Abstract
The objective of this study was to analyze the results of a multimodal therapeutic program during hospitalization in obese AECOPD patients.
This was a randomized, single-blind clinical trial conducted at two university hospitals in Granada, Spain. Forty-nine patients hospitalized due to AECOPD were randomly allocated to a control group (CG), in which patients received standard care, or to an intervention group (IG), in which patients were included in a multimodal therapeutic program, added to the standard care. The main outcome measures were pulmonary, physical (strength and exercise capacity) and perceived (dyspnea, quality of life and psychological distress) variables. Within-group significant improvements (p < 0.05) were found in physical and perceived variables in the IG after the treatment. In the CG, a significant decrease was found in lower limb strength and a significant improvement in dyspnea and in three subscales of the EuroQol-5D questionnaire. The between-groups analysis showed significant differences after the treatment on lower limb strength and exercise capacity values (p < 0.05), in three of the EuroQol-5D subscales, and in the total score and the depression subscale of the Hospital Anxiety and Depression Scale. A multimodal therapeutic program has a beneficial effect on physical functioning and perceived variables in hospitalized obese patients with AECOPD.
Introduction
Obesity in COPD has increased in the last decades and its prevalence varies from 10–20% in the European countries (Citation1). Obesity would be expected to amplify the abnormalities of dynamic ventilatory mechanics and ventilatory demand that characterise COPD. The combination of restrictive/obstructive deficits in obese patients with COPD increase the symptomatology and activity limitation (Citation2). Seres et al. (Citation3) determined that the reduced exercise capacity in morbid obesity was associated with greater oxygen uptake, heart rate, systolic blood pressure and minute ventilation.
The American Thoracic Society/European Respiratory Society Statement on Pulmonary Rehabilitation (PR) published in 2006, addressed the importance of the nutritional status. After this publication, different studies (Citation4,Citation5) have focused on the assessment and rehabilitation of obese COPD patients.
It has been reported a poorer exercise performance, a higher degree of functional impairment and fatigue levels in obese compared to non-obese COPD patients (Citation5).
As reported by the recent review of Chittal et al. (Citation6), over the last decade, there has been substantial focus on the paradox that exists among the obese with chronic diseases, where overweight and at least mild-moderately obese with these chronic diseases appear to have a better prognosis than do their leaner counterparts. Despite the growing interest on COPD therapeutics and obesity, to our knowledge, no previous studies have been focused on PR during acute exacerbation in obese COPD inpatients.
Most patients hospitalized due to acute COPD exacerbation (approximately 75%) show a progressive deterioration of muscle strength and endurance in both peripheral and respiratory muscles (Citation7). This has been attributed to prolonged bed rest and treatment with steroids (Citation8). Although numerous therapeutic programs have been proposed (Citation9, Citation10) for AECOPD inpatients, the results of a multimodal program in obese COPD exacerbated patients have not been previously assessed. The objective of this study was to evaluate the results of a multimodal therapeutic program including breathing training and lower and upper limb exercises during hospitalization due to acute exacerbation in obese COPD patients.
Methods
Study design
Randomized, single-blind clinical trial
The research assistant who collected the data was blinded to the hypothesis of the study and to the patient's allocation. The study was approved by the Hospital Ethics Committee (approval number 1107) and all the participants gave their written consent. The study was registered in www.clinicaltrials.gov, reference NCT01826682.
Randomization procedure
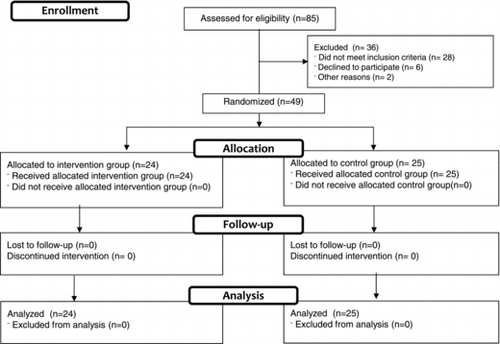
An independent nurse assigned the participants to the IG or CG according to a computer-generated randomization list. The nurse informed the physiotherapist once the participants had given their approval to participate in the study. The distribution of the participants is shown in the flow diagram (Figure ) (Citation11). First callout
Patients
Eighty-five patients among those admitted to the respiratory ward in San Cecilio and Virgen de las Nieves Hospitals, in Granada, Spain, were recruited during a 4-month period (July-October 2013). Thirty-six patients were excluded and finally forty-nine patients were randomized into the IG or the CG (Figure ).
