Abstract
Alpha-1-antitrypsin deficiency (AATD) is an inherited disorder responsible for early onset emphysema associated with a significant impairment of health-related quality of life (HRQoL). Our aim was to assess the usefulness of different instruments to evaluate the HRQoL in patients with AATD compared to non-AATD COPD. Observational, cross-sectional study in which all patients filled out a series of questionnaires: the COPD severity score (COPDSS), the EuroQoL 5-Dimensions (EQ-5D), the Living with COPD (LCOPD) and the COPD Assessment Test (CAT).
A total of 96 patients were included, 35 with AATD (mean age 56.5 yrs, 57.1% male and mean FEV1(%) 48.7% and 61 non-AATD COPD (70.3 yrs, 80.3% men and FEV1(%) 47%. The questionnaire scores were similar, with a tendency towards worse scores in AATD for the EQ-5D (VAS) (64.8 (20.2) vs. 71.6 (17.1); p = 0.08). The correlations between the different scores and FEV1(%) were significant in both groups for COPDSS and LCOPD, but not for CAT and EQ-5D. In general, the correlations of scores with FEV1(%) were stronger for AATD compared with non-AATD COPD patients: COPDSS r = −0.570, p < 0.01 for AATD and r = −0.260, p < 0.05 for COPD; LCOPD r = −0.502, p < 0.001 for AATD and r = −0.304, p < 0.05 for non-AATD COPD.
Patients with AATD have a similar degree of HRQoL impairment as older subjects with non-AATD COPD and showed a stronger correlation between HRQoL measurements and lung function impairment compared with non-AATD COPD. This may be related to the characteristics of the disease in these patients who are usually younger, with less co-morbidity and lower smoking consumption.
Introduction
Alpha-1-antitrypsin deficiency (AATD) is an autosomic codominant inherited disorder, characterized by a low serum concentration of alpha-1-antitrypsin, the most common antiprotease produced mainly by the liver and to a lesser extent by mononuclear phagocytes (Citation1). This protein is responsible for protecting the lung tissues against the potential destructive effect of neutrophil elastase, that can result in early onset panlobular emphysema (Citation2).The decline of lung function in patients with AATD may be accelerated by various risk factors, the most important being tobacco smoking (Citation3,4).
The impairment of airflow obstruction measured by the forced expiratory volume in one second (FEV1) is the most widely used marker of severity and prognosis in chronic obstructive pulmonary disease (COPD); however, the FEV1 alone is not enough to evaluate the impact of lung disease in patients with COPD (Citation5). The different questionnaires developed and validated to assess different aspects of health status in COPD provide more complete and complementary information on the consequences of the respiratory disease in the everyday life of patients with chronic respiratory disease (Citation6,7).
Many studies have been carried out on questionnaires assessing the impact of COPD on health status (Citation6,7). In contrast, the experience with HRQoL questionnaires in patients with emphysema due to AATD is more limited. In these studies, HRQoL was measured with the respiratory specific St. George's Respiratory Questionnaire (SGRQ) and the generic SF-36 questionnaire (Citation8–10). The introduction of new instruments which are shorter and more appropriate for routine clinical use require the evaluation of their performance in this particular phenotype of patients with COPD due to AATD. To our knowledge, there are no previous studies comparing the performance of these new questionnaires in patients with emphysema due to AATD and non-AATD COPD.
Therefore, we designed the current study to analyse the performance of a series of short questionnaires, developed and validated for use in patients with non-AATD COPD, in a group of patients with emphysema due to AATD.
Method
Study design
This was an observational and cross-sectional study, with the aim to evaluate the performance of a series of short severity and HRQoL questionnaires in a population of patients with emphysema due to AATD, and compare their scores and performance with a control group of non-AATD COPD.
Population
The study group included patients with a severe AATD characterized by low serum concentrations of AAT, below 50 mg/dl (less than 11 μM) and the PiZZ phenotype, and a diagnosis of COPD confirmed by spirometry (FEV1/FVC post-bronchodilatator of <70%).
The control group consisted of patients fulfilling the same criteria for COPD and being smokers or ex-smokers of at least 10 pack-years and with normal serum levels of AAT (non-AATD COPD). All patients were included in a stable condition, with no exacerbation or changes in baseline treatment over a period of at least 2 weeks prior to inclusion.
