Abstract
Evidence-based guidance for the use of airway clearance techniques (ACT) in chronic obstructive pulmonary disease (COPD) is lacking in-part because well-established measurements of pulmonary function such as the forced expiratory volume in 1s (FEV1) are relatively insensitive to ACT. The objective of this crossover study was to evaluate daily use of an oscillatory positive expiratory pressure (oPEP) device for 21–28 days in COPD patients who were self-identified as sputum-producers or non-sputum-producers.
COPD volunteers provided written informed consent to daily oPEP use in a randomized crossover fashion. Participants completed baseline, crossover and study-end pulmonary function tests, St. George's Respiratory Questionnaire (SGRQ), Patient Evaluation Questionnaire (PEQ), Six-Minute Walk Test and 3He magnetic resonance imaging (MRI) for the measurement of ventilation abnormalities using the ventilation defect percent (VDP).
Fourteen COPD patients, self-identified as sputum-producers and 13 COPD-non-sputum-producers completed the study. Post-oPEP, the PEQ-ease-bringing-up-sputum was improved for sputum-producers (p = 0.005) and non-sputum-producers (p = 0.04), the magnitude of which was greater for sputum-producers (p = 0.03). There were significant post-oPEP improvements for sputum-producers only for FVC (p = 0.01), 6MWD (p = 0.04), SGRQ total score (p = 0.01) as well as PEQ-patient-global-assessment (p = 0.02). Clinically relevant post-oPEP improvements for PEQ-ease-bringing-up-sputum/PEQ-patient-global-assessment/SGRQ/VDP were observed in 8/7/9/6 of 14 sputum-producers and 2/0/3/3 of 13 non-sputum-producers. The post-oPEP change in 3He MRI VDP was related to the change in PEQ-ease-bringing-up-sputum (r = 0.65, p = 0.0004) and FEV1 (r = –0.50, p = 0.009).
In COPD patients with chronic sputum production, PEQ and SGRQ scores, FVC and 6MWD improved post-oPEP. FEV1 and PEQ-ease-bringing-up-sputum improvements were related to improved ventilation providing mechanistic evidence to support oPEP use in COPD. Clinical Trials # NCT02282189 and NCT02282202.
Introduction
Chronic mucus hyper-secretion and impaired mucociliary clearance are hallmark features of the chronic bronchitis phenotype of chronic obstructive pulmonary disease (COPD). This is clinically important because chronic cough and exaggerated sputum production are both associated with exacerbation (Citation1), hospitalization (Citation2), accelerated pulmonary function decline (Citation3), and increased mortality (Citation4–Citation6). To treat chronic sputum production with cough in patients, airway clearance techniques (ACT) have been developed to facilitate mucus transport with the goal being improved respiratory symptoms and outcomes. Such approaches include conventional chest physiotherapy, active cycle breathing techniques, autogenic drainage, resistive inspiratory maneuvers, positive expiratory pressure (PEP), oscillatory positive expiratory pressure (oPEP) and high-frequency chest wall oscillation (Citation7–9).
Unfortunately, spirometry measurements are relatively insensitive to airway clearance methods (Citation10), and consequently, there are few studies (Citation9) that demonstrate ACT efficacy and effectiveness, making it difficult to make evidenced-based decisions about their use in patients, especially those with COPD. For example, in COPD, a significant post-PEP increase in the forced expiratory volume in 1 second (FEV1) was reported (Citation11), whereas other studies reported no change in FEV1 (Citation12,Citation13). In another investigation, a significant increase in sputum production without a change FEV1 was reported following postural drainage and oPEP therapy (Citation14), while another study reported that neither chest physiotherapy nor mechanical chest vibration improved pulmonary function in COPD patients who paradoxically reported feeling better (Citation15) after therapy.
Thoracic imaging methods such as x-ray computed tomography (CT) and magnetic resonance imaging (MRI) provide direct structural and functional measurements of pulmonary disease (Citation16). In particular, MRI of inhaled gases (O2, 19F, and hyperpolarized 129Xe, 3He) provides a way to visualize those regions of the lung that are ventilated and those that are not. Proof-of-concept COPD studies have reported significantly improved ventilation following salbutamol administration (Citation17), airway stent placement (Citation18) and acute exacerbation therapy (Citation19). MRI improvements in ventilation observed in the absence of clinically relevant changes in FEV1 have important consequences for both therapy development and efficacy evaluation (Citation16). Preliminary analysis in COPD patients suggested that oPEP therapy improved mucus clearance (Citation20,Citation21) and that MRI ventilation measurements may provide a way to objectively measure these effects.
