Abstract
Studies on interaction of tumor cells with ECM components showed increased extracellular protease activity mediated by the family of matrix metalloproteinases (MMPs). Here we studied the effect of human prostate adenocarcinoma PC-3 cells–fibronectin (FN) interaction on MMPs and the underlying signaling pathways. Culturing of PC-3 cells on FN-coated surface upregulated MMP-9 and MMP-1. This response is abrogated by the blockade of α5 integrin. siRNA and inhibitor studies indicate possible involvement of phosphatidyl-inositol-3-kinase (PI-3K), focal adhesion kinase (FAK) and nuclear factor-kappaB (NF-κB) in FN-induced upregulation of MMPs. FN treatment also enhanced phosphorylation of FAK, PI3K, protein kinase B (PKB or Akt), nuclear translocation of NF-κB, surface expression of CD-44, and cell migration. Our findings indicate that, binding of PC-3 cells to FN, possibly via α5β1 integrin, induces signaling involving FAK, PI-3K, Akt, NF-κB followed by upregulation of MMP-9 and MMP-1. CD-44 may have role in modulating MMP-9 activity.
INTRODUCTION
Tumor cells interact with extracellular matrix (ECM) by specific cell surface receptors and these interactions influence tumor development, cell survival, cell proliferation, and cell migration (CitationJuliano, 2002). ECM is mainly filled with intricate network of macromolecules like glycoproteins, fibrous proteins, etc. Some important examples of ECM components are fibronectin (FN), laminin (LN), vitronectin, collagen-IV, etc. Among them FN is well characterized having binding sites for other matrix macromolecules, and for receptors on cell surfaces. The motifs of cell–ECM interaction are Arg-Gly-Asp (RGD) and Pro-His-Ser-Arg-Asn (PHSRN) in FN molecule (CitationAkiyama et al., 1995). Among the cell-surface integrin receptors, α5β1 is major FN receptor on most cells. Integrin-mediated responses to FN include adhesion, downstream signaling to cause cell migration, assembly of cytoskeleton, invasion, etc. (CitationAkiyama et al., 1989; CitationSen et al., 2010).
Metastatic potential of cancer cells is determined by their ability to disrupt tissue architecture by ECM degradation and remodeling. Culturing cancer cells in presence of intact matrix proteins such as FN or LN or matrix-derived peptides was found to increase invasive and metastatic potential (CitationTerranova et al., 1984). Dysregulated proteolysis has been implicated in tumor invasion and metastasis in multiple model systems (CitationMunshi & Stack, 2006). Metastatic cells produce several extracellular proteinases, including matrix metalloproteinases (MMPs) that process ECM components. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of several human cancer cell lines to FN in serum-free medium was observed to be mediated by α5β1 integrin and involve participation of signaling proteins like focal adhesion kinase (FAK), phosphatidyl-inositol-3-kinase (PI3K), extracellular-signal-regulated kinases (ERK), Nuclear Factor- kappa B (NF-қB), etc. (CitationDas et al., 2008; CitationMaity et al., 2010). In several types of cancer, high expression of MMP-1 has been correlated with the depth of tumor invasion, angiogenesis, lymphangiogenesis, poor prognosis, and the presence of local and distant metastases (CitationMurray et al., 1998; CitationMurray et al., 1996; CitationShiozawa et al., 2000). In human prostate cancer cell DU-145, interaction of α5β1 integrin with the fibronectin PHSRN sequence was reported to induce activation of FAK, PI3K, and protein kinase C-δ (PKCδ) in the intracellular signaling pathway leading to MMP-1-dependent invasion (CitationZeng et al., 2006).
Prostate adenocarcinoma is now the most common malignancy in men and the second leading cause of cancer-related deaths in the United States (CitationGreenlee et al., 2001). In the European Union it is the second most common malignancy in men. Statistically significant increase in the incidence of prostate cancer has been registered in several cities in India (CitationYeole, 2008). The symptomatic phase of the disease is mainly due to occurrence of metastasis to the bone. PC-3 cell line, one of the classical cell lines of prostatic cancer, was originally derived from advanced androgen-independent bone metastasis. In this communication PC-3 cell line was used to study its response during interaction with ECM protein FN and decipher the intracellular signaling associated with the response.
METHODS
Cell culture
PC-3 (human prostate cancer cell line) was obtained from National Centre for Cell Sciences, Pune. This cell line was grown and maintained in Nutrient mixture F12 Ham medium, containing 10% FBS (GIBCO™-Invitrogen, Carlsbad, CA) in a 5% CO2 incubator at 37°C.
Treatment of cells with FN
PC-3 cells were grown on FN(10 μg/ml)-coated 35 mm petridishes (Corning, USA) in F12 SFCM or Serum-Free Culture Media (300 000 cells/ml) for required time at 37°C, 5% CO2.
Cell-adhesion assay
To assay the binding capacity of PC-3 cells to FN (440 kDa; Roche, Germany), microtitre plate wells (Corning, USA) were coated with the ligand (2.5 μg/ml, 5 μg/ml, 10 μg/ml) in triplicate. Rest of the steps were performed as previously described (CitationDas et al., 2008).
Gelatin zymography
The gelatinases were separated from the culture supernatants of control and experimental sets using gelatin sepharose 4B beads (GE Healthcare Biosciences AB, Uppsala, Sweden) with shaking for 2 h at 4°C. To assay the gelatinase activity, Gelatin Zymography was performed using a 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) co-polymerized with 0.1% gelatin (Sigma-Aldrich) as described in earlier report (CitationSen et al., 2009).