Exclusion criteria were an acute or recent exacerbation, diagnosis of asthma, pneumonia or dementia with serious mental illness that would prevent them from understanding and completing the questionnaires. The study protocol was approved by the Ethics Committee of the Hospital Clinic in Barcelona and all participants provided written informed consent.
Measurements
The sociodemographic characteristics obtained were: gender, age, BMI and civil status. Other variables were current and previous smoking history, date of diagnosis of COPD and the onset of respiratory symptoms, usual pharmacological treatment of COPD and the daily average physical activity in minutes walked per day self-reported by the patients.
Clinical characteristics were evaluated by pre- and post-bronchodilator spirometry, symptoms in the past month, number and severity of exacerbations during the last year. Dyspnoea was evaluated by the modified Medical Research Council (mMRC) scale of dyspnoea (Citation11).
Severity of COPD was evaluated by the COPD Severity Score (COPDSS) developed by Eisner et al. (Citation12), and translated and validated into Spanish (Citation13). This is a specific, simple, reliable and valid survey-based instrument to evaluate the severity of COPD beyond lung function. The questions include five overall aspects of COPD severity: respiratory symptoms, systemic corticosteroid use, other COPD medication use, previous hospitalisation or intubation for respiratory disease, and home oxygen use. Each item was assigned an a priori weight based on clinical aspects of the disease and its expected contribution to overall COPD severity. Missing values for medication use and other questions were defined as zero. The possible total score ranged from 0–35, with higher scores reflecting more severe COPD (Citation12,13).
The co-morbidities were evaluated by the Charlson Co-morbidity Index (CCI), a method for classifying co-morbid conditions that is considered to be reliable and able to provide a good correlation with mortality (Citation14).
The quality of life was evaluated by means of three questionnaires: a) the Spanish version of the EuroQol five-dimension questionnaire (EQ-5D), a generic, health-related quality of life questionnaire, that contains two sections, a descriptive and a valuation section (Citation15,16).The descriptive section consists of 5 questions about mobility, self-care, daily activities, pain/discomfort, and anxiety/depression. In the second section, patients are asked to assess their overall health status on a visual analogue scale (VAS). This VAS is a simple rating scale ranging from 0 (defined as the worst imaginable health state) to 100 (defined as the best imaginable health state).The data may be used to represent a profile of health status or converted into a single summary index (EQ-5D index) by applying scores from an evaluation set (Citation15); b) The Living with COPD Questionnaire (LCOPD) examines the everyday impact of living with COPD; it asserts that the quality of life is dependent on an individual's ability to fulfill his or her fundamental daily needs (Citation17). It consists of 22 items with 2 response options: true or not true (true scored 1; not true scored 0) with higher scores representing greater impairment of QoL; c) The COPD assessment test (CAT) was used to assess and quantify the symptoms and impact of COPD (Citation18). It consists of eight questions with each question scored from 0 to 5 giving a total score range from 0 to 40, where the worst condition is 40 (Citation18).
All the questionnaires were administered by the investigators in a face-to-face interview during the clinical visits.
Statistical analysis
We show n (%) for categorical variables and median (IQR) for continuous variables with non-normal distribution or mean (SD) for those with normal distribution. We compared categorical variables with the chi-square test or Fisher's exact test. Continuous variables were compared using the Student's t-test or the non-parametric Mann-Whitney U test. Linear regression analyses were performed to determine the relationship between post-bronchodilator FEV1 (% predicted) and the HRQoL questionnaires (LCOPD, CAT, EQ-5D global score). We investigated the association among LCOPD, CAT and EQ-5D global (as dependent variables) and age (years), sex, pack-years, post-bronchodilator FEV1(%) and Charlson co-morbidity index using multiple linear regression analysis with a stepwise backward model (pin < 0.10; pout < 0.05), respectively. The level of significance was set at 0.05 (two-tailed). All analyses were performed with IBM SPSS Statistics 18.0 (Armonk, New York, USA).