Inclusion criteria were defined as follows: diagnosis of COPD made according to the criteria of the American Thoracic Society (ATS) (Citation12); patients clinically diagnosed with AECOPD; patients with more than 30 kg/cm2 of BMI, patients free from exacerbations for at least 10 days prior to the AECOPD; patients with a hospital stay of at least 7 days. Patients with other organ failure, cancer or inability to cooperate were excluded.
Evaluation
For descriptive purposes, anthropometric measures and previous clinical history data were recorded at baseline. Additionally, a spirometry was performed in all the subjects following the criteria of the American Thoracic Society (Citation13). Other variables assessed at baseline were the quality of life measured by the St. George's Respiratory Questionnaire (SGRQ) (Citation14) and the level of independence (Barthel Index) (Citation15).
Outcome measures included physical (lower and upper limbs strength and exercise capacity) and perceived variables (dyspnea, quality of life and psychological distress).
Pulmonary, physical and perceived outcome measures were assessed at hospital admission and discharge.
Pulmonary outcomes
Respiratory function
The respiratory function was evaluated in all the patients following the criteria of the American Thoracic Society (Citation16). Forced expiratory volume in the first second (FEV1) % predicted and resting oxygen saturation (SO2) were assessed.
Physical outcomes
Lower limb strength
Quadriceps strength was assessed according to previous studies (Citation17, Citation18) with a portable hand-held dynamometer (Lafayette Manual Muscle Testing System, model 01163, Lafayette, IN, USA). The test was performed with the patient seated with his/her knees and hips flexed at 90º. Resistance was applied to the anterior tibia during 5 seconds of maximal muscle contraction. The test was repeated alternatively 3 times on the leg, allowing participants to rest between measurements. The highest value in Newton was selected for the analysis. This test has been previously used to assess muscle strength in COPD patients.
Upper limb strength
Handgrip strength is a reliable marker of peripheral muscle strength. The measurements were made with a handgrip dynamometer (TEC-60; Productos Técnicos, EE.UU.) individually adjusted for the size of the subject's handgrip. Three measurements were made in the hand; the peak force was recorded in each case (Citation19). There was a time to rest between measurements. This test has been previously used to measure muscle strength in people with COPD (Citation20).
Exercise capacity
Aerobic endurance was measured using the two-minute step-in-place (2MSP) test. This test can be used when there are space limitations or weather conditions that make difficult to perform the 6-minute walk test (Citation21). The patients were asked to raise each knee to a point midway between the patella (i.e., kneecap) and the iliac crest (i.e., top hip bone). The score of this test is the number of times that the right knee reaches the required height in 2 minutes.
Perceived outcomes
Dyspnea perception
Dyspnea was assessed at rest using the modified Borg scale. Patients classified their breathlessness from 0 to 10 (Citation22). This measure has been validated in healthy subjects and COPD patients.
Quality of life (QoL) measure
We used the Spanish version of the EuroQol-5D (EQ-5D) questionnaire (Citation23). Two values were obtained: the EQ-5D Visual Analogue Scale (VAS) and the EQ-5D index. The VAS consists of a rating scale of 0 to 100 points, taken as 0–100% (0%, death/worst possible health; 100%, best possible health). The EQ-5D index is a questionnaire composed of five items (mobility, self-care, usual activity, pain/discomfort and anxiety/depression). For each item, the patient selects one of three descriptive health states (from good to poor) and the number and percentage of patients selecting each state is recorded. The questionnaire has been previously used in COPD patients.
Psychological distress
It was measured with the Hospital Anxiety and Depression Scale (HADS). The HADS is a 14-items self-report questionnaire designed to detect psychological morbidity in medically ill patients (Citation24). It contains depression and anxiety subscales, each with scores ranging from 0 to 21.
Intervention
We compared the effects of a multimodal intervention program combined with standard medical and pharmacological care with those of a standard care intervention in obese patients hospitalized due to AECOPD. During the exacerbation, all patients were treated with standard medical therapy including systemic steroids (76%), inhaled bronchodilators (100%) and oxygen.
The IG received twice daily individualized multimodal PR during 30–45 minutes. The program included 15 minutes of deep breathing exercises and 20–30 minutes of limb exercises. The duration of the intervention was determined by the length of hospital stay of each patient, and all the sessions were conducted by a physiotherapist.
The first day of the treatment begins with the patient in bed and consisted of breathing exercises, active range of motion (ROM) exercises and muscle strengthening.
From the second to the fourth day the breathing program continues and the exercise treatment consisted of active ROM exercises and muscle strengthening while the patient is seated, including knee flexion-extension, and hip abduction-adduction and flexion-extension exercises. Upper limbs flexion-extension and abduction-adduction exercises were also included.