Study the effects of cell signaling inhibition on MMPs
PC-3 cells were untreated (con) or treated (exp) with anti-α3, anti-α4, and anti-α5 integrin antibodies (1 μg/ml) or with chemical inhibitors of PI3K, ERK, p38 (LY294002, PD98059, SB203580, respectively, in the concentration of 50 μM each; Promega, Madison, USA) in SFCM (CitationDas et al., 2008) for 1 h at 37°C, 5% CO2. Bay 117085 (Alexis Biochemicals, Lausen, Switzerland), the NF-κB inhibitor, was treated in 5 μM concentration (CitationDutta et al., 2010) for 24 h at 37°C, 5% CO2. Then both the control and experimental sets were grown on FN-coated (10 μg/ml) surface for 4 h in SFCM. The culture supernatant was subjected to gelatin zymography to visualize gelatinase activity and the cell extracts were subjected to western blot analysis to visualize MMP-1 expression.
Whole cell extraction
Control and treated cells were collected, washed with ice cold phosphate-buffered saline (PBS), and were extracted in lysis buffer [50 mM Tris pH 7.5, 150 mM NaCl, 1% NP40, 0.1% SDS, 0.5% Deoxycholate, Protease inhibitor cocktail tablets (Roche, Germany), 1 mM sodium orthovanadate, and 1 mM Sodium fluoride] on ice and clarified by centrifugation. The protein concentrations were estimated by Lowry method and equal amounts of proteins were loaded for western blot assay.
Nuclear fraction isolation
PC-3 cells were grown in absence and presence of FN (10 μg/ml) for 4 h. Cells were collected, washed twice with ice cold 1X PBS, and nuclear extract was prepared following previously described steps (CitationSen et al., 2010). Equal amounts of proteins from each fraction was loaded and analyzed by western blot assay.
Western blot analysis
Equal amounts (100 μg) of protein from whole cell or cytoplasmic or nuclear extracts from control and experimental sets were subjected to western blot analysis using primary antibodies [anti-α5, anti-FAK, anti-phospho-FAK (Tyr-397), anti-PI3K (p85), anti-phospho-PI3K (Tyr-508), anti-NF-κB, anti-Akt, anti-phospho-Akt (Thr-308), anti-phospho-Akt (Ser-473), anti-E-cadherin, anti-VEGF, anti-integrin-linked kinase (ILK), anti-phospho-ILK (Thr-173), and anti-MMP-1; 1 μg/2 ml dilution for each] followed by the respective secondary antibodies (1 μg/200 ml dilution) using methods discussed previously (CitationGanguly et al., 2012). Blots were reprobed with anti β-tubulin antibody as internal loading control. All of these antibodies were purchased from Santa Cruz Biotechnology, Inc., USA. Anti-TBP antibody, used for loading control for nuclear protein, was purchased from Abcam Inc, Cambridge, MA, USA. [ILK: Integrin-linked kinase, VEGF - Vascular endothelial growth factor, TBP: TATA box-binding protein]
Immunocytochemical analysis
PC-3 cells were grown on coverslips in absence and presence of 10 μg/ml FN for required time periods. After PBS wash, cells were fixed with 3.5% formaldehyde and treated with 0.5% Triton X-100 (Amresco, USA). Non-specific sites were blocked using 1% BSA solution. Cells were then incubated with anti-FAK and anti-CD-44 (Santa Cruz Biotechnology Inc., USA) primary antibodies (1 μg/ml), washed thrice with PBS and incubated with respective secondary antibodies (1 μg/ml dilution) in humid chamber. In the case of FAK, FITC labeled secondary antibody (Santa Cruz Biotechnology Inc., USA) was used. Cells were washed six times with PBS and coverslips were mounted on glass slides and observed under fluorescence microscope. In the case of CD-44, biotin coupled secondary antibody (Santa Cruz Biotechnology Inc., USA) was used. After PBS wash and 30 min incubation in an avidin–biotinylated peroxidase complex reagent (Vector Laboratories, Burlingame, CA), expressions were visualized with diaminobenzidin tetrahydrochloride (Pierce Biotechnology, USA) treatment. Cells were washed with PBS, coverslips were mounted on glass slides, viewed under bright field microscope.
Wound-healing assay
PC-3 cells (300 000 cells/ml) were cultured in a monolayer in absence and presence of FN (10 μg/ml, 4 h). The monolayer was then scratched with a sterile pipette tip, followed by washing with SFCM to remove cellular debris. The cells were maintained in fresh SFCM and cell migration was observed under microscope and photographed at successive time intervals (0, 15 and 24 h).
Transwell assay
24-well transwell plate (Corning, USA) with 12 inserts were taken and the lower chamber of each well was poured with 0.6 ml (as suggested by manufacturer) fresh F12 medium containing 5% FBS. Control and FN-treated (10 μg/ml, 4 h) PC-3 cells (100 000 cells/insert) were seeded in triplicate on membrane in the upper chamber of the insert and kept for 24 and 48 h 37°C, 5% CO2. Further steps were performed as previously described (CitationSen et al., 2010).
