Abstract
Dynamic hyperinflation (DH) during exercise is associated with both dyspnea and exercise limitation in COPD. Metronome-paced tachypnoea (MPT) is a simple alternative for studying DH. We compared MPT with exercise testing (XT) as methods of provoking DH, and assessed their relationship with dyspnea. We studied 24 patients with moderate COPD (FEV1 59 ± 9% predicted) after inhalation of ipratropium/salbutamol combination or placebo in a double-blind, crossover design. Inspiratory capacity (IC) was measured at baseline and after 30 seconds of MPT with breathing frequencies (fR) of 20, 30 and 40 breaths/min and metronome-defined I:E ratios of 1:1 and 1:2, in random sequence, followed by incremental cycle ergometry with interval determinations of IC. DH was defined as a decline in IC from baseline (∆IC) for both methods. Dyspnea was assessed using a Borg CR-10 scale. ∆IC during MPT was greater with higher fR and I:E ratio of 1:1 versus 1:2, and less when patients were treated with bronchodilator rather than placebo (P = 0.032). DH occurred during 19 (40%) XTs, and during 35 (73%) tests using MPT. Eleven of 18 (61%) non-congruent XTs (where DH occurred on MPT but not XT) terminated before fR of 40 breaths/min was reached. Although greater during XT, the intensity of dyspnea bore no relationship to DH during either MPT and XT. MPT at 40 breaths/min and I:E of 1:1 elicits the greatest ∆IC, and is a more sensitive method for demonstrating DH. The relationship between DH and dyspnea is complex and not determined by DH alone.
Introduction
Expiratory airflow limitation in chronic obstructive pulmonary disease (COPD) leads to air trapping and hyperinflation (Citation1, 2), which has both static and dynamic components. Static hyperinflation can be attributed to permanent structural changes affecting the lung parenchyma and chest wall, while dynamic hyperinflation (DH) is influenced by airway resistance, which, by slowing expiratory flow, prevents optimal lung emptying. This worsens when expiratory time is reduced, for example during exercise or other situations with tachypnea. DH increases operational lung volumes during exercise in patients with COPD and is thought to contribute to breathlessness and exercise limitation (Citation3).
Until recently the preferred method of studying DH has been the measurement of inspiratory capacity (IC) during exercise (Citation4); a fall in IC being associated with decreased exercise ability and exertional dyspnea (Citation5, 6). However, a recent study failed to show a close relationship between DH and dyspnea in exercising patients with COPD. Instead, dyspnea correlated more closely with the end-inspiratory lung volume (EILV) which is measured by taking into account end-expiratory lung volume (EELV) and tidal volume (VT) both of which increase during exercise (Citation7).
In the clinical setting, exercise testing is often impractical because it requires certain equipment, trained technicians, physician supervision, and is relatively difficult and uncomfortable for elderly and deconditioned patients. Metronome-paced tachypnea (MPT) is a simple alternative to exercise testing to induce dynamic hyperinflation (Citation8). The mechanism whereby paced tachypnea causes dynamic hyperinflation has not been fully studied, but in segments of lung with increased resistance to airflow, this might be simply the result of decreased time for expiration.
In previous reports of the MPT little attention has been paid to optimizing and standardizing the method. We have previously demonstrated that the expiratory time (TE) rather than the absolute level of minute ventilation is the principal determinant of DH in patients with COPD (Citation9). We reasoned that by manipulating both frequency and pattern of breathing during MPT, conditions for provoking DH would be improved and could be standardized, thereby permitting comparisons between patients and study populations, and possibly also the evaluation of treatments such as bronchodilators. By reducing the EELV at rest and during exercise, bronchodilators reduce DH (Citation2, Citation10, Citation11). However, to date the only studies investigating the effect of bronchodilators on DH provoked by MPT have reported conflicting results (Citation12, 13).
We report here an investigation of the performance and standardization of MPT as a method for diagnosing DH in patients with moderate COPD, comparing it with incremental exercise. We also studied the effect of a bronchodilator on MPT and the relationship of DH provoked by these two methods with dyspnea assessed using the Borg score.