From fifth day to last hospitalization day, in addition to breathing exercises, treatment included all the exercises done from second to fourth day and additionally they were encouraged to do standing exercises including knee flexion-extension, and hip flexion-extension and abduction-adduction exercises, they also had to do single leg stance, and sit to stand exercises, they had to try exercises with arms stationary at the beginning and try to raise hands to make it more difficult to keep standing.
A physiotherapist supervised these exercises. The number of repetitions was adapted to the subject's response taken into account the perceived dyspnea and fatigue during the exercise performance. The exercise program was based on methods that have been reported to increase the strength, the flexibility and the ROM (Citation25). The subjects were examined for adverse signs and symptoms such as increased pain, severe dyspnea, desaturation and increased skin temperature at each session. If any soreness lasted more than a few hours after the intervention, the regimen was decreased accordingly for that subject. Only were included in the analysis the patients who received at least 7 sessions of treatment.
Sample size calculation and power
The primary outcome measure for the study was the lower limb strength. On the basis of previous audit data (Citation26, Citation27), a small positive effect (10 Nm) was anticipated in the training group. Hence, in order to have 80% power using a two-sided α = 0.05, and a hypothetical drop-out rate of 20%, 20 patients in each group would be needed to show statistically significant differences in lower limb strength between the two groups.
Statistical analyses
Data were analyzed using SPSS software version 20.0. Descriptive statistics were used to determine the participant's characteristics. The Kolmogorov–Smirnov test was performed to assess the normality of continuous data prior to the statistical analysis. Normally distributed demographic variables were compared using Student's t-test. Non-normally distributed variables were compared using the Kruskal–Wallis test with an alpha level of 0.05. For each outcome variable measured, we performed a 2 (baseline and discharge) × 2 (multimodal therapeutic program + standard care vs. standard care group) two-way mixed ANOVA. If the two-way ANOVA showed a significant interaction for each variable, Scheffe's post-hoc test was used to identify the specific mean differences. Statistical significance was accepted at a p-value of 0.05.
Results
The final sample was composed of 49 patients (Figure ). The sample was composed of 4.8% (n = 2) women and 95.2% (n = 47) men. Age values were similar between intervention (72.36 ± 8.91) and control groups (73.7 ± 7.10), p = 0.597.
The groups were statistically equivalent on the clinical measures and importantly on the length of hospital stay. Means and standard deviations for pre-intervention sociodemographic and clinical characteristics of the sample are reported in First callout
Table 1. Descriptive characteristics of the sample at baseline
Table shows the characteristics of the patients included at the beginning of the study and randomly allocated in two groups. Outcome measures at baseline in the intervention and control groups are shown in First callout
Table 2. Outcome measures at baseline in the patients included in the study
Table shows both groups’ main outcome values. Not significant between groups differences were found at baseline. Within-group changes and between-group differences after the intervention on physical outcomes are shown in First callout
Table 3. Mean changes on pulmonary function, strength and exercise capacity by group.
Pulmonary variables improved significantly in both groups (p < 0.05). Physical variables improved significantly in the IG while lower limb strength showed a significant impairment in the CG (p < 0.05) (see Table ). First callout Between groups significant differences were found on lower limb strength and exercise capacity (p < 0.05).
Table 4. Mean changes on perceived outcomes by group
The dyspnea values showed a significant improvement in both groups (2.20 ± 2.6; p < 0.001 in the IG vs. 3.6 ± 2.21; p < 0.001 in the CG) with no between group differences (p = 0.785). In the IG, the quality of life measured with the EuroQol-5D questionnaire also showed a significant improvement (p < 0.05) in all the subscales. The CG improved significantly only in self-care, pain/discomfort and anxiety/depression subscales (p < 0.05). An improvement in the values of the EuroQol-5D VAS was recorded in both groups, although it was only significant in the IG (17.45 [-30.190, -4.920]; p = 0.006). Between-groups comparisons showed significant differences in the EuroQol-5D self-care, usual activities, anxiety and depression subscores and the EuroQol-5D VAS, with higher improvements in the IG (p < 0.05). The HADS subscores and the total score showed significant improvements in the IG after the treatment (p < 0.05). The CG also reported improvements but the differences were not significant. Between-groups comparisons also showed significant differences in HAD depression subscore and total score (p < 0.05).
Discussion
The objective of this study was to analyze the results of a multimodal therapeutic program during hospitalization in obese COPD exacerbated patients. Our results revealed that the patients who received a multimodal program consisting of breathing training and limb exercises experienced an improvement on strength, exercise capacity and psychological distress during AECOPD hospitalization. Moreover, our study showed a beneficial effect of physical treatment on QoL measures and dyspnea.