Semi quantitative reverse transcriptase-polymerase chain reaction
RNA was extracted from 1 × 106 PC-3 cells grown in absence and in presence of FN (10 μg/ml) for 4 h. Cells were washed in PBS and total RNA was extracted (RNAqeous, Ambion, USA). Two-step reverse transcriptase-polymerase chain reaction (RT-PCR) (Retroscript, Ambion, USA) was done with equal amounts of total RNA using specific primers (Operon, Germany) for human MMP-9 and TIMP-1 genes. Glyceraldehyde phosphate dehydrogenase (GAPDH) primers (Operon, Germany) were used as control to normalize for mRNA integrity and equal loading. Equal amounts of each PCR products were run on a 2% agarose gel and bands were visualized under UV. The primer sequences and PCR cycles/conditions for each primer are given below. The PCR cycles in all cases were started with Taq activation at 94°C for 5 min and ended with final extension of 72°C for 7 min. The primer sequences are as follows:
hMMP-9: 5′-CGCTACCACCTCGAACTTTG-3′ (forward);
5′GCCATTCACGTCGTCCTTAT-3′-(reverse);
hTIMP-1: 5′-CACCCACAGACGGCCTTCTGC-3′- (forward);
5′-AGTGTAGGTCTTGGTGAAGCC-3′-(reverse);
GAPDH: 5′-CGGAGTCAACGGATTTGGTCGTAT- 3′ (forward); and
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (reverse).
Conditions used for PCR consisted of 40 cycles for MMP-9, and TIMP-1 at 94°C for 30 s, 58°C for 30 s, and 72°C for 90 s in thermal cycler (CitationGanguly et al., 2012).
FAK inhibition by siRNA treatment
PC-3 cells were seeded on 35 mm dishes and grown to 60% confluence. For the transfection process, FAK siRNA and negative control siRNA were transfected using LipofectamineTM 2000 following the manufacturer's protocol. The transfection agent (Lipofectamine 2000; Invitrogen, Life Technologies, USA) was mixed with serum-free culture medium. Subsequently, respective siRNA [FAK siRNA (h), sense- GCAUGUGGCCUGCUAUGG; antisense- CCAUAGCAGGCCACAUGC; Santa Cruz Biotechnology] mixed with serum-free culture medium (SFCM) was added to the Lipofectamine medium and incubated at room temperature for additional 45 min. The mixtures were then diluted in serum-free culture medium and added to each dish so that the final concentration of the siRNA in each plate was 60 nM. The mixture was overlaid on the cells in full media without serum and without antibiotic and incubated at 37°C in presence of 5% CO2 for overnight. Then the transfection mixture was replaced with fresh media supplemented with 10% FBS and antibiotic and the transfected cells were allowed to grow for 24 h. Cells were exposed to FN in SFCM for 4 h. The SFCM was subjected to gelatin zymography and cells were extracted for western blot.
RESULTS
FN induces gelatinolytic activity and mRNA expression of MMP-9 with possible involvement of α5 integrin in PC-3 cells
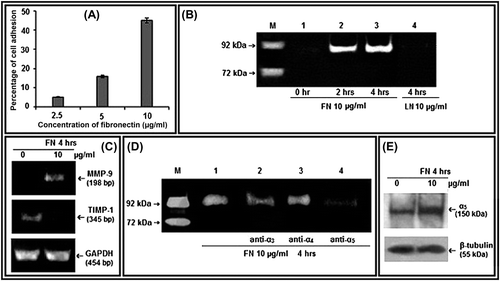
Cell-adhesion assay showed that PC-3 cells bind to FN and the percentage of adhesion increases with the increase in concentration of FN (). PC-3 cells grown on FN-coated surface showed increase in MMP-9 gelatinolytic activity in the culture supernatant with appreciable upregulation at 10 μg/ml for 4 h. Treatment of PC-3 cells with coated LN, another ECM component, at 10 μg/ml concentration for 4 h does not show detectable gelatinolytic activity in gelatin zymography. Thus it shows specificity of FN to upregulate secreted MMP-9 activity in PC-3 cells (). RT-PCR analysis of FN-treated PC-3 cells (10 μg/ml for 4 h) indicates increase in expression of MMP-9 mRNA and decrease in TIMP-1 mRNA (). α3, α4, α5 integrins were masked using their respective antibodies (1 μg/ml, for 1 h at 37°C in CO2 incubator) before culturing the PC-3 cells on FN-coated surface (10 μg/ml, 4 h). Gelatin zymography of culture supernatant showed appreciable downregulation of FN-induced MMP-9 activity in α5 integrin-blocked cells (). FN at 10 μg/ml for 4 h was also shown to enhance α5 protein expression in whole cell extracts by western blot assay ().