Methods
Study design and subjects
This was a single center, randomized, double blind, cross-over study in subjects with moderate COPD. Participants were aged 40 years or older and had a smoking history of at least 10 pack-years, a diagnosis of moderate COPD as defined by the GOLD classification (i.e. stage II and below: FEV1/FVC < 70%; FEV1 < 80%), and a total lung capacity (TLC) greater than 80% of predicted. Key exclusion criteria were a history of asthma, significant pleural disease, a history of lung resection, pleurodesis or lung volume reduction surgery, or the presence of any co-existing illness that prevented participation in the study or was likely to interfere with interpretation of the study results. The protocol was approved by the Human Research Ethics Committee of the Health Sciences Faculty, University of Cape Town, and all patients provided written consent. The trial was registered with the South African National Clinical Trial Registry (DH-27-0311-2342).
Procedures
Eligible subjects underwent baseline spirometry, body plethysmography and measurement of diffusing capacity according to American Thoracic Society guidelines (Citation14, 15) at study entry. Subjects were then randomized in a double blind, crossover design to receive either two inhalations of Combivent® pMDI (total dose: salbutamol 200 μg + ipratropium 36 μg, Boehringer Ingelheim Pharmaceuticals, Inc.) or two inhalations of an identical placebo inhaler, at two subsequent visits.
Metronome-paced tachypnea
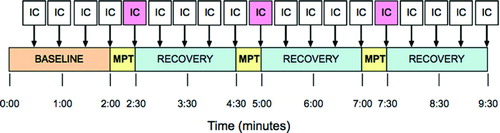
Subjects were randomly assigned to a sequence of metronome-paced breathing frequencies (20, 30 and 40 breaths/minute) at I:E ratios of both 1:1 and 1:2: a total of six patterns of breathing (). For each subject the same sequence was used on each of the two test days.
Ninety minutes after receiving either Combivent® or placebo, inspiratory capacity and breathing pattern were measured in accordance with ATS/ERS guidelines (Citation16), followed by MPT at each of the breathing frequencies for 30 seconds. Target breathing frequencies were achieved in each subject using a metronome that produced tones for alternating inspiration and expiration according to the predetermined respiratory frequency. The metronome also imposed an I:E ratio of 1:1 or 1:2, also in random sequence. A visual prompt on a computer screen displayed actual and target breath frequencies. Subjects were allowed to regulate their own tidal volumes, and end-tidal carbon dioxide levels were monitored. Inspiratory capacity (IC) was measured after 30 seconds of MPT and then during recovery (). A 60-second period of recovery was allowed between MPT intervals. A fall in IC, as defined below, was judged to represent dynamic hyperinflation. Intensity of dyspnea was assessed using the Borg CR-10 scale (Citation17) at the end of each 30-second period of MPT.
Exercise testing
DH during exercise was assessed using a symptom-limited, maximal incremental exercise protocol on a cycle ergometer (Ergoline 800S; Sensormedics Corp, Loma Linda, CA). The external work rate was incremented in “ramp” fashion by computer control. The ramp increment was judged for each individual subject by considering age, gender, height, weight and degree of functional impairment, with the intention of obtaining an exercise time of 8–12 minutes before exhaustion (Citation18).
An identical work rate protocol was used for subsequent testing on any given subject. The investigator monitored the respiratory rate during each test, and prompted the subject to perform an inspiratory capacity maneuver at regular intervals. of Dyspnea intensity was assessed using the Borg CR-10 scale (Citation17) at symptom limitation.
Statistical analysis
Descriptive statistics shown in text and tables are the means ± SD, unless otherwise indicated. The repeatability of baseline IC values, both within subjects and between subjects, was determined using repeated measures analysis of variance. The IC values after metronome-paced tachypnea were compared to baseline and were corrected for multiple comparisons using the Bonferroni method. Comparisons of differences in ∆IC between pre- and post- treatment variables were performed using paired t-tests. There is no consensus for cut-off values for DH; in this study it was judged to be present if the difference in IC post-MPT was more than 1.96 SD below the baseline IC measurements for that subject. A p-value < 0.05 was considered statistically significant for all analyses. All statistical analyses were performed using the PASW version 18.0 program (Statistic Solutions Inc., Palm Harbour, USA).
Results
Baseline characteristics of the study population
Baseline characteristics of the 24 COPD patients studied are provided in . Sixteen (67.4%) were men. Their ages ranged from 51 to 77 years; FEV1 was 1.70 ± 0.45L (59.3 ± 8.8% of predicted) and TLC, FRC, RV were 110.2 ± 18.7%, 130 ± 29.9% and 169 ± 39.7% of predicted, respectively. Twenty three patients were in GOLD stage II, and one patient in stage III (FEV1 43% predicted). Nine patients had static hyperinflation at baseline: seven with a TLC greater than 120% predicted, and two additional patients had an IC/TLC ratio of less than 25%. Mean resting IC at baseline assessment at visit 1 was 2.46 ± 0.65L (89 ± 24% predicted).