The sample of subjects included in this study was representative of the general population with COPD and obesity (i.e., similar age range, and COPD severity). This homogeneity was useful in order to reduce the possibilities of including confounding factors that could have affected the value of our results. Additionally, the final sample of participants was similar to previous studies (Citation28).
Peripheral muscle weakness is a well-known systemic feature in patients with clinically stable COPD (Citation29) that became more important with obesity (Citation4). Different studies (Citation6, Citation30) focused on PR have found a relationship in obese COPD patients with baseline status, but this seems not to affect PR outcomes: lower extremity exercise performance, health status, and functional status. Garrod et al. (Citation30) showed that BMI was not related to changes in the 6 minute walking distance (6MWD) or health status following PR. These studies have compared the results of PR by BMI groups but no previous studies have been focused on specific therapeutic proposals for obese COPD patients. This study evaluates the results of a multimodal therapeutic program on AECOPD patients.
The study of Spruit et al. (Citation26) showed a detrimental effect of hospitalization on peripheral muscle strength and systemic inflammation on the third day of hospitalization in AECOPD patients compared to stable COPD patients and healthy elderly subjects. That study also revealed a significant decrease in peripheral muscle strength during hospitalization. Similar results were shown in the studies of Martínez-Llorensa et al. (Citation7) and Pitta et al. (Citation27). Our results in the control group are in the same line, showing a decrease on muscle strength during hospitalization. No previous studies have compared the repercussion of inactivity and hospitalization in COPD by BMI group, but obese COPD patients seem to have poorer prediction after hospitalization due to the negative influence of their increased metabolic and ventilatory requirements (Citation31).
In this study we proposed a multimodal therapeutic program to prevent hospital impairment. Other authors (Citation29, Citation32) have suggested various interventions in order to prevent such impairment. As demonstrated in the study conducted by Giavedoni et al. (Citation32), neuromuscular electrical stimulation (NMES) is a feasible and effective treatment to prevent the impairment of lower limb strength during AECOPD and may be used to supplement early post-exacerbation PR. Electrical stimulation may be an useful alternative treatment in patients with severe COPD, who are unable to perform usual exercise on a regular basis, as required in rehabilitation programs (Citation17).
Troosters et al. (Citation33) concluded that resistance training during AECOPD is a safe and effective strategy to counterbalance loss of musculoskeletal function. Resistance training does generate a protective stimulus to the muscle and may facilitate functional recovery after an acute exacerbation. In their study, they found a significant increase in lower limb strength compared to a control group.
Our treatment proved to be effective not only in preventing hospital impairment but also improving muscle strength and exercise capacity in hospitalized obese AECOPD patients. This treatment is cost-efficient, taking into account that other treatments that have been proposed (e.g., neuromuscular electrical stimulation programs) imply a greater cost.
While it has been well established that pulmonary rehabilitation improves quality of life, exercise tolerance and dyspnea, these recommendations do not support pulmonary rehabilitation for the prevention of hospitalizations in COPD patients > 4 weeks post recent hospitalization (Citation35). However, our study has shown an additional effect of a multimodal therapeutic program on psychological distress during AECOPD. Psychological distress has been related to AECOPD influencing its clinical course (Citation34). The effectiveness of a PR program in reducing stress and depressive symptoms experienced by patients with COPD has been proven regardless of disease stage, patients’ gender, age or education level (Citation36).
Our study shows similar results in psychological distress, with higher improvements in depression compared to the anxiety levels. This improvement in our study may be influenced by the hospitalization “per se,” the more aggressive pharmacological treatment and the clinical profile of the obese COPD subjects.
However, no previous studies have compared the effects of a rehabilitation program on psychological distress during acute exacerbation in obese COPD patients.
The limitations of this study include the variability of the length of hospital stay of the patients and the imbalance in the sex distribution. In addition, we used a dynamometer to measure muscle condition, while other studies used a biopsy.
Conclusion
A multimodal therapeutic program has a beneficial effect on muscle strength and exercise capacity in obese AECOPD hospitalized patients, also improving the perceived variables. Future research should explore other modalities of physical therapy in obese COPD subjects.
Additional information
Funding
References
- Franssen FME, O'Donnell DE, Goossens GH, et al.Obesity and the lung: 5. Obesity and COPD. Thorax 2008; 63(12):1110–1117.
- Schols AM, Mostert R, Soeters PB, et al.Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax 1991; 46(10):695–699.
