Abstract
Background: COPD is associated to increased fatigue, decreased health status and mortality. However, these relationships are rarely evaluated in population-based studies. Aims: To describe the relationship between health status, respiratory symptoms and fatigue among subjects with and without COPD. Further, to evaluate whether fatigue and/or health status predicts mortality in these groups. Methods: Data were collected in 2007 from the population-based OLIN COPD study. Subjects participated in lung function tests and structured interviews, and 434 subjects with and 655 subjects without COPD were identified. Fatigue was assessed by FACIT-Fatigue and health status by the generic SF-36 questionnaire including physical (PCS) and mental (MCS) components. Mortality data until February 2012 were collected. Results: Fatigue greatly impacts the physical and mental dimensions of health status, both among subjects with and without COPD. Among subjects with clinically significant fatigue, COPD subjects had significantly lower PCS-scores compared to non-COPD subjects. Fairly strong correlations were found between FACIT-F, SF-36 PCS and MCS, respectively. In multivariate models adjusting for covariates, increased fatigue, decreased physical and mental dimensions of health status were all associated to mortality in subjects with COPD (OR 1.06, CI 1.02–1.10, OR 1.04, CI 1.01–1.08 and OR 1.06, CI 1.02–1.10), but not in non-COPD. Conclusions: Fatigue and decreased health status were closely related among subjects with and without COPD. Not only physical health status, but also fatigue and mental health predicted mortality among subjects with COPD. Fatigue assessed by FACIT-F, can be a useful instrument of prognostic value in the care of subjects with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a growing public health disease that has a considerable impact on health status and mortality (Citation1). Besides respiratory symptoms (Citation2) also fatigue (Citation3) is common among subjects with COPD. Respiratory symptoms contribute to a reduced health status regardless of COPD diagnosis (Citation4), but the presence of respiratory symptoms in mild COPD may be a predictor for poor long-term outcomes, such as rapid decline in FEV1 and decreased health status (Citation5). Among COPD subjects with respiratory symptoms, fatigue has been shown to be present already in mild COPD (Citation6, 7), and increased fatigue may be associated with impaired health status among subjects with moderate to severe COPD (Citation8–10).
Mortality is increased among subjects with COPD compared to the general population (Citation11), and cardiovascular co-morbidities (Citation12, 13) contribute to the increased mortality. Concomitant heart disease in COPD has also been shown to be associated with a decreased health status (Citation14) and increased fatigue (Citation7). In studies including outpatients and mainly men with COPD, dyspnea (Citation15) as well as decreased health status (Citation16) predicted mortality. Also in a primary health-care based study including both sexes, health status assessed by a disease specific questionnaire predicted mortality among subjects with COPD (Citation17).
As described above, fatigue has been associated with decreased health status in subjects with COPD (Citation8–10) but all studies are based on highly selected study populations recruited from health care. There is a lack of population-based data on the relationship between health status and mortality, and fatigue has never been evaluated in this context. The well-known under-diagnosis of COPD (Citation18, 19) has to be taken into account when interpreting results from register-based studies, while population-based studies can contribute with representative data including all severity grades of COPD. The aim of this population-based study was to describe the relationship between health status, respiratory symptoms and fatigue among subjects with and without COPD. Further, to evaluate whether fatigue and/or health status predicts mortality in these groups.
Methods
Study population
Between 2002 and 2004, nearly 4200 subjects from four adult cohorts within the Obstructive Lung Disease in Northern Sweden (OLIN) studies participated in clinical re-examinations. Based on these examinations, all subjects with COPD (n = 993), and the same number of age-and sex-matched subjects without obstructive lung function impairment formed the OLIN COPD study. The study population (n = 1986) has been invited to annual examinations since 2005 with a basic programme including a structured interview and spirometry with reversibility testing (Citation20).