Results
Patient characteristics
A total of 96 patients with chronic airflow obstruction were included of which 35 were AATD patients (PI*ZZ) and 61 non-AATD COPD. There were significant differences in the demographic and clinical characteristics between the groups; patients in the non-AATD COPD group were more frequently male (80.3% versus 57.1%, p < 0.01), older (70.3 years (SD = 9.2) versus 56.5 years (SD = 10.6); p < 0.001) and with a higher smoking consumption (56.3 pack-years (SD = 43) versus 18.4 pack-years (SD = 15.8); p < 0.001). Patients in the AATD group had a younger age at onset of respiratory symptoms (42 years (SD = 11) versus 60 years (Citation13), p < 0.01), had fewer co-morbidities with a mean Charlson index of 0.4 (interquartile range (IQR) 0-1) compared with 1.3 (IQR: 0-2) (p < 0.001) and a lower BMI (24.5 (SD = 4) versus 27.4 (4.7), p < 0.001). There were no significant differences in lung function parameters, prevalence of respiratory symptoms, frequency of exacerbations and patterns of treatment, except for augmentation therapy with AAT that was only prescribed to 62.9% of patients in the AATD group ().
Table 1. Demographic and clinic characteristics of the study population
The characteristics of both groups of patients are presented in and a detailed distribution of co-morbidities included in the Charlson index in both groups of patients is presented in .
Table 2. Co-morbidities included in the Charlson index in both groups of patients with COPD with and without AATD (values in%)
Evaluation of health-related quality of life and severity questionnaires
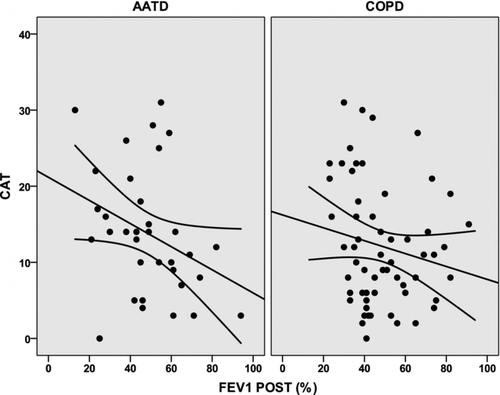
The scores of the COPDSS, LCOPD and CAT questionnaires were not significantly different for the two groups ().There was only a trend towards a better score in the EQ-5D VAS in the non-AATD COPD group (71.6 (SD = 17.1) versus 64.8 (SD = 20.2), p = 0.08).
Table 3. Scores of the different HRQoL and severity questionnaires in patients with AATD and non-AATD COPD
Correlations between post-bronchodilator FEV1(%) and questionnaire scores
In general, there was a stronger correlation between post-bronchodilator FEV1(%) and COPDSS and HRQoL questionnaire scores in the AATD group compared with the non-AATD COPD group. In the AATD group there was a significant correlation between FEV1(%) and COPSS, LCOPD and the EQ-5D VAS (r values of −0.57, −0.502 and 0.562 respectively) at a level of p < 0.001. In contrast, in the non-AATD COPD group a significant correlation was only found between FEV1(%) and the LCOPD (r = −0.324; p < 0.05). The correlations between the scores of the different questionnaires were, in general, also stronger in the AATD group ().
Table 4. Analysis of the correlation between lung function (FEV1% post-bronchodilator) and the scores of the different questionnaires
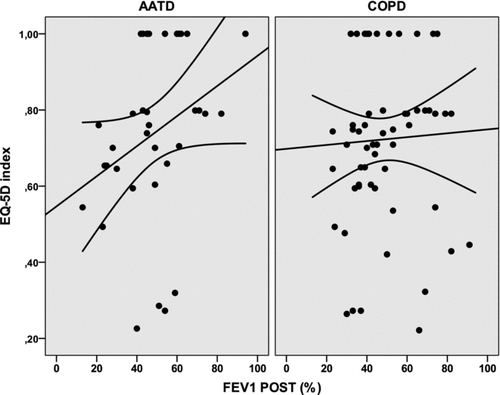
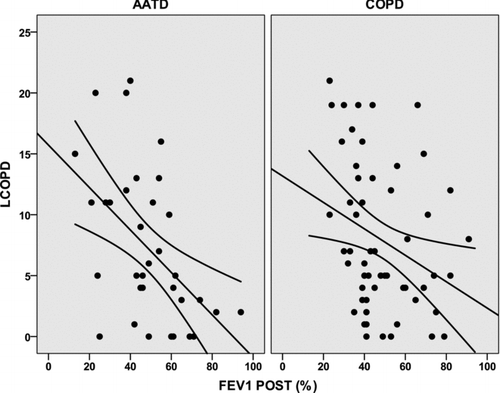
The relationship between post-bronchodilator FEV1(%) and scores of the HRQoL questionnaires was further analysed by linear regression analysis. Only the LCOPD had a significant relationship with FEV1(%) in both groups of patients, being stronger in the AATD group (r2 = 0.252; p = 0.002 compared with r2 = 0.092; p = 0.017) (). The relationships between FEV1(%) and CAT and global EQ-5D scores approached statistical significance (r2 = 0.108; p = 0.054 and r2 = 0.098; p = 0.067, respectively) and were weaker and non-significant for the non-AATD COPD group ( and ).