Figure 1. Effect of FN on MMP-9, TIMP-1, and α5 integrin in PC-3 cells: A. Cell-adhesion assay was done with different concentrations (2.5, 5, and 10 μg/ml) of FN and percentages of adhesion were plotted. B. PC-3 cells were grown in SFCM in absence (Lane 1) and in presence of 10 μg/ml of FN for 2 h (Lane 2), for 4 h (Lane 3), and 10 μg/ml of LN for 4 h (Lane 4). Culture supernatant of HT-1080 cells grown in SFCM for 24 h was used as MMP-9 (92 kDa)/MMP-2 (72 kDa) marker (Lane -M). The culture supernatants in all the cases were subjected to gelatin zymography. C. PC-3 cells were grown in serum-free culture medium (SFCM) in absence (0) and in presence of 10 μg/ml FN for 4 h. 2 steps RT-PCR was done with equal amounts of total RNA, using MMP-9 and TIMP-1 specific primers for PCR. GAPDH primers were used to confirm equal loading. D. PC-3 cells were treated without (Lane 1) or with 1 μg/ml anti- α3 (Lane 2), anti-α4 (Lane 3), and anti-α5 (Lane 4) integrin antibodies for 1 h and then all the sets were grown in presence of 10 μg/ml FN for 4 h. Gelatin zymography of the culture supernatants were performed. E. Following FN treatment (10 μg/ml for 4 h), PC-3 cells were collected, extracted, and equal amounts of protein (100 μg) was subjected to western blot analysis with anti-α5 antibody (1 μg/2 ml dilution) and respective HRP-coupled secondary antibody (1 μg/200 ml dilution) for ECL method. β-tubulin was used as internal control.
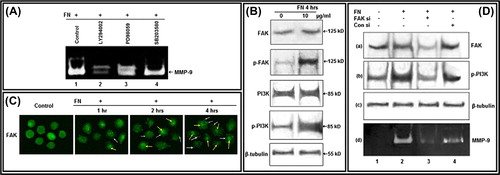
Involvement of FAK and PI3K in FN-induced MMP-9 activity
To identify the cell signaling components involved in FN-induced MMP-9 upregulation, specific chemical inhibitors were used to treat the cells prior to culturing with FN. FN-induced MMP-9 gelatinolytic activity was considerably decreased with treatment of the cells with PI-3K inhibitor (LY 294002), whereas no appreciable effect was observed upon treatment with ERK inhibitor (PD 98059) and p38 inhibitor (SB 203580) (). Western blot analysis reveals that, FN treatment appreciably induces phosphorylation of FAK at Tyr-397 and phosphorylation of PI3K at its p85 subunit within 4 h (). FN treatment on PC-3 cells for various time periods induces gradual migration of FAK to the focal complex (white arrow) and nucleus (yellow arrow) as visualized by immunocytochemistry (). When FAK was silenced by siRNA treatment, endogenous FAK expression was reduced in FN-treated cells. FN-mediated upregulation of MMP-9 was decreased in these cells, as visualized in zymography of the culture supernatant. FAK silencing was also found to reduce PI3K phosphorylation at p85 in FN-treated cells indicating position of FAK upstream of PI3K in this signaling pathway ().
Figure 2. Effect of FN on FAK and PI3K, and their involvement in FN-induced MMP-9 activity: A. PC-3 cells, untreated (Lane 1) or treated with PI-3K inhibitor LY 294002 (Lane 2); ERK inhibitor PD 98059 (Lane 3) and p38 inhibitor SB 203580 (Lane 4), were grown in presence of FN and gelatin zymography of the culture supernatants were performed. B. PC-3 cells were grown in SFCM in absence (0) and in presence of 10 μg/ml FN for 4 h. Equal amounts of proteins (100 μg) of cell extracts were subjected to western blot analysis with anti-FAK, anti-p-FAK, anti-PI3K, anti-p-PI3K antibodies. β-tubulin was used as internal control. C. PC-3 cells were grown on coverslips in absence (Control) and presence of FN (10 μg/ml) for 1, 2, 4 h. Immunocytochemistry was preformed with anti-FAK (1 μg/ml dilution) primary and respective FITC-labeled secondary antibody (1 μg/ml dilution). White arrows and yellow arrows show localization of FAK at focal points and nucleus, respectively. D. PC-3 cells were transfected with control and FAK siRNA (60 nM each) followed by FN treatment and then culture supernatant was subjected to zymography (d) and cell extracts were subjected to western blot analysis of FAK (a), p-PI3K (b). β-tubulin (c) was used as internal control for western blot. Lane 1 is control lane without FN or siRNA treatment, Lane 2 denotes treatment of FN (10 μg/ml for 4 h) without siRNA treatment, Lane 3 is with FN and FAK siRNA treatment, Lane 4 is with FN and control siRNA treatment.
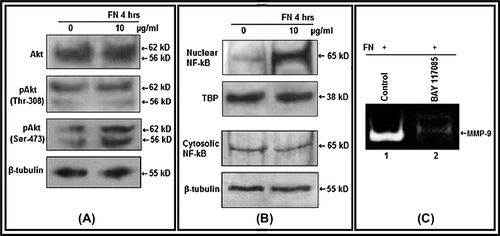
Effect of FN on Akt, NF-κB in PC-3 cells
Akt expression and its phosphorylation status at Thr- 308 position remains almost same, but appreciably enhanced at Ser-473 position in FN-treated PC-3 cells than the untreated cells as found in western blot analysis (). Western blot of nuclear fraction isolated from FN-treated PC-3 cells shows presence of elevated level of NF-κB than the untreated cells (). FN-induced MMP-9 gelatinolytic activity was considerably decreased in cells treated with NF-κB inhibitor (BAY-11-7085) before growing on FN ().