Therefore, the objective of this randomized crossover study was to evaluate daily oPEP use in COPD patients, self-identified as sputum or non-sputum-producers. We hypothesized that in chronic sputum-producers with COPD, oPEP use would improve sputum movement out of the airways resulting in clinically relevant symptom improvements. We also investigated whether functional imaging measurements (ventilation) would be related to improvements in symptoms, thereby generating evidence to support the use of oPEP in certain COPD patients.
Methods
Ethics and study design
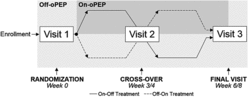
Study participants provided written informed consent to a protocol approved by a local review board (The University of Western Ontario Health Sciences Research Ethics Board approvals 102789, 103969, Clinical Trials # NCT02282189 and NCT02282202). Participants enrolled in one of two randomized, unblinded, crossover studies (run consecutively) to evaluate the efficacy of four-times daily oPEP using an Aerobika device (Trudell Medical International, London Canada). Treatment-time was 28 ± 5 days for the first study and 21 ± 5 days for the second study while the treatment procedure and all study evaluations were the same. As shown in Figure , subjects were randomized to oPEP or standard-of-care on a 1:1 allocation basis and crossed-over to the other option after 28 ± 5 days or 21 ± 5 days. Study participants and the investigating team were not blinded to group allocation, but all evaluations were performed blinded to clinical status and treatment group.
Figure 1. Randomized crossover study design. At visit 1 participants were randomized to oPEP (dark-gray) or baseline care (light-gray) on a 1:1 allocation basis and after 3 or 4 weeks were crossed-over to the other option at visit 2 and evaluated a final time at visit 3. On-Off Treatment (—), participants randomized to oPEP and crossed-over to baseline care; Off-On Treatment (– –), participants randomized to baseline care and crossed-over to oPEP.
Participants were provided a hand-held mechanical oPEP device (Citation22) and instructed by a pulmonary technologist to inhale normally through the device which provided a linear pathway with an inhalation valve. Upon exhalation, a one-way valve within the device mechanism opens and closes intermittently resulting in positive pressure oscillations. The participant was enabled to use five different resistance indicator settings on the device that were adjusted to a setting based on the participant's comfort in exhaling through the device. All participants were trained by a pulmonary function technologist to use the device four-times daily with each session consisting of 10–20 blows into the device, followed by 2–3 “huff” coughs. Treatment sessions were documented at home by the patient using a daily diary. Treatment adherence was defined as the percent of days when oPEP was performed four-times. There was no sham therapy, nor blinding to treatment. Participants underwent study visits at baseline, crossover and study-end when they completed the St. George's Respiratory Questionnaire (SGRQ) (Citation23,Citation24), spirometry, plethysmography, MRI and Six-Minute Walk Test (6MWT) (Citation25). The Patient Evaluation Questionnaire (PEQ) (Citation26) was completed weekly. Thoracic CT was acquired at visit 1.
Study subjects
Male and female patients with a clinical diagnosis of COPD, aged 40–85 years, were recruited from a tertiary care practice. Sputum-producers reported they had coughed and brought up sputum “several days a week” (>2 days a week) or “almost every day” in the month prior to the study. Non-sputum-producers reported they had coughed and brought up sputum “a few days a month,” “only with lung/respiratory infects” or “not at all” in the month prior to the study. Baseline pulmonary medications and smoking habits were not modified during the study. Subjects were to be withdrawn in the case of a pulmonary exacerbation or because of any change in pulmonary medication deemed medically required.