Table 1. Baseline characteristics of the subjects
Dynamic hyperinflation induced by metronome-paced tachypnea
All 24 patients were able to perform the MPT protocol after instruction and practice. There were no significant differences between the four baseline IC measurements performed at each visit (repeated measures ANOVA p = 0.907). The mean time between test days 1 and 2 (visit 2 and 3) was 12 days (range 1 to 112 days).
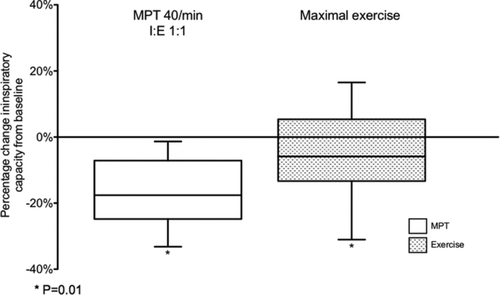
With breathing frequencies of 20, 30 and 40 breaths per minute, there were step-wise reductions of IC, which were significantly different to baseline (). The decrease in IC was also greater for I:E ratio of 1:1 versus 1:2 (p = 0.032) (). The greatest decline in IC (between baseline and 40 breaths per minute at I:E ratio 1:1 for placebo) was 0.37 ± 0.30 L.
Figure 2. Time course and degree of dynamic hyperinflation produced by MPT. Columns represent mean inspiratory capacity (% predicted) ± SEM for each condition of testing. There is a graded decline in inspiratory capacity with increasing respiratory rates for both I:E ratios. *P < 0.05 for same I:E ratio compared to baseline. †P < 0.05 versus same respiratory rate at different I:E ratio.
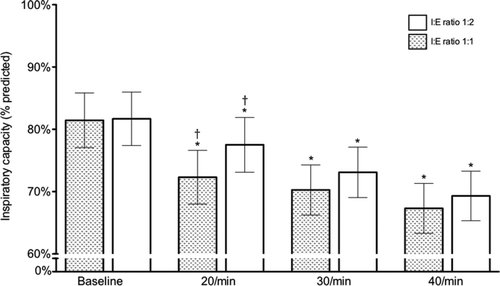
Table 2. Change in inspiratory capacity (∆IC) for each condition of MPT
Dynamic hyperinflation induced by exercise testing
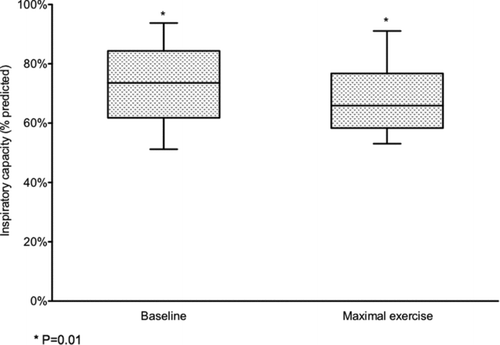
Baseline IC prior to XT was not significantly different from that measured before MPT. The mean respiratory rate achieved at maximal exercise was 35 ± 7 breaths/minute. IC declined from 73.2 ± 15.3% of predicted at baseline to 67.9 ± 14.4% of predicted at maximal exercise (p = 0.01) (). The mean decline in IC was 0.13 ± 0.39 L, and this was significantly less that achieved during peak MPT at 40/min (p = 0.003).
Effect of bronchodilators on dynamic hyperinflation
For each condition of testing, dynamic hyperinflation was less for Combivent® than for placebo (). The changes between matched conditions were not statistically significant. The mean difference between ∆IC for Combivent® versus placebo for MPT 40/min and I:E 1:1 was 79 ml which was not statistically significant by paired t-test (P = 0.364). However, for all six conditions of measurement, ∆IC following Combivent® was numerically lower than with placebo, and analysis using the sign rank test was statistically significant (p = 0.032).
By contrast, when comparing the effects of Combivent® and placebo on DH during exercise, the differences between changes in IC from baseline to maximal exercise were not statistically significant ().