Figure 3. Effect of FN on Akt, NF-κB: A. PC-3 cells were grown in SFCM in absence (0) and in presence of 10 μg/ml FN for 4 h. Equal amounts of protein (100 μg) of cell extracts were subjected to western blot analysis with anti-Akt, anti-p-Akt (Thr-308), anti-p-Akt (Ser-473) antibodies. β-tubulin was used as internal control. B. Nuclear and cytoplasmic extracts of control (0) and FN-treated (10 μg/ml) cells were subjected to western blot analysis with anti-NF-κB antibody. TBP was used as internal control for nuclear proteins. C. PC-3 cells, untreated (Control) or treated with NF-κB inhibitor BAY-11-7085 (5 μM for 24 h), were allowed to grow on FN (10 μg/ml for 4 h in SFCM). The culture supernatants were collected and gelatin zymography was performed.
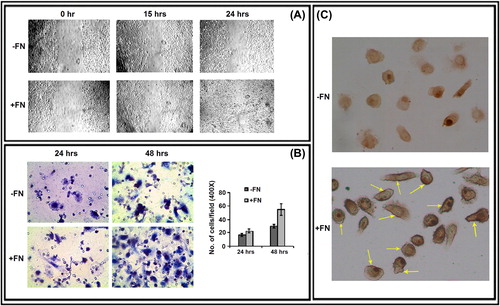
Effect of FN on migration, invasion, and CD-44 surface expression of PC-3 cells
Photographs taken at 0, 15, and 24 h during wound-healing assay of control (-FN) and FN-treated PC-3 cells indicate that cells in presence of FN are able to migrate and heal experimentally scratched wounds more efficiently than the cells in absence of FN can do (). When control and FN-treated PC-3 cells were seeded on the membrane of transwell inserts, the FN-treated cells were observed to migrate more efficiently through the membrane after 24 and 48 h (). In FN-treated PC-3 cells, CD-44 molecules were observed to be localized throughout the surface making the cell periphery darker and more prominent than the untreated control cells ().
Figure 4. Effect of FN on PC-3 cell migration, invasion and cell surface expression of CD-44: A. Wound-healing assay of PC-3 cells grown without (-FN) or with FN (+ FN). B. Transwell assay. C. Immunocytochemistry was preformed with anti-CD-44 (1 μg/ml dilution) primary and respective biotin-labeled secondary antibody (1 μg/ml dilution).
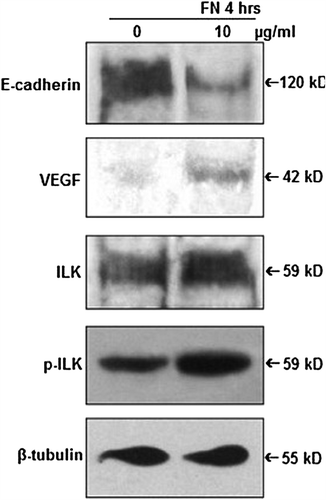
Effect of FN on E-cadherin, VEGF, ILK
PC-3 cells were grown without (0) or with 10 μg/ml FN for 4 h and cell lysates were subjected to western blot assay, which shows downregulation of E-cadherin expression, upregulation of expression of VEGF and phosphorylation of ILK in FN-treated cells in comparison with the untreated cells ().
Figure 5. Effect of FN on E-cadherin, VEGF and ILK: PC-3 cells were grown in SFCM in absence (0) and in presence of 10 μg/ml FN for 4 h. Equal amounts of protein (100 μg) of cell extracts were subjected to western blot analysis with anti-E-cadherin, anti-VEGF, anti-ILK, anti-p-ILK antibodies. β-tubulin was used as internal control.
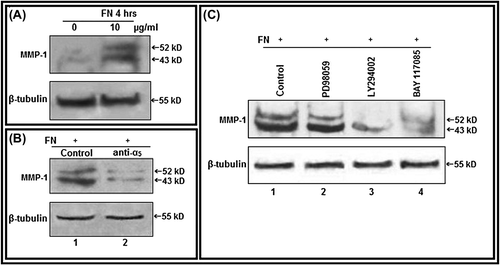
Effect of FN on MMP-1 expression and associated cell signaling proteins
In western blot assay of cell lysates of PC-3 cells, both the pro (52 kDa) and active form (43 kDa) of MMP-1 expressions were found to be upregulated in FN-treated (10 μg/ml for 4 h) cells in comparison with control cells, grown without FN (). Appreciable downregulation of FN-induced MMP-1 expression was observed in PC-3 cells, where α5 integrin was masked (). FN-induced MMP-1 expression was considerably decreased with treatment of the cells with PI-3K inhibitor (LY 294002) and NF-κB inhibitor (BAY-11-7085), whereas little or no appreciable effect was observed upon treatment with ERK inhibitor (PD 98059) ().
Figure 6. Culture of PC-3 cells on FN-coated surface modulate MMP-1: A. PC-3 cells (300 000 cells/ml) were grown in SFCM in absence (0) and in presence of 10 μg/ml FN for 4 h. Equal amounts of protein (100 μg) of cell extracts were subjected to western blot analysis with anti-MMP-1 antibody. B. PC-3 cells, untreated or treated with anti-α5 integrin antibody (1 μg/ml for 1 h), were allowed to grow in presence of FN (10 μg/ml for 4 h). Cell extracts (100 μg) were subjected to western blot analysis with anti-MMP-1 antibody. C. PC-3 cells, untreated (Lane 1) or treated with ERK inhibitor PD98059 (Lane 2), PI-3K inhibitor LY294002 (Lane 3) (50 μM each in SFCM for 1 h) and NF-κB inhibitor (Lane 4) BAY-11-7085 (5 μM for 24 h), were allowed to grow in presence of FN (10 μg/ml for 4 h). Cell extracts (100 μg) were subjected to western blot analysis with anti-MMP-1 antibody. β-tubulin was used as internal control.