Pulmonary function, six-minute walk test and questionnaires
Study participants performed spirometry (MedGraphics Corporation, St. Paul, Minnesota, USA) according to American Thoracic Society (ATS) guidelines to quantify FEV1 and the forced vital capacity (FVC) (Citation27). A whole body plethysmograph (MedGraphics Corporation, St. Paul, Minnesota, USA) and the attached gas analyzer was used to measure diffusing capacity of the lung for carbon monoxide (DLCO). The 6MWT was performed according to ATS guidelines (Citation28) to measure the six-minute-walk distance (6MWD). The SGRQ (Citation23,Citation24) and PEQ (Citation26) were used with permission. The PEQ was previously used in the National Mucolytic Study (Citation26) and evaluates cough frequency, cough severity, chest discomfort, dyspnea, bronchodilator use, ease-bringing-up-sputum and patient-global-assessment. The PEQ global score is a cumulative symptom score comprised of cough frequency, cough severity, chest discomfort and dyspnea.
Image acquisition
MRI was performed on a whole body 3.0 Tesla Discovery MR750 (General Electric Health Care, Milwaukee, Wisconsin, USA) system. For both 1H and 3He MRI, subjects were instructed to inhale a gas mixture from a 1.0 L Tedlar bag (Jensen Inert Products, Coral Springs, Florida, USA) from functional residual capacity and image acquisition was performed during a 16-second inspiration breath-hold (Citation29). Coronal 1H MRI was performed prior to 3He MRI, both previously described (Citation29). CT was performed on a 64-slice Lightspeed VCT scanner (General Electric Health Care) as previously described (Citation30), and the total effective dose for an average adult was 1.8 mSv.
Image analysis
Thoracic CT images were analyzed (Pulmonary Workstation 2.0, VIDA Diagnosis Inc., Coralville, Iowa, USA) to quantify the relative area of the parenchyma density histogram with attenuation values ≤ –950 Hounsfield Units (RA950). CT images were qualitatively assessed for evidence of emphysema, bronchiectasis and mucus plugging by an expert chest radiologist (RER) with 25-years’ experience. Hyperpolarized 3He MRI ventilation defect percent (VDP), a measurement of ventilation abnormalities, was generated for the whole lung as previously described (Citation31).
Statistical analysis
As shown in Figure , for the participants randomized to On-Off treatment, pre- and post-oPEP measurements were acquired at Visit 1 and Visit 2 respectively. For the participants randomized to Off-On treatment, pre- and post-oPEP measurements were acquired at Visit 2 and Visit 3 respectively. For the sputum-producer and non-sputum-producer subgroups, we evaluated the effect of oPEP treatment in two ways for all measured outcomes (spirometry, 6MWD, SGRQ, mean PEQ and 3He MRI VDP). First, for all measurements, the mean pre- and post-oPEP measurements for each subgroup were compared using paired t-tests. Second, for each subgroup, the mean change in measured outcomes during the on- and off-oPEP periods were compared using paired t-tests. All paired t-tests were performed in GraphPad Prism version 6 (La Jolla, California, USA). A Shapiro–Wilk normality test was performed and when data were not normal, non-parametric Wilcoxon matched-pairs tests were performed.
Previously published minimum-clinically important-differences (MCID) for FEV1 (Citation32), 6MWD (Citation33) and SGRQ (Citation34) were used to identify subjects with clinically relevant improvements post-oPEP. For PEQ, a single point change (Citation26) in score and for VDP, the smallest detectable difference (SDD) generated on the basis of inter-visit variance (Citation35) were used because no MCID are published. Fisher's exact test determined the significance of the difference in the proportion of sputum-producers versus non-sputum-producers reporting changes ≥ MCID and SDD. Relationships were evaluated using linear regression (r2), Pearson correlation (r) and when the data were not normal, using Spearman correlations (ρ). Results were considered significant when the probability of making a type 1 error was < 5% (p < 0.05).
Results
Study subjects
Thirty-two participants (Table S1 online) including 16 sputum-producers and 16 non-sputum-producers provided written informed consent. Two sputum-producers and three non-sputum-producers withdrew; one subject withdrew because of a pulmonary exacerbation (S15), two withdrew due to back pain (S31, S32), one subject withdrew because he was MRI incompatible (S16) and one subject withdrew with no reason (S30). There were no adverse events related to the use of the oPEP device, nor were there any serious or severe adverse events during the study. As shown in Table S1 (online), the intent-to-treat and efficacy populations were not different at baseline (visit 1).