Figure 1. Relationship between post-bronchodilator FEV1(%) and scores of LCOPD in AATD and non-AATD COPD patients. Results of simple regression model. For AATD r2 = 0.252 (p = 0.002), for non-AATD COPD r2 = 0.092 (p = 0.017).
Multiple regression analysis of factors associated with the scores of the questionnaires
The results of multiple regression analysis in the AATD group demonstrated a significant association of the LCOPD score and the post-bronchodilator FEV1(%), and a significant association of the CAT and global EQ-5D scores with the pack-years of smoking (). Regarding the non-AATD COPD, only the LCOPD scores were significantly associated with the Charlson co-morbidity index ().
Table 5. Multivariate model of the variables in both groups
Discussion
The results of our study have shown the differences in the demographic and clinical characteristics existing between the non-AATD COPD and emphysema due to AATD. Patients with the deficiency were younger, with a lower smoking consumption and with fewer co-morbidities, despite a similar severity of impairment in lung function. Regarding HRQoL, the scores obtained in both groups of COPD patients were not significantly different, but correlations between FEV1(% predicted) and the scores of the different questionnaires were stronger in the AATD group. In multiple regression analysis, the scores of LCOPD in patients with AATD were significantly associated with the FEV1(%), and the scores of CAT and EQ-5D with the number of pack-years. In contrast, in the group of non-AATD COPD only the LCOPD scores were significantly associated with the Charlson co-morbidity index.
The differences found in the demographic and clinical characteristics of patients with non-AATD COPD or with AATD were those already described in previous studies (Citation19,20) and corresponded, in general, to a more severe impairment in lung function or a similar impairment but at a younger age and with lower exposure to causative factors such as smoking. More interestingly and unique to our study is the comparison of the performance of the different severity and HRQoL questionnaires in both groups of patients with COPD. The first observation is that global scores were not significantly different between groups, and despite the differences in demographic and clinical characteristics; there was only a trend towards a worse score of the visual-analogue scale of the EQ-5D in patients with AATD.
The evaluation of HRQoL using standardised and validated questionnaires is a frequent outcome in COPD (Citation6,7); however, less information is available in patients with emphysema due to AATD. The existing studies have used the specific St George's Respiratory Questionnaire (SGRQ) and the generic SF-36. Both are amongst the most widely used HRQoL questionnaires in COPD (Citation21,22). Interestingly, the results observed in the previously mentioned studies in individuals with COPD associated with AATD were similar to those obtained in previous studies in non-AATD COPD. The scores of the SGRQ were worse in patients experiencing frequent exacerbations, either while on augmentation therapy (Citation23) or not (Citation24).
The scores of the SGRQ were also worse in patients with more severe impairment in lung function, as observed in non-AATD COPD (Citation24), and more interestingly, the changes in SGRQ scores correlated with changes in lung density over time (Citation25), suggesting that monitoring health status would be a good way of monitoring the progression of the disease in patients with emphysema due to AATD. Our results support this observation, demonstrating a better correlation of HRQoL scores with lung physiology in patients with AATD compared with non-AATD COPD.