DISCUSSION
Many physiological processes viz wound healing, tissue remodeling, angiogenesis, embryo development, etc. are mediated by regulated degradation of ECM proteins by MMPs (CitationYan & Boyd, 2007). A loss of activity control of MMPs, which are precisely regulated at the level of transcription, activation of the precursor zymogens, interaction with specific ECM components, and inhibition by endogenous inhibitors, may result in a range of diseases including cancer (CitationVisse & Nagase, 2003). Our interest is to study the effect of tumor cell–ECM interaction and its downstream signaling that control the expression and activity of MMPs in various cancer cell lines (CitationDas et al., 2008; CitationSen et al., 2010; CitationMaity et al., 2011a,Citationb). In the present study highly metastatic prostate adenocarcinoma cell line PC-3 is found to bind ECM protein FN in dose-dependent manner. As a result of this adhesion-mediated signaling expression and activity of MMP-9 and MMP-1 are enhanced appreciably.
The pathways involving the FN-induced upregulation of MMP-9 and MMP-1 in PC-3 cell line were not fully elucidated. To decipher it we initially studied α5 integrin, which is normally expressed in these cells but upregulated further in presence of FN. This is consistent with our previous finding in a different cell line (CitationSen et al., 2010). Masking of integrin α5 drastically reduce the MMP-9 expression. In earlier reports α5α1 integrin, receptor for FN, was shown to play major role in FN-mediated downstream signaling leading to upregulation of MMPs in different other cell lines (CitationDas et al., 2008; CitationJia et al., 2004; CitationMaity et al., 2010; CitationPal et al., 2012; CitationSen et al., 2010). Integrins activate various protein tyrosine kinases including FAK, which is an important component of focal adhesion. Localization of FAK at focal adhesion points in presence of FN was observed in our study. Upon activation, FAK is phosphorylated at Tyr-397 (CitationGiancotti & Ruoslahti, 1999) and is evident in the current study in presence of FN. Activation of FAK is coupled to assembly of focal adhesions, which plays major role in cell attachment and migration (CitationGilmore & Romer, 1996; CitationMitra et al., 2005). In our study, FN-treated cells showed enhanced migration. FAK is also a potent regulator of MMP-9 expression and activity (CitationGanguly et al., 2012; CitationSein et al., 2000). Silencing the FAK by siRNA appreciably reduced gelatinolytic activity of FN-induced MMP-9. Thus FAK may play a very pivotal role in FN-induced upregulation of MMP-9 in PC-3 cells. Apart from these, FAK may have some other effects also in these cells. Some recent findings implicate involvement of FAK in regulating gene expression directly (CitationGolubovskaya et al., 2009). Nuclear translocation of FAK (in addition to its localization to the focal complex), in presence of FN in our study indicatates possibility of its additional involvements in gene regulation, but further research is needed in this field.
Among the downstream cellular signaling targets of activated FAK, PI3K is found to be phosphorylated at its p85 regulatory subunit in response to FN treatment in our study. The major autophosphorylation site of FAK (Tyr-397) is one of the binding sites for the SH2 domains of p85 and is responsible for the in vivo association of FAK with PI3K (CitationChen et al., 1996). Inhibition of PI3K downregulates MMP-9 activity in FN-treated PC-3 cells. Silencing FAK also reduced PI3K phosphorylation and MMP-9 activity. These may indicate position of FAK upstream of PI3K in the FN-induced FAK/PI3K signaling leading to MMP-9 upregulation.
The serine threonine kinase Akt (Protein kinase B or PKB) is one of the downstream targets of PI3K (CitationCorvera & Czech, 1998). Activation of Akt is thought to involve a conformational change and phosphorylation on two residues. The major phosphorylation site lies within the kinase domain activation loop (Thr 308). In addition, a second phosphorylation site in the C-terminus (Ser 473) is required for full or maximal activity. We observed that FN-upregulated phosphorylation of Akt (Ser 473) appreciably. This phosphorylation event may be done by ILK directly or indirectly by favoring PKB/Akt autophosphorylation (CitationPersad et al., 2001). ILK is a Ser/Thr kinase that can interact with the cytoplasmic domains of β1 integrins and couple integrins and growth factor receptors to downstream signaling pathways promoting suppression of apoptosis, cell survival, cell migration, and invasion (CitationYoganathan et al., 2002). FN-induced upregulation of p-ILK, found in our study, may also contribute to activation of Akt in presence of FN in PC-3 cells in addition to FAK/PI3K. PI3K-Akt signals control several transcription factors, including NF-κB (CitationBader et al., 2005). In human cancer cells, NF-κB positively regulates cell survival, proliferation, chemoresistance, angiogenesis, cellular invasion, and oncogenesis (CitationKarin et al., 2002; CitationNakanishi & Toi, 2005). NF-κB is negatively regulated by IκB, which binds NF-κB in the cytoplasm. Positive regulation occurs through IκB kinase (IKK), which may be activated by active Akt. When IKK phosphorylates IκB, NF-κB is released and translocated to the nucleus to form a transcriptional activator of several regulatory proteins (CitationKane et al., 1999). The present work shows that, FN treatment induces nuclear translocation of NF-κB in PC-3 cells and NF-κB inhibition reduces FN-induced MMP-9 activity. In several cell lines MMP-9 upregulation was found to be dependent on transcriptional modulation of MMP-9 gene, requiring involvement of NF-κB (CitationTai et al., 2008). Taken together these informations indicate that, NF-κB may be an essential component in FN-mediated signaling in PC-3 cells and possibly involved in transcriptional modulation to upregulate MMP-9 level. Comparative RT-PCR assay indicates increase in MMP-9 gene expression and decrease in TIMP-1 gene expression, which is a negative regulator of MMP-9, in FN-treated PC-3 cells and cumulatively exhibits enhanced gelatinolytic activity of MMP-9.