The efficacy population of 27 COPD patients is summarized in Table including 14 sputum-producers and 13 non-sputum-producers. There was CT evidence of bronchiectasis in 8 of 14 sputum-producers and no evidence of bronchiectasis in non-sputum-producers. There was CT evidence of emphysema in 13 of 14 sputum-producers and all non-sputum-producers.
Table 1. Subject demographic and baseline measurements
Post-oPEP measurements
We evaluated oPEP treatment effects in two ways and the potential influence of randomization order on these results, as shown in Tables 2, S3 and S4. Table shows mean pre- and post-oPEP measurements for sputum-producers and non-sputum-producers and Table S2 (online) provides a by-subject list of all measurements. Table shows that the PEQ-ease-bringing-up-sputum was significantly improved post-oPEP for sputum-producers (p = 0.005) and non-sputum-producers (p = 0.04). It is important to note that the magnitude of improvement was significantly greater for sputum-producers (p = 0.03). In sputum-producers only, post-oPEP FVC (p = 0.01), 6MWD (p = 0.04), SGRQ total score (p = 0.01) and PEQ-patient-global-assessment (p = 0.02) were also significantly improved. Table S3 shows for each subgroup, the mean change in measured outcomes during the on- and off-oPEP periods. For the sputum-producers only, the change while on-oPEP was significantly different than the change while off-oPEP for FVC (p = 0.02), PEQ-ease-bringing-up-sputum (p = 0.01) and PEQ-patient-global-assessment (p = 0.03). Table S4 shows that potential differences in post-oPEP measurements were not influenced by randomization order.
Table 2. Pre- and Post-oPEP measurements
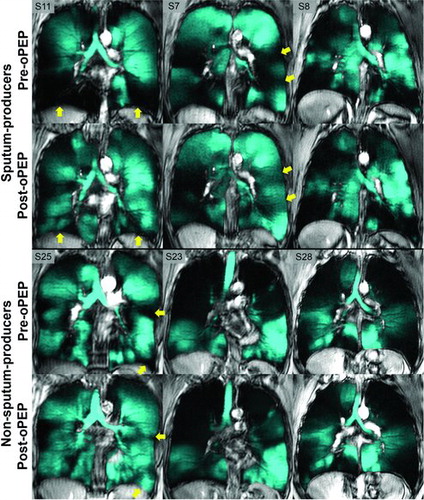
Figure provides 3He MRI coronal ventilation images for three representative sputum-producers (S7, S8 and S11) and three representative non-sputum-producers (S23, S25 and S28). Figure shows that ventilation defects were visually obvious in all subjects prior to oPEP use and for individual subjects, there were regional 3He ventilation improvements post-oPEP. For example, as shown in Figure , sputum-producers (S7, S11) showed improved ventilation and exercise capacity and SGRQ total-score with FEV1 and FVC improvement.
Figure 2. 3He MRI ventilation in representative sputum and non-sputum-producers. Pre- and post-oPEP ventilation (in cyan) registered to 1H anatomical MRI (in grey-scale) for sputum- and non-sputum-producers. Yellow arrows identify regional differences in 3He ventilation post-oPEP. Sputum-producers: S11, 56 year-old female, Δ FEV1 = −11%pred, Δ FVC = −13%pred, Δ 6MWD = −78m, Δ SGRQ = 20, Δ VDP = 6%. S7, 72 year-old female, Δ FEV1 = −6% pred, Δ FVC = −24% pred, Δ 6MWD = −24m, Δ SGRQ = 19, Δ VDP = 4%. S8, 79 year-old female, Δ FEV1 = 2%pred, Δ FVC = 3%pred, Δ 6MWD = −63m, Δ SGRQ = 2, Δ VDP = −3%. Non-sputum-producers: S25, 72 year-old female, Δ FEV1 = −5%pred, Δ FVC = 0%pred, Δ 6MWD = −18m, Δ SGRQ = −3, Δ VDP = 3%. S23, 77 year-old female, Δ FEV1 = −2%pred, Δ FVC = −2%pred, Δ 6MWD = 12m, Δ SGRQ = 2, Δ VDP = 1%. S28, 51 year-old male, Δ FEV1 = −1%pred, Δ FVC = −1%pred, Δ 6MWD = −6m, Δ SGRQ = −11, Δ VDP = −1%.