Dowson et al. (Citation10) also observed that SGRQ scores in patients with AATD were significantly associated with the impairment in FEV1, in addition to the number of exacerbations and the severity of basal emphysema measured by the voxel index of the lower pulmonary lobes. The extent of emphysema in the lower lobes in AATD was also correlated to the decrease in exercise capacity and both variables were significantly related to the impairment in the SGRQ scores in another study from the same group (Citation26).
A recent study has compared the HRQoL of a group of patients with COPD due to AATD with a group of non-AATD COPD using the SGRQ. The results of this study showed that patients with AATD had, on average, a total score 4.75 points higher (worse) than individuals with non-AATD COPD when demographic and health characteristics were adjusted for (Citation27). All these studies highlight the importance of the evaluation of the HRQoL in individuals with emphysema due to AATD.
The most widely used questionnaires, SGRQ and SF-36, are long and time consuming (Citation21,22). The current tendency is to replace them with shorter questionnaires that can provide the same information and can be used in routine clinical practice, such as the CAT, LCOPD or EQ-5D (Citation28). In the current study we compared the performance of these questionnaires in two groups of patients with and without AATD, but no direct comparison was performed with the SGRQ of SF-36.
In a recent study, published as an abstract, Edgar et al. (Citation29) found an excellent correlation between the scores of SGRQ and CAT in a group of 157 patients with AATD. The CAT is a short, self-administered questionnaire that can be completed in only 2 minutes and has good measurement properties, is sensitive to differences in state and provide a valid, reliable and standardised measure of COPD health status (Citation18,Citation30). Our results have demonstrated a better correlation of the CAT scores with scores of other health status measurements such as the LCOPD and EQ-5D in patients with AATD compared with non-AATD COPD.
Furthermore, correlations of CAT scores with other measures of severity of COPD such as the COPDSS and the FEV1(%) were also stronger in AATD patients compared with non-AATD COPD. In simple regression analysis, the variation in FEV1(%) explained 11% of the variation of CAT scores in AATD patients (p = 0.054), but these variables were not significantly associated in non-AATD COPD. On multivariate analysis, only the number of pack-years was significantly associated with the CAT scores in AATD, but none of the variables analysed was significantly and independently associated with changes in CAT scores in non-AATD COPD.
These differences may be related to the characteristics of COPD in AATD patients, in whom the impairment in health status may be more closely related to the severity of impairment of the lung physiology and less influenced by other aspects such as co-morbidities, aging or others. In a large study, Pillai et al. (Citation31) found that CAT score 10 was on average equivalent to mMRC scale 0 and CAT 15 to mMRC scale 1 in patients with AATD; which is relevant for the new GOLD classification of patients with COPD. In contrast, other guidelines propose the use of changes in CAT scores to modulate the intensity of treatment but not to classify individuals in different categories (Citation32).
The LCOPD is a short, self-administered, specific HRQoL questionnaire that has demonstrated good measurement properties in patients with COPD (Citation17,Citation33,Citation34). Similar to the results observed with the CAT, we observed that the correlation of LCOPD scores with FEV1(%) in AATD patients was stronger than in non-AATD COPD patients. On simple regression analysis, the variation in FEV1(%) explained 25% of the variation in LCOPD scores (p = 0.002), compared with only 9.2% in COPD (p = 0.017). On multivariate analysis, the FEV1(%) was the only variable significantly related to the LCOPD scores in AATD patients, whereas the Charlson co-morbidity index was significantly related to LCOPD in non-AATD COPD patients. These results again underscore the stronger relationship between HRQoL and lung physiology in AATD patients compared with non-AATD COPD, in which co-morbidities may play a major role. Similar results were found for the generic questionnaire EQ-5D with a stronger correlation of the scores and other measures of lung impairment in AATD patients compared with non-AATD COPD.
The COPDSS is a comprehensive measure of COPD severity that was developed and validated by Eisner and co-workers (Citation12) and its validity was demonstrated in a population of 383 U.S. adults with self-reported physician-diagnosed COPD. The Spanish version of the questionnaire was validated by our group in a population of 827 patients with spirometry-diagnosed COPD (Citation13). In a subsequent study Eisner et al. (Citation35) prospectively demonstrated that higher COPDSS was associated with a greater risk of acute exacerbation of COPD requiring emergency room visit, hospitalisation or either indicator of hospital-based care for COPD, and our group extended these observations by demonstrating that evaluation of COPDSS at the onset of an exacerbation was significantly related to the clinical success of the treatment and was the best independent predictor of outcome (Citation36). In accordance with the results obtained with the HRQoL questionnaires, we found a stronger correlation of the COPDSS with FEV1(%) and HRQoL scores in the AATD population compared with the patients with non-AATD COPD.