Data collected in 2007 was used in the present study, where in addition to the basic program, questionnaires for assessing fatigue and health status were distributed. From the original study population (n = 1986), 143 were deceased, 180 declined to participate and 277 were not able to attend to the examination. Of the 1386 subjects who participated in the clinical examinations, 1089 subjects had a complete response to the health status questionnaire, and they constituted the study population. Mortality data was collected from the Swedish national mortality register beginning on the date of examination in 2007 until February 2012. Information regarding the cause of death was not available. The Regional Ethical Review Board at Umeå University, Sweden, approved the study.
Questionnaires
The structured interview questionnaire includes well-validated questions about respiratory symptoms, smoking habits and co-morbidities (Citation7, Citation20). The modified Medical Research Council (mMRC)- dyspnea scale was used for assessing dyspnea, and a higher score indicate more dyspnea (0 to 4) (Citation21). The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) is a self-completed symptom-specific questionnaire that measures subjective fatigue. FACIT-F was designed for chronic diseases (Citation22, 23) and has previously been used (Citation6, Citation24) and validated (Citation25) among subjects with COPD. The FACIT-F questionnaire contains 13 items measuring the intensity of fatigue and its impact on daily life during the last 7 days. A higher score indicate less fatigue (0–52) (Citation22).
The short form 36 (SF-36) is a generic standardised instrument for self-rated health status that provides insight into individuals’ conditions and limitations in daily life in the prior four weeks. The Swedish version of the SF-36 has high validity and reliability (Citation26). The instrument contains 36 items divided into eight domains, Physical Functioning (PF), Roll-Physical (RP), Bodily Pain (BP), General Health (GH), Vitality (VT), Social Functioning (SF), Role-Emotional (RE) and Mental Health (MH). Two summary scores can be combined, the Physical Component Summary (PCS), which includes PF, RP, BP, and GH and the Mental Component Summary (MCS), which includes VT, SF, RE and MH. A higher score indicates better health status (0–100). In accordance with the SF-36 manual, the scores were transformed into a mean score at 50, with a standard deviation of 10 (Citation27).
Spirometric classification
Lung function was measured according to the ATS-standards (Citation28) using the Dutch Mijnhardt Vicatest 5 dry spirometer. Swedish reference values were applied for FEV1 (Citation29). Definition of COPD and classification disease severity followed the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (Citation1); COPD: FEV1/best of FVC or VC < 0.7; GOLD grade I (mild): FEV1 ≥ 80% predicted; GOLD grade II (moderate): 50% ≤ FEV1 < 80% predicted; GOLD grade III (severe): 30% ≤ FEV1 < 50% predicted and GOLD grade IV (very severe): FEV1 < 30% predicted. Non-COPD was classified as FEV1/best of FVC or VC ≥ 0.7. The highest values pre- or post-bronchodilator were used.
Definitions
Smoking habits were defined as non-smokers, ex-smokers and current smokers. Respiratory symptoms were defined as a self-reported history of at least one of the following: mMRC-dyspnea ≥2, chronic cough, chronic productive cough or recurrent wheeze. Clinically significant fatigue was defined as a FACIT-F questionnaire score of ≤43, based on the criteria previously used in population-based studies (Citation7, Citation23). Subjects with COPD were also classified by presence of respiratory symptoms and clinically significant fatigue. Heart disease was defined as a self-reported history of at least one of the following: angina pectoris, myocardial infarction, cardiac insufficiency, coronary artery bypass or receiving a Percutaneous Coronary Intervention procedure.
Statistical analyses
Statistical Package for the PASW Statistics (version 20.0; SPSS Inc., Chicago IL, USA) was used for the statistical analyses. GOLD grade III and IV were grouped together because of the limited number of cases. Univariate comparisons were assessed using Chi-square tests and Independent sample t-test. Because of a skewed distribution of the SF-36 scores, Mann–Whitney and Kruskal–Wallis tests were used to test for differences between the groups. When significant differences were found by Kruskal–Wallis test, post hoc analysis was carried out using Mann–Whitney tests with Bonferroni correction. Due to the non-normal distribution of the SF-36 scores, median values are presented. Spearman's rho was used to examine the degree of correlation between SF-36 and FACIT-F. A p value < 0.05 was considered statistically significant for all tests. Odds ratios (OR) with 95% confidence intervals (CI) were calculated using multiple logistic regression analysis. Mortality was used as a dependent variable, and the independent variables were sex, age, BMI, heart disease, FACIT-F score (Model A), SF-36 PCS score (Model B) and SF-36 MCS score (Model C). Smoking habits and FEV1 were also added to the models as covariates.