- Seres L, Lopez-Ayerbe J, Coll R, et al.Cardiopulmonary function and exercise capacity in patients with morbid obesity. Rev Esp Cardiol 2003; 56:594–600.
- Sabino PG, Silva BM, Brunetto AF. Nutritional status is related to fat-free mass, exercise capacity and inspiratory strength in severe chronic obstructive pulmonary disease patients. Clinics 2010; 65(6):599–605.
- Ramachandran K, McCusker C, Connors M, et al.The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis 2008; 5(4):205–209.
- Chittal P, Babu AS, Lavie CJ. Obesity paradox: does fat alter outcomes in chronic obstructive pulmonary disease? COPD 2015; 12(1):14–18.
- Martínez-Llorensa JM, Orozco-Levi M, Masdeu MJ, et al.Disfunción muscular global durante la exacerbación de la EPOC: un estudio de cohortes. Med Clin (Barc) 2004; 122:521–527.
- Decramer M, Lacquet LM, Fagard R, et al.Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med 1994; 150:11–16.
- Harth L, Stuart J, Montgomery C, et al.Physical therapy practice patterns in acute exacerbations of chronic obstructive pulmonary disease. Can Respir J 2009; 16(3):86–92.
- Yohannes AM, Connolly MJ. A national survey: percussion, vibration, shaking and active cycle breathing techniques used in patients with acute exacerbations of chronic obstructive pulmonary disease. Physiotherapy 2007; 93(2): 110–113.
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med 2010; 152:726–732.
- ATS statement. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152(Suppl):S77–S120.
- Miller MR, Hankinson J, Brusasco V, et al.Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005; 2:75–79.
- Collin C, Wade DT, Davies S, et al.The Barthel ADL Index: a reliability study. Disabil Rehabil 1988; 10(2):61–63.
- Miller MR, Hankinson J, Brusasco V, et al.ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Walker PP, Burnett A, Flavahan PW, Calverley PM. Lower limb activity and its determinants in COPD. Thorax 2008; 63:683–689.
- Martin HJ, Yule V, Syddall HE, et al.Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard biodex dynamometry. Gerontology 2006; 52:154–159.
- Shephard RJ, Montelpare W, Plyley M, et al.Handgrip dynamometry, Cybex measurements and lean mass as markers of the ageing of muscle function. Br J Sports Med 1991; 25(4):204–208.
- O'Shea SD, Taylor NF, Paratz JD. Measuring muscle strength for people with chronic obstructive pulmonary disease: retest reliability of hand-held dynamometry. Arch Phys Med Rehabil 2007; 88(1):32–36.
- Jones CJ, Rikli RE. Measuring functional fitness of older adults. J Active Aging 2002 ( March-April); 24–30.
- Borg GAV. Psychophysical basis of perceived exertion. Med Sci Sports Exerc 1982; 14:377–381.
- Anon. EuroQol–a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Pol 1990; 16:199–208.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67:361–370.
- DiNubile NA. Strength training. Clin Sports Med 1991; 10:33–62.
- Spruit MA, Gosselink R, Troosters T, et al.Muscle force during an acute exacerbation in hospitalised COPD patients and its relationship with CXCL8 and IGF-1. Thorax 2003; 58:752–756.
- Pitta F, Troosters T, Probst VS, et al.Physical activity and hospitalization for exacerbation of COPD. Chest 2006; 129:536–544.
- Sava F, Laviolette L, Bernard S, et al.The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med 2010; 10(1):55.
- Miravitlles M, Ferrer M, Pont A, et al.Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004; 59:387–395.
- Garrod R, Marshall J, Barley E, et al.Predictors of success and failure in pulmonary rehabilitation. Eur Respir J 2006; 27:788–794.
- Ora J, Laveneziana P, Wadell K, et al.Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol 2011; 111(1):10–19.
- Giavedoni S, Deans A, McCaughey P, et al.Neuromuscular electrical stimulation prevents muscle function deterioration in exacerbated COPD: a pilot study. Respir Med 2012; 106:1429–1434.
- Troosters T, Probst VS, Crul T, et al.Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181:1072–1077.
- Laurin C, Moullec G, Bacon SL, et al.Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med 2012; 185(9):918–923.
- Criner GJ, Bourbeau J, Diekemper RL, et al.Executive Summary: Prevention of Acute Exacerbation of Chronic Obstructive Pulmonary Disease: American College of Chest Physicians and Canadian Thoracic Society Guideline. Published online October 16, 2014. doi:10.1378/chest.14-1677.
- Tselebis A, Bratis D, Pachi A, et al.A pulmonary rehabilitation program reduces levels of anxiety and depression in COPD patients. Multidiscip Respir Med 2013; 8(1): 41.