oPEP responders
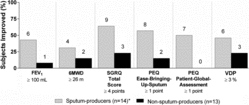
Figure shows the number and proportion of sputum-producer and non-sputum-producer subjects that exceeded minimum clinically important-difference (MCID) measurements for FEV1 (Citation32), 6MWD (Citation33) and SGRQ (Citation34), and a single point score improvement for PEQ (Citation26) and SDD for VDP (≥3%), post-oPEP. A significantly greater proportion of sputum-producers versus non-sputum-producers reported clinically relevant post-oPEP improvements for PEQ-ease-bringing-up-sputum (p = 0.04) and PEQ-patient-global-assessment (p = 0.006).
Figure 3. Clinically relevant post-oPEP improvements. Values = n improved ≥ minimum-clinically important-difference (FEV1, 6MWD, SGRQ Total Score), smallest detectable difference (VDP) or 1-point score improvement (PEQ). FEV1, forced expiratory volume in 1 second; 6MWD, 6-minute walk distance; SGRQ, St. George's Respiratory Questionnaire total score; PEQ, Patient Evaluation Questionnaire; VDP, ventilation defect percent; * n = 13 for 6MWD and VDP.
Relationships
Table shows correlations for participant measurements at baseline with the post-oPEP change in PEQ-ease-bringing-up-sputum and PEQ-patient-global-assessment. In sputum-producers only, there was a strong relationship for baseline VDP with the post-oPEP change in PEQ-ease-bringing-up-sputum (r = 0.75, p = 0.003) and the post-oPEP change in PEQ-patient-global-assessment (r = 0.66, p = 0.01).
Table 3. Correlations for post-oPEP changes in PEQ Ease-Bringing-Up-Sputum and PEQ Patient-Global-Assessment
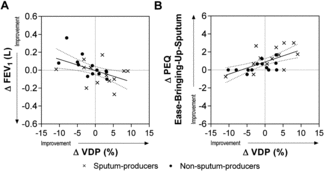
As shown in Figure , there was a moderate relationship for the post-oPEP change in VDP with the post-oPEP change in FEV1 (r = −0.50, r2 = 0.25, p = 0.009) and PEQ-ease-bringing-up-sputum (r = 0.65, r2 = 0.39, p = 0.0004).
Figure 4. Relationships for post-oPEP changes in clinical measurements and change in 3He MRI VDP. Significant relationship for post-oPEP changes in 3He MRI VDP with A) FEV1 (r = −0.50, r2 = 0.25, p = 0.009), and B) PEQ Ease-Bringing-Up-Sputum (r = 0.65, r2 = 0.39, p = 0.0004). Dotted lines = 95% confidence intervals.
Discussion
We evaluated daily oPEP use in patients with COPD and observed: 1) a significant improvement for ease-bringing-up-sputum based on the PEQ questionnaire for sputum- and non-sputum-producers, 2) significantly improved PEQ and SGRQ scores, 6MWD and FVC in sputum-producers only, 3) clinically relevant improvements for PEQ-ease-bringing-up-sputum, PEQ-patient-global-assessment, SGRQ and ventilation in 8/7/9/6 of 14 sputum-producers, and, 4) the post-oPEP change in PEQ-ease-bringing-up-sputum was related to MRI measurements of ventilation.
A previous study reported increased sputum weight and volume clearance after ACT (Citation14), so we were not surprised that COPD patients who were self-reported sputum-producers also reported improved ease-in-bringing-up-sputum. Somewhat more surprising was the small mean improvement in COPD patients who were not sputum-producers. This apparently paradoxical result suggests that when crossed over to daily oPEP use, non-sputum-producers sensed a modest improvement in how easily they could cough up any sputum at all. This observation is consistent with a previous study reporting cephalad movement of a radio-aerosol following unproductive cough and forced “huff” coughing in patients with airflow obstruction without sputum production (Citation36). Importantly, in non-sputum-producers, there was no CT evidence of bronchiectasis or chronic bronchitis, and this is consistent with emphysema-dominant COPD, for which ACT is not typically indicated.