Results
Table shows the characteristics of the study population. There were no significant differences regarding the prevalence of clinically significant fatigue, heart disease or mortality when comparing subjects with and without COPD. Comparisons between the study population that completed SF-36 in 2007 (n = 1089) and those not participating and/or answering the SF-36 (n = 897) showed that the non-participants were significantly older (72.0 vs. 64.0, p < 0.001), but there were no differences regarding sex or presence of COPD.
Table 1. Study population characteristics (n = 1089)
SF-36 Physical Component Summary Score (PCS)
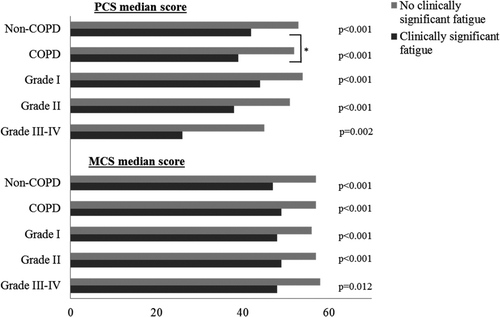
Subjects in the groups COPD, COPD with respiratory symptoms and COPD with clinically significant fatigue all had significantly lower median PCS scores compared to subjects without COPD (49.2, 46.9, and 39.1 vs. 50.1, p = 0.002, p < 0.001, p < 0.001). The PCS scores decreased by disease severity, and in all groups GOLD ≥ II had significantly lower scores compared to non-COPD (47.9, 46.9, 37.8 vs. 50.1, all p < 0.001). Among subjects with clinically significant fatigue, also GOLD I had significantly lower PCS scores than subjects without COPD (43.5 vs. 50.1, p < 0.001) (Table ).
Table 2. SF-36 Physical (PCS) and Mental (MCS) component summary scores in non-COPD, COPD and by severity of COPD (grade I, II and III-IV). COPD subjects are subdivided into; spirometric classified COPD, COPD with reported respiratory symptoms and COPD with reported clinically significant fatigue, respectively
In non-COPD and in all severity grades of COPD, subjects with respiratory symptoms (Figure ) respectively clinically significant fatigue (Figure ) had significantly lower PCS scores compared to asymptomatic subjects. Among subjects with clinically significant fatigue, those with COPD had significantly lower PCS scores than those without COPD (39.0 vs. 42.0, p = 0.027) (Figure ). In non-COPD subjects, women had significantly lower PCS scores than men, while there was no sex differences observed among subjects with COPD (data not shown). Among subjects with COPD, there were significant differences when comparing PCS scores by smoking habits; non-smokers 51.7, ex-smoker 48.1, respectively current smoker 48.2 (p = 0.023), while there were no such differences among non-COPD subjects.
Figure 1. Median SF-36 physical (PCS) and mental (MCS) component summary scores, comparing subjects without and with respiratory symptoms in the groups non-COPD, COPD and by GOLD grades I and II. GOLD, the Global Initiative for Chronic Obstructive Lung Disease.
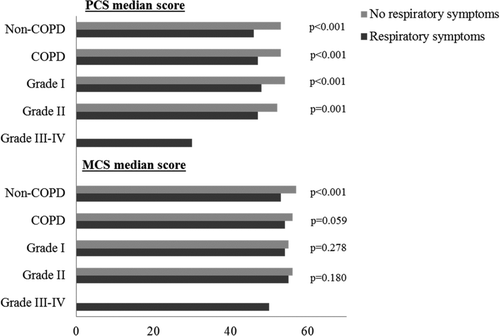
Figure 2. Median SF-36 physical (PCS) and mental (MCS) component summary scores, comparing subjects without and with clinically significant fatigue in the groups non-COPD, COPD and by GOLD grades I, II and III-IV. *p Value for comparing non-COPD and COPD, p = 0.027. GOLD, the Global Initiative for Chronic Obstructive Lung Disease.