In summary, our results indicate that the questionnaires evaluated are useful to monitor the severity and impairment in health status in patients with emphysema due to AATD. The scores of these questionnaires are more closely related to the usual measures of severity of lung function impairment in patients with AATD compared with individuals with non-AATD COPD. This probably reflects the particular characteristics of the disease in carriers of this deficiency, who are typically younger, with fewer co-morbidities and lower smoking consumption.
Acknowledgments
The authors wish to thank Albert Gabarrús (IDIBAPS. Hospital Clínic, Barcelona) for his support in the statistical analysis.
Grifols has provided support through an unrestricted grant for the development of the Catalan Center of Excellence for AATD. Sandra Manca from the Institute of Respiratory Diseases, Sassari University (Italy) has been a recipient of an Erasmus Placement fellowship.
Declaration of Interest Statement
Marc Miravitlles has received speaker fees from Boehringer Ingelheim, Pfizer, AstraZeneca, Bayer Schering, Novartis, Talecris-Grifols, Takeda-Nycomed, Merck, Sharp & Dohme and Novartis, and consulting fees from Boehringer Ingelheim, Pfizer, GSK, AstraZeneca, Bayer Schering, Novartis, Almirall, Merck, Sharp & Dohme, Talecris-Grifols and Takeda-Nycomed. The other authors have no conflict of interest to disclosure related to this article. The authors are responsible for the content and writing of this paper.
References
- Luisetti M, Seersholm N. α1-Antitrypsin deficiency·1: Epidemiology of α1-antitrypsin deficiency. Thorax 2004; 59: 164–169.
- Miravitlles M. Alpha-1-antitrypsin and other proteinase inhibitors. Curr Opin Pharmacol 2012; 12: 309–314.
- Piitulainen E, Eriksson S. Decline in FEV1 related to smoking status in individuals with severe a1-antitrypsin deficiency (PiZZ). Eur Respir J 1999; 13: 247–251.
- Tirado-Conde G, Lara B, Casas F, Blanco I, Bustamante A, Cadenas S, Factors associated with the evolution of lung function in patients with alpha-1 antitrypsin deficiency in the Spanish Registry. Arch Bronconeumol 2011; 47: 495–503.
- Jones PW, Donohue JF, Nedelman J, Pascoe S, Pinault G, Lassen C. Correlating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysis. Respir Res 2011; 12: 161.
- Renwick DS, Connolly MJ. Impact of obstructive airways disease on quality of life in older adults. Thorax 1996; 51: 520–525.
- Ferrer M, Alonso J, Morera J, Marrades RM, Khalaf A, Aguar C, Chronic obstructive pulmonary disease stage and health-related quality of life. Ann Intern Med 1997; 127: 1072–1079.
- Campos MA, Alazemi S, Zhang G, Salathe M, Wanner A, Sandhaus RA, Clinical characteristics of subjects with symptoms of alpha1-antitrypsin deficiency older than 60 years. Chest. 2009; 135: 600–608.
- Dowson LJ, Guest PJ, Stockley RA. Longitudinal changes in physiological, radiological, and health status measurements in alpha-1 antitrypsin deficiency and factors associated with decline. Am J Respir Crit Care Med 2001; 164: 1805–1809.
- Dowson LJ, Guest PJ, Stockley RA. The relationship of chronic sputum expectoration to physiologic, radiologic, and health status characteristics in alpha-1 antitrypsin deficiency (PiZ). Chest 2002; 122: 1247–1255.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54: 581–586.
- Eisner MD, Trupin L, Katz PP, Yelin EH, Earnest G, Balmes J, Development and validation of a survey-based COPD Severity Score. Chest 2005; 127: 1890–1897.
- Miravitlles M, Llor C, de Castellar R, Izquierdo I, Baró E, Donado E. Validation of the COPD severity score for its use in Primary Care. The NEREA study. Eur Respir J 2009; 33: 519–527.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383.