Comparison between MPT and XT
Of 48 matched measurements (including placebo and Combivent®), 35 (73%) demonstrated DH on MPT while only 19 (40%) showed DH on exercise testing. When compared with XT, the sensitivity and specificity for MPT in demonstrating DH was 90% and 38%, respectively. When compared with MPT, the sensitivity and specificity for XT in demonstrating DH was 50% and 85%, respectively. Overall, the negative predictive value for MPT was 85%, and that for exercise was 38%.
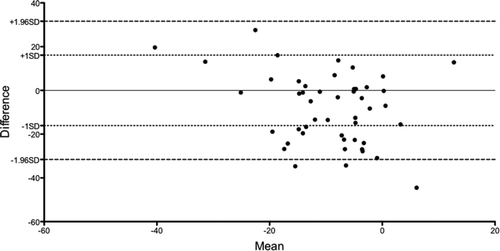
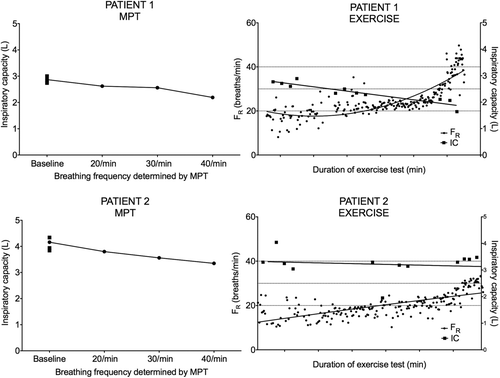
In 18 of 48 comparisons, there was non-congruence, with DH demonstrated by MPT but not during XT. In 11 (61%) of these, exercise terminated before breathing frequencies equal to or higher than those achieved during MPT were reached. Examples of congruent and non-congruent patient observations are shown in . If only the matched observations in which comparable respiratory rates in ET and MPT were achieved are analysed, the specificity for MPT in detecting DH increased to 61%. The negative predictive value overall for MPT was 85%. Using Bland-Altman analysis, a bias for a greater decline in IC during MPT was found ().
Figure 5. Comparison between DH induced during MPT and XT for two separate patients. Patient 1 demonstrates DH during both MPT and XT; a respiratory rate above 40 breaths/min is achieved during XT. Patient 2 demonstrates DH during MPT but not during XT, which is terminated prematurely due to exhaustion; the highest respiratory frequency reached during XT is only approximately 30 breaths/minute.
Exertional dyspnea
The intensity of dyspnea induced by MPT at maximal conditions (frequency of 40 breaths per minute, I:E ratio 1:1) was very low (mean Borg CR-10 scale score 1.12, 95% CI 0.70-1.54). During XT, the mean modified Borg scale score was significantly higher (2.94, 95% CI 2.22–3.67 p < 0.001). There was no correlation between the magnitude of DH and the mean dyspnea score for either MPT or XT.
Discussion
The results of our study confirm that the optimal conditions of MPT for inducing DH is a rapid breathing frequency (40 /min) with an I:E ratio of 1:1. While previous studies have interpreted changes in IC during metronome-pacing by referencing cardiopulmonary exercise testing as the gold standard (Citation12, Citation19), we have shown that MPT performed using this methodology is actually more sensitive than incremental exercise in eliciting DH in patients with moderate COPD.
In part, this can be attributed to the fact that patients may not reach such rapid breathing frequencies during incremental exercise, cannot maintain them, or have exercise limitation due to factors other than lung mechanics (e.g., poor motivation, muscle fatigue, poor conditioning and the development of metabolic acidosis, or cardiac disease). In addition, complex neurohormonal changes, like increased circulating catecholamines (that might not be present during MPT), may mitigate dynamic hyperinflation and lessen the fall in IC during exercise. By contrast, in our study all patients were able to reproducibly perform the required MPT breathing routine.
A further advantage of MPT is that it simulates other conditions in which DH may occur; such as the tachypnea associated with strong emotions, panic attacks and hypoxaemia. We have shown elsewhere that this methodology (which controls expiratory time) can also elicit DH in normal non-smoking subjects without airflow limitation; however, for the same conditions, the magnitude of DH is considerably less than for subjects with COPD, and the determinants of DH in these two groups are different (Citation20).