Effect of upregulated MMP-9 expression is enhanced by its increased activation, which is seemed to be mediated by CD-44 because of its elevated cell-surface localization in FN-induced cells. CD-44 can function as docking molecule for MMP-9 activity and help in cell migration (CitationKaradag et al., 2005; CitationSamanna et al., 2007). Reduction of surface level of CD-44 reduces MMP-9 activity, whereas MMP-9 knocked down PC-3 cells show reduced CD-44 at cell surface. Hence CD-44 surface expression and MMP-9 activation on cell surface are interdependent (CitationDesai et al., 2009). Treatments that enhance surface localization of CD-44, which is FN induction in our study, should favor enhanced MMP-9 activity. Such activity may become crucial for cell invasion and metastasis through degradation of the basement membrane required for tumor cell intravasation or extravasation. This fact is further supported by the transwell assay.
It has been reported that α5β1 integrin-mediated signaling upregulate MMP-1 in human breast cancer (CitationJia et al., 2004). In PC-3 cells we have observed appreciable upregulation of MMP-1 expression and activity in FN-induced cells, but not in α5 integrin masked cells. Interestingly, inhibition of PI3K and NF-κB have downregulated FN-induced MMP-1 expression indicating similar pathway of MMP-9 regulation may be involved in modulation of MMP-1 activity. We extended our study to find the effect of FN-induced signaling on some of the key proteins which play important roles in angiogenesis and metastasis, thereby cancer progression namely VEGF, E-cadherin. VEGF is known to be a potent inducer of angiogenesis. The switch from vascular quiescence to angiogenesis involves MMP-9, which is upregulated in angiogenic islets and tumors, rendering VEGF more available to its receptors (CitationBergers et al., 2000). MMP-9 is capable to release biologically active VEGF from the ECM of cancer cells (CitationHawinkels et al., 2008). In our system we have showed upregulation of both MMP-9 and VEGF in response to FN, drawing a possible connection between FN-mediated signaling and induction of angiogenic switch. E-cadherin is a calcium-dependent cell–cell adhesion molecule which with its cytoplasmic tail forms a powerful invasion suppressor complex (CitationNoë et al., 2001). In ovarian cancer, loss of E-cadherin followed by upregulation of α5 integrin was found to enhance metastasis (CitationSawada et al., 2008). Downregulation of E-cadherin and upregulation of α5 integrin in presence of FN in our study may increase invasive property of cancer cells.
CONCLUSION
Therefore it may be concluded that, FN induces signaling through α5β1 integrin in PC-3 cells and possibly mediates signaling through FAK/PI3K/Akt/NF-κB pathway leading to upregulation of MMP-9 and MMP-1. Apart from the FAK/PI3K signaling, full activation of Akt may require involvement of ILK. FN treatment induces cell-surface localization of CD-44, which may enhance MMP-9 activity. Upregulation of intracellular VEGF levels and downregulation of E-cadherin level in presence of FN may favor angiogenesis and metastasis, respectively. This model study may be implicated in broader sense to understand the role of tumor microenvironment in progression of prostate cancer and to provide scopes for future clinical interventions by targeting these signaling pathways of FN-induced MMP upregulation and invasion of prostate cancer.
ACKNOWLEDGMENTS
The authors wish to express their thanks to Director, Chittaranjan National Cancer Institute, for academic, financial, and infrastructural support.
Declaration of Interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Akiyama SK, Olden K, Yamada KM (1995). Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 14: 173–189.
- Akiyama SK, Yamada SS, Chen WT, Yamada KM (1989). Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 109: 863–875.
- Bader AG, Kang S, Zhao L, Vogt PK (2005). Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 5: 921–929.
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D (2000). Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2: 737–744.
- Chen HC, Appeddu PA, Isoda H, Guan JL (1996). Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 271: 26329–26334.
- Corvera S, Czech MP (1998). Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 8: 442–446.
- Das S, Banerji A, Frei E, Chatterjee A (2008). Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci. 82: 467–476.
- Desai B, Ma T, Zhu J, Chellaiah MA (2009). Characterization of the expression of variant and standard CD44 in prostate cancer cells: identification of the possible molecular mechanism of CD44/MMP9 complex formation on the cell surface. J Cell Biochem. 108: 272–284.
- Dutta A, Sen T, Chatterjee A (2010). Culture of K562 human myeloid leukemia cells in presence of fibronectin expresses and secretes MMP-9 in serum-free culture medium. Int J Clin Exp Pathol. 3: 288–302.
- Ganguly KK, Sen T, Pal S, Biswas J, Chatterjee A (2012). Studies on Focal Adhesion Kinase in Human Breast Cancer cell MDA-MB-231. Adv Biol Chem. 2: 29–42.