- EuroQoL Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208.
- Badia X, Roset M, Montserrat S, Herdman M, Segura A. The Spanish version of EuroQol: a description and its applications. European quality of life scale. Med Clin (Barc) 1999; 112(Suppl 1):e79–e85.
- McKenna SP, Meads DM, Doward LC, Twiss J, Pokrzywinski R, Revicki D, Hunter CJ, Glendenning GA. Development and validation of the living with chronic obstructive pulmonary disease questionnaire. Qual Life Res 2011; 20: 1043–1052.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Leidy KN. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34: 648–654.
- Silverman EK, Pierce JA, Province MA, Rao DC, Campbell EJ. Variability of pulmonary function in alpha-1 antitrypsin deficiency: clinical correlates. Ann Intern Med 1989; 111: 982–991.
- Piras B, Ferrarotti I, Lara B, Martinez MT, Bustamante A, Ottaviani S, Characteristics of Italian and Spanish patients with alpha-1-antitrypsin deficiency: Identifying clinical phenotypes. Eur Respir J 2013; 42: 54–64.
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991; 85(Suppl B): 25–31.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483.
- Campos MA, Alazemi S, Zhang G, Wanner A, Salathe M, Baier H, Exacerbations in subjects with alpha-1 antitrypsin deficiency receiving augmentation therapy. Respir Med 2009; 103: 1532–1539.
- Needham M, Stockley RA. Exacerbations in alpha-1 antitrypsin deficiency. Eur Respir J 2005; 25: 992–1000.
- Stolk J, Ng WH, Bakker ME, Reiber JHC, Rabe KF, Putter H, Stoel BC. Correlation between anual change in health status and computer tomography derived lung density in subjects with alpha-1 antitrypsin deficiency. Thorax 2003; 58: 1027–1030.
- Dowson LJ, Newall C, Guest PJ, Hill SL, Stockley RA. Exercise capacity predicts health status in alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2001; 163: 936–941.
- Holm KE, Borson S, Sandhaus RA, Ford DW, Strange C, Bowler RP, Differences in adjustement between individuals with alpha-1 antitrypsin deficiency (AATD)-associated COPD and non-AATD COPD. COPD 2013; 10: 226–234.
- Jones P, Miravitlles M, van der Molen T, Kulich K. Beyond FEV1 in COPD—A review of patient-reported outcomes and their measurement using a new generation of instruments. Int J Chron Obst Pulm Dis 2012; 7: 697–709.
- Edgar RG, Griffiths D, Stockley RA. Validations of the COPD Assessment Test (CAT) whithin alpha-1 antitrypsin deficiency (AATD). Thorax 2010; 65: A141.
- Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J 2011; 38: 29–35.
- Pillai AP, Turner AM, Stockley RA. Global initiative for chronic obstructive lung disease 2011 symptom/risk assessment in α1-antitrypsin deficiency. Chest 2013; 144: 1152–1162.
- Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA Spanish COPD guidelines (GesEPOC). Pharmacological treatment of stable COPD. Arch Bronconeumol 2012; 48: 247–257.
- Miravitlles M, Iriberri M, Barrueco M, Lleonart M, Villarrubia E, Galera J. Usefulness of the LCOPD, CAFS, and CASIS scales in understanding the impact of COPD on patients. Respiration 2013; 86: 190–200.
- McKenna SP, Twiss J, Crawford SR, Oprandi NC, Tammaru M, Miravitlles M. Adapting the Living with Chronic Obstructive Pulmonary Disease (LCOPD) for use in Southern European (Italian and Spanish) and Eastern European (Russian) cultures. J Clin Epidemiol 2012; 65: 906–914.
- Eisner MD, Omachi TA, Katz PP, Yelin EH, Iribarren C, Blanc PD. Measurement of COPD severity using a survey-based score: validation in a clinically and physiologically characterized cohort. Chest 2010; 137: 846–851.
- Miravitlles M, Izquierdo I, Herrejon A, Torres VJ, Baró E, Borja J. On behalf of the ESFERA investigators COPD severity score as a predictor of failure in exacerbations of COPD. The ESFERA study. Respir Med 2011; 105: 740–747.