Secondly, we demonstrated that DH induced by MPT was associated with less dyspnea than with exercise, which might in part explain their ability to persist with MPT at high breathing frequencies than achieved during exercise. The shorter duration of MPT may have been a factor; resulting in insufficient time for perceiving the respiratory muscle mechanical disadvantage caused by DH. However, with both methods, we observed a poor correlation between DH and dyspnea, supporting the findings of O'Donnell et al. (Citation2, Citation7) during exercise testing. The recent study by Guenette et al. (Citation7) reported similar findings, and in addition showed that dyspnea and exercise limitation were more closely associated with an increase in end-inspiratory lung volume (EILV) during exercise. EILV is a combination of both the change in EELV and the increasing tidal volume (VT) during exercise, and a critical increase in EILV results in particularly unfavourable respiratory system mechanics perceived subjectively by the patient as dyspnea.
The effects of inhaled short- and long-acting anticholinergic agents and B2-agonists on reducing both static and dynamic hyperinflation during exercise are well documented (Citation2, Citation5, Citation10, Citation21) but their effect during MPT is less well studied. Gelb et al., using a different MPT method to ours, failed to show an increase in IC with ipratropium at baseline or after hyperventilation in patients with moderate-to-severe COPD.
Fujimoto and colleagues (Citation13) showed a reduced fall in IC during MPT after administering the short-acting beta-agonist (SABA), salbutamol, but not after the short-acting muscarinic antagonist (SAMA), oxytropium. In our study, the effect of the SABA plus SAMA combination inhaler was only evident by trend analysis of all test conditions. However, the failure of this treatment to significantly reduce ∆IC for each test comparison may be explained by differences in study populations, as well as differences in methodology between the studies, First, we reduced TE by controlling I:E ratio and fR, whereas Fujimoto et al. induced hyperventilation by setting tidal volume and fR. TE was not reported in their study. Secondly, the Fujimoto study patients were older and had more severe COPD (almost half were GOLD stage III or IV).
Previous reports of MPT have employed different methodologies: Gelb et al attempted to double the resting breathing frequency and to control tidal volume by encouraging subjects to follow a graphic display of their breathing pattern (Citation12, Citation22–24). Fujimoto et al. used stepwise increases in absolute breathing frequency and controlled tidal volumes (Citation13), while a further study controlled for breathing frequency but allowed patients to regulate their own tidal volumes (Citation8). None of these methods controlled for breathing pattern or expiratory time. We recommend simply comparing IC measured at baseline with that measured at a breathing frequency of 40 breaths per minute and I:E ratio of 1:1.
Last, whereas in the Fujimoto study patients underwent step-wise MPT sequentially before and after treatment with the bronchodilators on the same day, we randomized the order of breathing frequencies during MPT and randomized treatments in a blinded manner on different study days to eliminate any possibility of a sequence effect. For unavoidable logistical reasons, this methodology resulted in a relatively long time between test days (mean = 12 days, range 1 to 112 days); a delay that may have permitted random changes in bronchomotor tone and baseline lung function between the visits.
As we did not measure baseline spirometry before administration of placebo or bronchodilator on test days 2 and 3, we were unable to correct for such variation, and this was a weakness of our study. Nevertheless, baseline ICs did not differ significantly between visits and this finding may suggest that any reduction in dynamic hyperinflation achieved with a single dose of combination bronchodilator in patients with moderate COPD is insufficient to be detected above the noise of random variation in baseline lung function.
Conclusions
We have described a simple standardised method for inducing and measuring dynamic hyperinflation in patients with COPD; the difference between IC at rest, and after 30 seconds of MPT at 40 breaths per minute at an I:E ratio of 1:1 (measured in mL). We have demonstrated that MPT has superior performance (high sensitivity and negative predictive value) to incremental exercise testing in patients with moderate COPD in demonstrating dynamic hyperinflation. We propose that MPT may be useful in all severities of COPD, and might be informative as a method for assessing the efficacy of bronchodilators and other treatments.
Declaration of Interest Statement
This work was supported by a research grant from Boehringer Ingelheim Pharmaceuticals, Inc. to the University of Cape Town Lung Institute. South African National Clinical Trial Registry (DH-27-0311-2342).
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc 2006 Apr; 3(2):176–179. PubMed PMID: 16565428. Epub 2006/03/28. eng.
- O'Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999 Aug; 160(2):542–549. PubMed PMID: 10430726.
- O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001 Sep 1; 164(5):770–777. PubMed PMID: 11549531. Epub 2001/09/11. eng.