SF-36 Mental Component Summary Score (MCS)
COPD subjects with clinically significant fatigue had significantly lower median MCS scores than non-COPD subjects (48.5 vs. 54.9, p < 0.001). The MCS scores did not decrease by disease severity (Table ). Among subjects without COPD, those with respiratory symptoms had lower MCS scores compared to those without symptoms (Figure ). In non-COPD and in all severity grades of COPD, subjects with clinically significant fatigue had significantly lower MCS scores compared to those without fatigue (Figure ). There were no significant differences when comparing MCS scores by smoking habits neither among subjects with COPD (non-smokers 55.6, ex-smoker 54.3 respectively current smoker 53.3, p = 0.088), nor without COPD (non-smokers 55.1, ex-smoker 54.4 respectively current smoker 54.9, p = 0.206). Nor could significant sex differences be detected.
Correlations between FACIT-F and SF-36
Among subjects with respiratory symptoms fairly strong correlations were found between FACIT-F and SF-36 PCS (Spearman's rho 0.56–0.65) as well as SF-36 MCS (Spearmans's rho 0.48-0.72). Analysis stratified by the SF-36 domains (Physical Functioning, Roll-Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role-Emotional and Mental Health), revealed that all domains correlated fairly strong to FACIT-F, and Vitality had the strongest correlations out of all eight domains (Spearmans's rho 0.68–0.93) (Table ).
Table 3. Spearmans’ rho correlations between FACIT-Fatigue scores and SF-36 physical (PCS), mental (MCS) component summary scores, and the MCS-domain Vitality (VT), respectively in non-COPD, COPD, and severity of COPD
Fatigue and health status in relation to mortality
In multiple logistic regression analyses, adjusting for confounders, FACIT-F, SF-36 PCS and MCS scores were each analysed as risk factors for mortality (model A-C) among subjects with and without COPD (Table ). Among subjects with COPD, lower scores in FACIT-F, SF-36 PCS and MCS each, was a significant risk factor for mortality as shown in the models A-C (OR 1.06, CI 1.02-1.10, OR 1.04, CI 1.01-1.08 and OR 1.06, CI 1.02-1.10). Neither, FACIT-F, SF-36 PCS, nor MCS were associated with mortality among subjects without COPD. Adding the variables FEV1 and smoking habits to the models yielded similar results.
Table 4. Multiple logistic regression model analysing risk factors for mortality in separate models for FACIT-Fatigue, SF-36 physical (PCS) and mental (MCS) component summary scores (Models A–C, respectively), adjusting for sex, age, BMI and heart disease, expressed as Odds Ratio (OR) with 95% confidence interval (CI)
Discussion
In this population-based study, fatigue had a great impact on physical as well as mental health status both among subjects with and without COPD. Among subjects with clinically significant fatigue, those with COPD had significantly lower physical health status compared to those without COPD. Increased fatigue, decreased physical and mental health status, each marked an increased risk for death among subjects with, but not among subjects without COPD.
In accordance with earlier findings, we found that health status was associated to presence of respiratory symptoms and not merely fulfilling the spirometric criteria of COPD (Citation4, Citation30). According to a recent publication there is an association between breathlessness and muscle weakness independent of obstructive lung function impairment, and this relationship was stronger among physical inactive subjects (Citation31). However, fatigue increase by COPD severity (Citation7), and in our study physical, but not mental health status deteriorated by disease severity. Similar relationships between disease severity and physical, but not mental health status assessed by SF-36 have been reported from another population-based study (Citation32). As demonstrated among patients with Rheumatoid Arthritis (Citation33), we also found fairly strong correlations between FACIT-F and SF-36, indicating that fatigue is a multidimensional symptom including both physical and mental aspects of health status. Thus, not only respiratory symptoms but also fatigue seems to be of importance when evaluating health status among subjects with COPD.