- Giancotti FG, Ruoslahti E (1999). Integrin signaling. Science. 285: 1028–1032.
- Gilmore AP, Romer LH (1996). Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 7: 1209–1224.
- Golubovskaya VM, Zheng M, Zhang L, Li JL, Cance WG (2009). The direct effect of focal adhesion kinase (FAK), dominant-negative FAK, FAK-CD and FAK siRNA on gene expression and human MCF-7 breast cancer cell tumorigenesis. BMC Cancer. 9: 280.
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001). Cancer statistics, 2001. CA Cancer J Clin. 51: 15–36.
- Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, Fontijn RD, David G, Hommes DW, Lamers CB, Sier CF (2008). VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer. 44: 1904–1913.
- Jia Y, Zeng ZZ, Markwart SM, Rockwood KF, Ignatoski KM, Ethier SP, Livant DL (2004). Integrin fibronectin receptors in matrix metalloproteinase-1-dependent invasion by breast cancer and mammary epithelial cells. Cancer Res. 64: 8674–8681.
- Juliano RL (2002). Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 42: 283–323.
- Kane LP, Shapiro VS, Stokoe D, Weiss A (1999). Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 9: 601–604.
- Karadag A, Fedarko NS, Fisher LW (2005). Dentin matrix protein 1 enhances invasion potential of colon cancer cells by bridging matrix metalloproteinase-9 to integrins and CD44. Cancer Res. 65: 11545–11552.
- Karin M, Cao Y, Greten FR, Li ZW (2002). NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2: 301–310.
- Maity G, Choudhury PR, Sen T, Ganguly KK, Sil H, Chatterjee A (2011a). Culture of human breast cancer cell line (MDA-MB-231) on fibronectin-coated surface induces pro-matrix metalloproteinase-9 expression and activity. Tumour Biol. 32: 129–138.
- Maity G, Fahreen S, Banerji A, Roy Choudhury P, Sen T, Dutta A, Chatterjee A (2010). Fibronectin-integrin mediated signaling in human cervical cancer cells (SiHa). Mol Cell Biochem. 336: 65–74.
- Maity G, Sen T, Chatterjee A (2011b). Laminin induces matrix metalloproteinase-9 expression and activation in human cervical cancer cell line (SiHa). J Cancer Res Clin Oncol. 137: 347–357.
- Mitra SK, Hanson DA, Schlaepfer DD (2005). Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 6: 56–68.
- Munshi HG, Stack MS (2006). Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 25: 45–56.
- Murray GI, Duncan ME, O’Neil P, McKay JA, Melvin WT, Fothergill JE (1998). Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 185: 256–261.
- Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE (1996). Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 2: 461–462.
- Nakanishi C, Toi M (2005). Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 5: 297–309.
- Noë V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M (2001). Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 114: 111–118.
- Pal S, Ganguly KK, Moulik S, Chatterjee A (2012). Modulation of MMPs by Cell Surface Integrin Receptor α5β1. Anticancer Agents Med Chem. 12: 726–732.
- Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S (2001). Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 276: 27462–27469.
- Samanna V, Ma T, Mak TW, Rogers M, Chellaiah MA (2007). Actin polymerization modulates CD44 surface expression, MMP-9 activation, and osteoclast function. J Cell Physiol. 213: 710–720.
- Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, Lengyel E (2008). Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 68: 2329–2339.
- Sein TT, Thant AA, Hiraiwa Y, Amin AR, Sohara Y, Liu Y, Matsuda S, Yamamoto T, Hamaguchi M (2000). A role for FAK in the Concanavalin A-dependent secretion of matrix metalloproteinase-2 and -9. Oncogene. 19: 5539–5542.
- Sen T, Dutta A, Maity G, Chatterjee A (2010). Fibronectin induces matrix metalloproteinase-9 (MMP-9) in human laryngeal carcinoma cells by involving multiple signaling pathways. Biochimie. 92: 1422–1434.
- Sen T, Moulik S, Dutta A, Choudhury PR, Banerji A, Das S, Roy M, Chatterjee A (2009). Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 84: 194–204.
- Shiozawa J, Ito M, Nakayama T, Nakashima M, Kohno S, Sekine I (2000). Expression of matrix metalloproteinase-1 in human colorectal carcinoma. Mod Pathol. 13: 925–933.
- Tai KY, Shieh YS, Lee CS, Shiah SG, Wu CW (2008). Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-κB and Brg-1. Oncogene. 27: 4044–4055.
- Terranova VP, Williams JE, Liotta LA, Martin GR (1984). Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science. 226: 982–985.
- Visse R, Nagase H (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 92: 827–839.
- Yan C, Boyd DD (2007). Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 211: 19–26.
- Yeole BB (2008). Trends in the prostate cancer incidence in India. Asian Pac J Cancer Prev. 9: 141–144.
- Yoganathan N, Yee A, Zhang Z, Leung D, Yan J, Fazli L, Kojic DL, Costello PC, Jabali M, Dedhar S, Sanghera J (2002). Integrin-linked kinase, a promising cancer therapeutic target: biochemical and biological properties. Pharmacol Ther. 93: 233–242.
- Zeng ZZ, Jia Y, Hahn NJ, Markwart SM, Rockwood KF, Livant DL (2006). Role of focal adhesion kinase and phosphatidylinositol 3’-kinase in integrin fibronectin receptor-mediated, matrix metalloproteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer Res. 66: 8091–8099.