- Dodd DS, Brancatisano T, Engel LA. Chest wall mechanics during exercise in patients with severe chronic air-flow obstruction. Am Rev Respir Dis. 1984 Jan; 129(1):33–38. PubMed PMID: 6230971.
- O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998 Nov; 158(5 Pt 1):1557–1565. PubMed PMID: 9817708.
- O'Donnell DE, Webb KA. Breathlessness in patients with severe chronic airflow limitation. Physiologic correlations. Chest 1992 Sep; 102(3):824-31. PubMed PMID: 1516410.
- Guenette JA, Webb KA, O'Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J 2012 Aug; 40(2):322–329. PubMed PMID: 22183485.
- Weigt SS, Abrazado M, Kleerup EC, Tashkin DP, Cooper CB. Time course and degree of hyperinflation with metronome-paced tachypnea in COPD patients. COPD 2008 Oct; 5(5):298–304. PubMed PMID: 18972278. Epub 2008/10/31. eng.
- Eshaghian P, Quinn M, Coskun F, Raine R, Bateman E, Cooper C. Metronome-paced tachypnea for measurement of dynamic hyperinflation in COPD patients and normal controls. Am J Respir Crit Care Med 2009; 179(1_MeetingAbstracts):A2904.
- O'Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J 2004; 24(1):86–94. PubMed PMID: 15293609.
- Peters MM, Webb KA, O'Donnell DE. Combined physiological effects of bronchodilators and hyperoxia on exertional dyspnoea in normoxic COPD. Thorax 2006 Jul; 61(7):559–567. PubMed PMID: 16467067. Pubmed Central PMCID: 2104668.
- Gelb AF, Gutierrez CA, Weisman IM, Newsom R, Taylor CF, Zamel N. Simplified detection of dynamic hyperinflation. Chest 2004 Dec; 126(6):1855–60. PubMed PMID: 15596684.
- Fujimoto K, Yoshiike F, Yasuo M, Kitaguchi Y, Urushihata K, Kubo K, Effects of bronchodilators on dynamic hyperinflation following hyperventilation in patients with COPD. Respirology 2007 Jan; 12(1):93–99. PubMed PMID: 17207032. Epub 2007/01/09. eng.
- ATS. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis 1991 Nov; 144(5):1202–1218. PubMed PMID: 1952453.
- ATS. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995 Sep;152(3-D):1107-36. PubMed PMID: 7663792.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Standardisation of spirometry. Eur Respir J 2005 Aug; 26(2):319–338. PubMed PMID: 16055882. Epub 2005/08/02. eng.
- Wilson RC, Jones PW. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnoea during exercise. Clin Sci 1989 Mar; 76(3):277–282. PubMed PMID: 2924519.
- Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol 1983 Nov; 55(5):1558–1564. PubMed PMID: 6643191.
- Lahaije AJ, Willems LM, van Hees HW, Dekhuijzen PN, van Helvoort HA, Heijdra YF. Diagnostic accuracy of metronome-paced tachypnea to detect dynamic hyperinflation. Clin Physiol Funct Imaging 2013 Jan; 33(1):62–69. PubMed PMID: 23216767.
- Cooper CB, Calligaro GL, Quinn MM, Eshaghian P, Coskun F, Abrazado M, Determinants of dynamic hyperinflation during metronome-paced tachypnea in COPD and normal subjects. Respir Physiol Neurobiol 2013 (In press).
- O'Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004 Jun; 23(6):832–840. PubMed PMID: 15218994.
- Gelb AF, Taylor CF, Cassino C, Shinar CM, Schein MJ, Zamel N. Tiotropium induced bronchodilation and protection from dynamic hyperinflation is independent of extent of emphysema in COPD. Pulm Pharmacol Ther 2009 Jun; 22(3):237–242. PubMed PMID: 19138754. Epub 2009/01/14. eng.
- Gelb AF, Taylor CF, McClean PA, Shinar CM, Rodrigues MT, Gutierrez CA, Tiotropium and simplified detection of dynamic hyperinflation. Chest 2007 Mar; 131(3):690–695. PubMed PMID: 17356081. Epub 2007/03/16. eng.
- Hannink J, Lahaije A, Bischoff E, van Helvoort H, Dekhuijzen R, Schermer T, Dynamic hyperinflation after metronome-paced hyperventilation in COPD—A 2 year follow-up. Respir Med 2010 May 7. PubMed PMID: 20452759. Epub 2010/05/11. Eng.