In a primary care study of older patients with different diagnoses, the simple question ‘do you feel tired all the time?’ could identify patents with a higher risk of death (Citation34). We found that fatigue assessed by FACIT-F as well as physical and mental health status assessed by SF-36 were associated with mortality among subjects with COPD, independent of age and other confounders. In a previous study of male COPD-patients, the SF-36 physical, but not the mental dimension of health status was associated with mortality (Citation16); however, in another study of mainly male COPD-patients health status assessed by SF-36 was not associated with mortality (Citation15).
In population-based studies as ours, the results are not distorted by the known under-diagnosis of COPD, which may explain the observed differences when compared to the referred register-based studies. FACIT-F has, as far as we known, never been evaluated in this context, and there are no previous reports that SF-36 mental score is associated to mortality. However, both instruments seem to be of prognostic importance when applied in a population-based COPD-cohort. Also disease specific questionnaires such as the St. George's Respiratory Questionnaire (SGRQ) (Citation16) and the Clinical COPD Questionnaire (CCQ) (Citation17), as well as complex measures like the Dyspnea, Obstruction, Smoking and Exacerbation (DOSE) index (Citation35) have demonstrated prognostic significance and associations with mortality in COPD. The COPD Assessment Test (CAT) could be an easy-to-use instrument in the clinic, including only eight questions comprising both respiratory symptoms and energy. CAT correlates strongly with the SGRQ (Citation36), but there are no clear data on the association between CAT and mortality.
The increased risk for death related to fatigue and the two dimensions of SF-36 was only observed among subjects with COPD. Important confounders such as age and sex distribution, and presence of heart disease did not differ between COPD and non-COPD subjects, and can thus not explain the different results between the groups. Even though mortality was similar in subjects with and without COPD, we lack data on cause of death among the deceased, and cause of death may contribute to the observed differences regarding the prognostic value of FACIT-F and SF-36.
There are also studies suggesting that a systemic inflammation may contribute to mortality in COPD (Citation37), and a modest correlation has been found between systemic inflammation and fatigue (Citation38). A systemic effect may have an impact on the observed differences between non-COPD and COPD, but this is merely a speculation; the reason for the observed differences remains unclear.
Possible mechanisms and/or factors related to fatigue among subjects with COPD, such as systemic inflammation, reduced muscle strength, physical activity and co-morbidities needs to be further studied. We have previously, in the same population, shown that heart disease and smoking habits are related to increased fatigue independent of COPD (Citation7), but the relationship to other common condition such as anxiety/depression also needs to be evaluated.
Strengths of this study are the use of well-validated instruments (Citation20–22, Citation27) and the use of both symptom specific and generic health status questionnaires (Citation39). Other strengths include the ability to evaluate the relationships between health status, fatigue and mortality in a large population-based sample, including comparisons between subjects with and without COPD. The distribution of COPD disease severity in our cohort is comparable to what has been reported from population-based studies (Citation19), and the cohort is considered representative for COPD in the general population. The under-diagnosis of COPD is related to disease severity (Citation18), and population-based studies as our study, enables investigation of also mild disease, which is rarely studied. In this study, a ‘healthy survivor bias’ cannot be ignored, also supported by the non-participator analysis. Despite this, the shown significant relationships are strong enough to overcome a healthy survivor effect.