In addition to improved ease-in-bringing-up-sputum, there was significantly improved FVC, 6MWD and symptoms in sputum-producers. Moreover there were clinically relevant improvements in SGRQ, PEQ-ease-bringing-up-sputum and PEQ-patient-global-assessment for half of sputum-producers, and improvements in FEV1, 6MWD, and VDP in more than ¼. Such clinically -relevant improvements were not observed in non-sputum-producers, except in three patients (S22, S24, S29) with improved SGRQ, two (S21, S29) with improved PEQ-ease-bringing-up-sputum, two (S19, S24) with improved 6MWD and one (S25) with improved FEV1. Notably, three of the six non-sputum-producers (S19, S25, S29) with clinically relevant improvements also showed improved ventilation post-oPEP. This reinforces the notion that with improved ventilation, there were improved symptoms or exercise capacity.
In keeping with these findings, there were moderate-to-strong relationships for baseline VDP with post-oPEP change in ease-bringing-up-sputum and patient-global-assessment, and this may reveal clues as to the mechanisms of how and why patients feel better after ACT. In other words, subjects with worse baseline ventilation, but not worse FEV1, had a greater improvement in sputum clearance and felt better. Changes in FEV1 and PEQ-ease-bringing-up-sputum were also modestly but significantly related to changes in 3He MRI VDP. This raises the intriguing suggestion that movement of mucus improved ventilation and this resulted in improved FEV1 and certainly there was evidence of this in specific cases.
We must acknowledge a number of study limitations including the small sample size and the fact that for logistical reasons, study data were retrospectively pooled across two consecutive crossover trials that could not be blinded. In addition, we stratified participants on the basis of self-reported cough and sputum production rather than a quantitative measure of daily sputum volume. Although this can be viewed as a shortcoming of the study, self-identification of chronic cough and sputum is likely more representative of the typical clinical scenario when ACT is considered as a therapy option. We also recognize that the self-administered daily diary accounts of oPEP use may have over-estimated adherence and therefore the adherence reported here should be considered an estimate. This study interrogated a relatively short 21–28 days of oPEP use, so longer term inferences regarding outcomes, cannot be readily ascertained or inferred and warrant further investigation. Finally, a previous study suggested that “huff” coughing alone could result in mucus movement (Citation36) whereas here, all participants were instructed to use oPEP and then “huff” cough, so we could not elucidate the independent contribution of oPEP here. Regardless, this study showed that COPD patients with chronic sputum production showed improved symptoms and quality of life following a relatively short course of daily oPEP use. Moreover these improvements were related to improved ventilation which may help identify the mechanisms of patient response.
As compared to their non-sputum-producer counterparts, patients with COPD accompanied by chronic sputum production have worse outcomes and accelerated pulmonary function decline (Citation3–Citation6) and yet there are still few options available to support these patients. Despite the well-known relationship between sputum-production and clinically important outcomes, the current standard of care for stable COPD patients with chronic bronchitis does not include physiotherapeutic regimens or pharmacological therapies to combat mucus hypersecretion or impaired mucus clearance. Numerous oPEP devices are available and widely used to assist the clearance of airway secretions in patients with cystic fibrosis, but there is much lower use in COPD and the reasons for this are not clear. As compared to more conventional physiotherapeutic approaches, oPEP devices are portable and treatment can be performed at home by the patient although age, independence and disease severity must be considered.
It should be acknowledged that in addition to the Aerobika oPEP device evaluated here, there are numerous options available to the COPD population including the Flutter (Axcan Scandipharm, Birmingham, Alabama, USA), Acapella (Smiths Medical, Watford, UK), Quake (Thayer Medical, Tucson, Arizona, USA), RC-Cornet (Curaplex Medical, Dublin, Ohio, USA) and the Lung Flute (Medical Acoustics, Buffalo, New York, USA). With adequate adherence to the proposed treatment regimens improved mucus clearance may decrease the frequency and severity of respiratory tract infections/exacerbations resulting in improved pulmonary function and an overall improvement in quality of life. One reason for their limited uptake may stem from the current lack of objective evidence to support the mechanism of action of oPEP in such patients.
