-years. Thus, for former and current smokers at age 25 years the absolute model predicts a scary annual decline of 137 ml and 139 ml, respectively, whereas the prediction of the relative model is 43 ml and 56 ml, respectively, which is much more physiologic. These figures underscore the importance of primary preventive strategies against smoking at an early age.
Limitations
Our study has several limitations. First of all, we studied a selected population. The inclusion of participants in response to advertisements in the written media pose a potential bias for selection that favour the more enlightened and possibly less sick smokers. Compared to the general smoking population in Denmark the participants did not differ in smoking history in pack years but more participants were current smokers and more were women (Citation19). We included only current or former heavy smokers in the age range from 50 to 70 years and with a smoking history of at least 20 pack-years, and thus cannot predict lung function decline in younger, never or more moderate smokers. Furthermore, former smokers had quit relatively recently (that is after the age of 50 years and less than 10 years before inclusion) which may have influenced the effect of smoking cessation (). In addition, participants were relatively healthy. Even though we used FEV1/FVC < 0.7 instead of LLN, more than half had no AFL, and of those with AFL most were mild (GOLD I), few were severe (GOLD III), and subjects with FEV1 < 30% predicted at baseline were excluded. Therefore, our results cannot necessarily identify factors of importance for rate of decline in people with very severe COPD such as those included in recent clinical trials (Citation4–6). Furthermore, the exclusion from this analysis of participants, who changed their smoking habit, might have introduced some selection bias. We did not perform reversibility testing which could have biased the FEV1/FVC ratio. However, because all spirometries were performed in the same location and according to ATS/ERS standards, we believe that this has not biased our results. The study is purely observational, and we did not include possible effects of treatments in our analysis.
Conclusion
In summary, measuring changes in relative terms might have important implications for the interpretation of results from clinical trials where FEV1 is the primary outcome, because the effect of treatments on rate of decline (and statistical significance) may differ considerably between measuring changes in absolute or relative terms. Also when looking for correlations between decline in lung function and changes in other variables such as frequency of exacerbations or quality of life based on questionnaires, it could be of great importance whether rate of decline is measured in ml or%. This may explain why such correlations often have been difficult to prove, and from a clinical point of view, they are usually surprisingly weak. Furthermore, when searching for genes that predispose to the development of AFL, some consider rate of decline of lung function as an interesting phenotype, and again results will depend very much, on how the rate of decline is calculated. In such situations, it may be more productive using relative decline as a marker instead of the more conventional absolute decline.
In conclusion, our data show that relative changes seem to explain the decline in lung function better than the more conventional absolute measurements. Smoking and severity of airflow obstruction are associated with an increased rate of decline in lung function, and the cumulative effect of smoking (pack-years) primarily works through potentiating the accelerated decline due to aging, and this emphasizes the importance of abstaining from smoking, the sooner the better.
Declaration of Interests Statement
DLCST was funded in full by the Danish Ministry of Interior and Health. The Danish Ministry of Interior and Health had no influence on the study design, the collection, analysis and interpretation of data, on the writing of the report or in the decision to submit the paper. LHT, AD, SBS, LTS and JHP declare no conflicts of interest. MD is employed at AstraZeneca R&D Mölndal, Mölndal Sweden.
The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors thank the investigators, staff, and participants of the Danish Lung Cancer Screening Trial for their valuable contributions.
References
- Fletcher C, Peto R, Tinker C, Speizer FE. The Natural History of Chronic Bronchitis and Emphysema. London: Oxford University Press; 1976.
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977 Jun 25; 1(6077):1645–1648.
- Burrows B, Knudson RJ, Camilli AE, Lyle SK, Lebowitz MD. The “horse-racing effect” and predicting decline in forced expiratory volume in one second from screening spirometry. Am Rev Respir Dis 1987 Apr; 135(4):788–793.
- Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011 Sep 29; 365(13):1184–1192.
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008 Aug 15; 178(4):332–338.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008 Oct 9; 359(15):1543–1554.
- Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis 2012; 7:95–99.
- Pedersen JH, Ashraf H, Dirksen A, Bach K, Hansen H, Toennesen P, The Danish randomized lung cancer CT screening trial–overall design and results of the prevalence round. J Thorac Oncol 2009 May; 4(5):608–614.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993 Mar; 16:5–40.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Interpretative strategies for lung function tests. Eur Respir J 2005 Nov; 26(5):948–968.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013 Feb 15; 187(4):347–365.
- Lee PN, Fry JS. Systematic review of the evidence relating FEV1 decline to giving up smoking. BMC Med 2010; 8:84.
- Drummond MB, Hansel NN, Connett JE, Scanlon PD, Tashkin DP, Wise RA. Spirometric predictors of lung function decline and mortality in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012 Jun 15; 185(12):1301–1306.
- Mohamed Hoesein FA, Zanen P, Boezen HM, Groen HJ, van GB, de Jong PA, Lung function decline in heavy male smokers relates to baseline airflow obstruction severity. CHEST 2012 Dec; 142(6):1530–8.
- The Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am J Respir Crit Care Med 1998 Jul; 158(1):49–59.
- Wang ML, Avashia BH, Petsonk EL. Interpreting periodic lung function tests in individuals: the relationship between 1- to 5-year and long-term FEV1 changes. CHEST 2006 Aug; 130(2):493–499.
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004 Jun 24; 350(26):2645–2653.
- Burrows B. An overview of obstructive lung diseases. Med Clin North Am 1981 May; 65(3):455–471.
- Hestbech MS, Siersma V, Dirksen A, Pedersen JH, Brodersen J. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer 2011 Sep; 73(3):325–331.
Appendices
Statistical analysis:
We used a random coefficients model with linear and quadratic time/age effects, allowing the intercept, slope and curvature to vary between subjects. It takes the form Yij = αi + β1itij + β2itij2 + eij where αi, β1i and β2i are parameters specific to subject i, tij is the age of subject i at the time of the jth lung function test, and Yij is the corresponding FEV1 at this time. The eij are independent random errors associated with Yij and are normally distributed with mean 0. The effects of covariates on the intercept, slope and curvature are modeled via αi = α + δ11 × X1i + δ12 × X2i +…+ δ1k ´ Xki + ai and β1i = β1 + δ21 × X1i + δ22 × X2i +…+ δ2k × Xki + b1i and likewise for β2i where Xm are the m = 1,…, k covariates of interest. The random effects ai, b1i and b2i for the same subject are assumed to be normally distributed with mean 0 across subjects, and are allowed to be dependent. All analyses were conducted with the use of R software, version 2.13.1.