It is known that the fixed ratio contributes to an overestimation of COPD among elderly and an underestimation among younger subjects, and today it is recommended to use the lower limit of normal to define COPD in epidemiological studies (Citation40). However, the fixed ratio was accepted to define COPD after the launch of the GOLD guidelines in 1997, and the current study was designed just after the shift of the millennium. As a consequence of using the fixed ratio, it has to be considered that GOLD grade I may include non-smokers without respiratory symptoms when interpreting the results, and this may be especially notably among elderly. The fixed ratio is usually used in health care, and the results can thus be interpreted also in relation to clinical practice, but one must always take into account the weakness of fixed ratio, as discussed here. Further, spirometric data in our study is limited to FEV1, FVC and VC, without other known prognostic spirometric measures, such as carbon monoxide transfer factor (TLCO) (Citation41).
Conclusions
In this population-based study, fatigue was strongly related to physical as well as mental dimensions of health status, both among subjects with and without COPD. However, physical health status was lower among subjects with COPD compared to subjects without COPD, and decreased by disease severity. Increased fatigue, decreased physical and mental dimensions of health status could each predict mortality among subjects with, but not without COPD. Thus, the FACIT-F can be a useful instrument in the clinic, evaluating both physical and mental dimensions of health status of prognostic importance.
Declarations of Interest Statement
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing of the paper.
Acknowledgement
Norrbotten county council, the Swedish Heart-Lung Foundation, the Northern Sweden Regional Health Authorities, Umeå University (Visare Norr and ALF) and the Swedish Research Council are acknowledged for funding. Professor Bo Lundbäck is acknowledged for initiating the study, Ann-Christine Jonsson, RN and Sigrid Sundberg, RN, for data collecting, Viktor Johansson for computerising the data, and Helena Backman and Ola Bernhoff for data management.
References
- The Global Initiative for Chronic Obstructive Lung Disease (GOLD). Updated 2014 [Cited 1 Mars 2014]: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available from: http://</υρλ>www.goldcopd.org.
- Blinderman C, Homel P, Billings A, Tennsteds S, Portenoy R. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manag 2009; 38:115–123.
- Theander K, Unosson M. Fatigue in patients with chronic obstructive pulmonary disease. J Adv Nurs 2004; 45:172–177.
- Voll-Aanerud M, Eagan TM, Wentzel-Larsen T, Gulsvik A, Bakke PS. Respiratory symptoms, COPD severity, and health related quality of life in a general population sample. Respir Med 2008; 102:399–406.
- Bridevaux P, Gerbase MW, Probst NM, Schindler C, Gaspoz J, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax 2008; 63:768–774.
- Jones PW, Brusselle G, Dal Negro RW, et al. Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med 2010; 1:57–66.
- Stridsman C, Muellerova H, Skär L, Lindberg A. Fatigue in COPD and the impact of respiratory symptoms and heart disease—A population-based study. COPD 2013; 10:125–132.
- Breslin E, van der Schans C, Breukink S, et al. Perception of fatigue and quality of life in patients with COPD. Chest 1998; 114:958.
- Peters JB, Heidra YF, Dandey L, et al. Course of normal and abnormal fatigue in patients with chronic obstructive pulmonary disease, and its relationship with domains of health status. Patient Educ Couns 2011; 85:281–285.
- Theander K, Jakobsson P, Torstensson O, Unosson M. Severity of fatigue is related to functional limitation and health in patients with chronic obstructive pulmonary disease. Int J Nurs Pract 2008; 14:455–462.
- Lindberg A, Larsson LG, Muellerova H, Rönmark E, Lundbäck B. Up-to-date on mortality in COPD -report from the OLIN COPD study. BMC Pulm Med 2012; 12:1.
- Lundbäck B, Eriksson B, Lindberg A, et al. A 20-year follow-up of a population study-based COPD cohort -report from the Obstructive Lung Disease in Northern Sweden studies. COPD 2009; 6:263–271.
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: Role of comorbidities. Eur Respir J 2006; 28:1245–1257.
- de Miguel-DÌez J, Carrasco-Garrido P, Rejas-Gutierrez J, et al. The influence of heart disease on characteristics, quality of life, use of health resources, and costs of COPD in primary care settings. BMC Cardiovasc Disord 2010; 10:8.