FEV1 has been the most frequently used outcome measure in obstructive lung disease despite being notorious for its lack of sensitivity to abnormalities in the small airways (Citation37). Therefore, it is not surprising that movement of secretions from the smaller conducting airways may go undetected by measurements such as FEV1, which are made at the mouth and dominated by the large airways. Because of these considerations, here we used less well-validated methods including the PEQ and functional MRI to probe and interrogate the effects of oPEP in COPD. While novel imaging measurements cannot yet be routinely used in the clinical setting, for some patients, such measurements provide a way to better understand response to therapy and avoid therapies that may not be helpful.
In conclusion, we evaluated daily oPEP use in patients with COPD and provide evidence to support the use of oPEP in sputum-producers based on significant improvements in the ease-in-bringing-up-sputum, FVC, quality-of-life and 6MWD. Post-oPEP changes in the PEQ-ease-in-bringing-up-sputum were related to improved ventilation, providing mechanistic evidence to support oPEP use in COPD patients with chronic sputum production.
Acknowledgments
We thank Andrew Wheatley, Sandra Blamires, and Trevor Szekeres all based in the Parraga Lab, Imaging Research Laboratories at Robarts Research Institute, Western University, London, Canada for all participant evaluations, hyperpolarized gas dispensing and MRI/CT acquisition.
Declaration of Interests Statement
This study was supported by grant-in-aid funding from Trudell Medical International (London, Canada). Dr. Parraga acknowledges salary support from a Canadian Institutes of Health Research (CIHR) New Investigator Award and operating funding from a CIHR Team Grant (Thoracic Imaging Network of Canada). The authors declare there is no conflict of interest. Supporting sources had no involvement in the study design including: the collection, analysis, and interpretation of data, in the writing of the report and in the decision to submit the report for publication.
ICOP_1043523_SUPPLEMENTARY_TABLES.pdf
Download PDF (462.9 KB)References
- Corhay JL, Vincken W, Schlesser M, Bossuyt P, Imschoot J. Chronic bronchitis in COPD patients is associated with increased risk of exacerbations: a cross-sectional multicentre study. Int J Clin Pract 2013; 67:1294–1301.
- Burgel PR, Nesme-Meyer P, Chanez P, Caillaud D, Carré P, Perez T, Roche N, Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 2009; 135:975–982.
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996; 153:1530–1535.
- Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J 1995; 8:1333–1338.
- Prescott EI, Lange P, Vestbo J. Chronic expectoration and risk of death in chronic obstructive lung disease. Ugeskr Laeger 1996; 158:6456–6460.
- Ekberg-Aronsson M, Pehrsson K, Nilsson JA, Nilsson PM, Löfdahl CG. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res 2005; 6: 98.
- Fagevik Olsen M, Westerdahl E. Positive expiratory pressure in patients with chronic obstructive pulmonary disease—a systematic review. Respiration 2009; 77:110–118.
- Bhowmik A, Chahal K, Austin G, Chakravorty I. Improving mucociliary clearance in chronic obstructive pulmonary disease. Respir Med 2009; 103:496–502.
- Osadnik CR, McDonald CF, Jones AP, Holland AE. Airway clearance techniques for chronic obstructive pulmonary disease. Cochrane Database Syst Rev [online] 2012. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008328.pub2/abstract. Accessed November 9, 2014.
- van der Schans CP. Airway clearance: assessment of techniques. Paediatr Respir Rev 2002; 3:110–114.
- Wolkove N, Kamel H, Rotaple M, Baltzan MA. Use of a mucus clearance device enhances the bronchodilator response in patients with stable COPD. Chest 2002; 121:702–707.
- Cegla UH, Bautz M, Fröde G, Werner T. Physical therapy in patients with COPD and tracheobronchial instability–comparison of 2 oscillating PEP systems (RC-Cornet, VRP1 Desitin). Results of a randommized prospective study of 90 patients. Pneumologie 1997; 51:129–136.
- Christensen HR, Simonsen K, Lange P, ClementsenP, Kampmann JP, Viskum K, Heideby J, Koch U. PEEP-masks in patients with severe obstructive pulmonary disease: a negative report. Eur Respir J 1990; 3:267–272.
- Bellone A, Lascioli R, Raschi S, Guzzi L, Adone R. Chest physical therapy in patients with acute exacerbation of chronic bronchitis: effectiveness of three methods. Arch Phys Med Rehabil 2000; 81:558–560.