- Esteban C, Quintana JM, Aburto M, et al. Predictors of mortality in patients with stable COPD. J Gen Intern Med 2008; 23:1829–1834.
- Domingo Salvany A, Lamarca R, Ferrer M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166:680–685.
- Sundh J, Janson C, Lisspers K, Montgomery S, Stallberg B. Clinical COPD questionnaire score (CCQ) and mortality. Int J Chron Obstruct Pulmon Dis 2012; 7:833–842.
- Lindberg A, Bjerg A, Ronmark E, Larsson L, Lundbäck B. Prevalence and underdiagnosis of COPD by disease severity and attributable fraction of smoking. Report from the Obstructive Lung Disease in Northern Sweden studies. Respir Med 2006; 100:264–272.
- Miravitlles M, Soriano JB, Garcia-Rio F. Prevalence of COPD in Spain: Impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64:863–868.
- Lindberg A, Lundbäck B. The Obstructive Lung Disease in Northern Sweden Chronic Obstructive Pulmonary Disease study: Design, the first year, participation and mortality. Clin Respir J 2008; 2:64–71.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93:580–586.
- Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System. Evanston, IL: Center on Outcomes, Research and Education (Core), Evanston Northwestern Healthcare and Northwestern University, 1997.
- Cella D, Lai J, Chang C, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002; 94:528–538.
- Baghai-Ravary R, Quint J, Goldring J, Hurst J, Donaldson G, Wedzicha J. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med 2009; 103:216–223.
- Al-shair K, Muellerova H, Yorke J, et al. Examining fatigue in COPD: Development, validity and reliability of a modified version of FACIT-F scale. Health Qual Life Outcomes 2012; 10:100.
- Sullivan M, Karlsson J, Ware JE. The Swedish SF-36 health survey—I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med 1995; 41:1349–1358.
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey. Manual and Interpretation Guide. Boston: New England Medical Center, 1993.
- ATS. American thoracic society: Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995; 152:1107–1136.
- Berglund E, Birath G, Grimsby G, et al. Spirometric studies in normal subjects. Forced expirograms in subjects between 7 and 70 years of age. Acta Med Scand 1963; 173:185–192.
- Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T, Izumi T. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPD. Chest 1999; 116:1632–1637.
- Kelly JL, Elkin SL, Fluxman J, Polkey MI, Soljak MA, Hopkinson NS. Breathlessness and skeletal muscle weakness in patients undergoing lung health screening in primary care. COPD 2013; 10:40–54.
- Ståhl E, Lindberg A, Jansson SA, Rönmark E, Svensson K, Andersson F, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes 2005; 3:56.
- Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005; 32:811–819.
- Hardy SE, Studenski SA. Fatigue predicts mortality in older adults. J Am Geriatr Soc 2008; 56:1910–1914.
- Sundh J, Janson C, Lisspers K, Stallberg B, Montgomery S. The dyspnoea, obstruction, smoking, exacerbation (DOSE) index is predictive of mortality in COPD. Prim Care Respir J 2012; 21:295–301.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J 2009; 34:648–654.
- Sin DD, Man SF. Systemic inflammation and mortality in chronic obstructive pulmonary disease. Can J Physiol Pharmacol 2007; 85:141–147.
- Al-shair K, Kolsum U, Dockry R, Morris J, Singh D, Vestbo J. Biomarkers of systemic inflammation and depression and fatigue in moderate clinically stable COPD. Respir Res 2011; 12:3.
- Mahler DA, Mackowiak JI. Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with COPD. Chest 1995; 107:1585–1589.
- Bakke PS, Rönmark E, Eagan T, Pistelli F, Annesie-Maesono I, Maly M, et al. Recommendations for epidemiological studies on COPD. Eur Respir J 2011; 38:1261–1277.
- Boutou AK, Shrikrishna D, Tanner RJ, Smith C, Kelly JL, Ward SP, et al. Lung function indices for predicting mortality in COPD. Eur Respir J 2013; 42:616–625.