- Mohsenifar Z, Rosenberg N, Goldberg HS, Koerner SK. Mechanical vibration and conventional chest physiotherapy in outpatients with stable chronic obstructive lung disease. Chest 1985; 87:483–485.
- Coxson HO, Leipsic J, Parraga G, Sin DD. Using pulmonary imaging to move chronic obstructive pulmonary disease beyond FEV1. Am J Respir Crit Care Med 2014; 190:135–144.
- Kirby M, Mathew L, Heydarian M, Etemad-Rezai R, McCormack DG, Parraga G. Chronic obstructive pulmonary disease: quantification of bronchodilator effects by using hyperpolarized 3He MR imaging. Radiology 2011; 261:283–292.
- Mathew L, Kirby M, Farquhar D, Licskai C, Santyr G, Etemad-Rezai R, Parraga G, McCormack DG. Hyperpolarized 3He functional magnetic resonance imaging of bronchoscopic airway bypass in chronic obstructive pulmonary disease. Can Respir J 2012; 19:41–43.
- Kirby M, Kanhere N, Etemad-Rezai R, Wheatley A, McCormack DG, Parraga G. Hyperpolarized helium-3 magnetic resonance imaging of chronic obstructive pulmonary disease exacerbation. J Magn Reson Imaging 2013; 37:1223–1227.
- Svenningsen S, Kirby M, Suggett J, Kanhere N, Hasany A, McCormackDG, Parraga G. Oscillatory Positive Expiratory Pressure (oPEP) Treatment in Chronic Obstructive Pulmonary Disease [abstract]. Chest 2013; 741A.
- Svenningsen S, Paulin G, Wheatley A, Pike D, Suggett J, McCormack DG, Parraga G. Oscillating positive expiratory pressure (oPEP) therapy in chronic obstructive pulmonary disease and bronchiectasis [abstract]. Eur Respir J 2014; P3679.
- Suggett J,Meyer A, Costella S, Morton R, Mitchell J. et al. Assessment Of Oscillating Positive Expiratory Pressure (OPEP) devices by means of adult expiratory waveforms: A laboratory study [abstract]. Am J Respir Crit Care Med 2014; 189:A3036.
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991; 85 Suppl B: 25–31; discussion 33–37.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145:1321–1327.
- Enright PL. The six-minute walk test. Respir Care 2003; 48:783–785.
- Petty TL. The National Mucolytic Study. Results of a randomized, double-blind, placebo-controlled study of iodinated glycerol in chronic obstructive bronchitis. Chest 1990; 97:75–83.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117.
- Parraga G, Ouriadov A, Evans A, McKay S, Lam WW, Fenster A, Etemad-Rezai R, McCormack D, Santyr G. Hyperpolarized 3He ventilation defects and apparent diffusion coefficients in chronic obstructive pulmonary disease: preliminary results at 3.0 Tesla. Invest Radiol. 2007; 42:384–391.
- Kirby M, Pike D, Coxson HO, McCormack DG, Parraga G. Hyperpolarized 3He ventilation defects used to predict pulmonary exacerbations in mild to moderate chronic obstructive pulmonary disease. Radiology 2014; 273:887–896.
- Kirby M, Heydarian M, Svenningsen S, Wheatley A, McCormack DG, Etemad-Rezai R, Parraga G. Hyperpolarized 3He magnetic resonance functional imaging semiautomated segmentation. Acad Radiol 2012; 19:141–152.
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005; 2:111–124.
- Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, Wise RA, Sciurba F, National Emphysema Treatment Trial (NETT) Research Group. The minimal important difference of exercise tests in severe COPD. Eur Respir J 2011; 37:784–790.
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005; 2:75–79.
- Eliasziw M, Young SL, Woodbury MG, Fryday-Field K. Statistical methodology for the concurrent assessment of interrater and intrarater reliability: using goniometric measurements as an example. Phys Ther 1994; 74:777–788.
- Hasani A, Pavia D, Agnew JE, Clarke SW. Regional mucus transport following unproductive cough and forced expiration technique in patients with airways obstruction. Chest 1994; 105:1420–1425.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.

