Abstract
In the present study, we have investigated the effect of the cannabinoid R(+)methanandamide (MET) in the androgen-resistant prostate cancer PC3 cells. MET induced a dose-dependent decrease in PC3 cell viability as well as a dose-dependent increase in the secretion of the cytokine IL-6. Looking deeper into the mechanisms involved, we found that MET-induced de novo synthesis of the lipid mediator ceramide that was blocked by the ceramide synthase inhibitor Fumonisin B1. Pre-incubation of cells with the cannabinoid receptor CB2 antagonist SR 144528 (SR2), but not the CB1 antagonist Rimonabant or the TRPV1 antagonist capsazepine, partially prevented the anti-proliferative effect, the ceramide accumulation, and the IL-6-induced secretion, suggesting a CB2 receptor-dependent mechanism. Fumonisin B1 did not have any effect in the IL-6 secretion increase induced by MET. However, even an incomplete down-regulation of (i.e., not a total silencing of) ceramide kinase expression by specific siRNA prevented the MET-induced IL-6 secretion. These results suggest that MET regulates ceramide metabolism in prostate PC3 cells which is involved in cell death as well as in IL-6 secretion. Our findings also suggest that CB2 agonists may offer a novel approach in the treatment of prostate cancer by decreasing cancer epithelial cell proliferation. However, the interaction of prostate cancer cells with their surrounding, and in particular with the immune system in vivo, needs to be further explored.
Introduction
Prostate cancer is the most frequently diagnosed cancer and the second leading cause of cancer-related death in Western men (Jemal et al., Citation2008; Crawford, Citation2009). Early in their development, prostate tumors require androgen stimulation for growth and survival. Thus, they respond to androgen deprivation therapy (Crawford, Citation2009; Simmons and Klein, Citation2009). Following remission, however, tumors frequently recur in an androgen-independent form refractory to current treatment modalities (Yuan and Balk, Citation2009). Accumulated epidemiological studies support the idea that chronic inflammatory diseases are frequently associated with prostate cancer. Many recent reports indicate that uncontrolled, constitutive pro-inflammatory cytokine expression is induced in prostate cancer, but their function in tumors remains controversial (Germano et al., Citation2008).
Prostate epithelial cells contribute to this local cytokine secretion that could modulate cell-to-cell interactions and host anti-tumor responses in a paracrine manner (Mechergui et al., Citation2009). In particular, the cytokine interleukin (IL)-6 is secreted in great quantity in prostatic tumor glandular tissue, with a significant higher rate during any hormono-refractory phase (Mechergui et al., Citation2009). Moreover, IL-6 is also secreted by highly metastatic DU145 and PC3 cells, while the androgen-sensitive LNCaP cells did not produce a significant IL-6 level. Although IL-6 levels correlate with prostate cancer progression, its role in tumor growth still remains unrevealed and could be complex. It has been recently shown that IL-6 acts in concert with transforming growth factor (TGF)-β to induce differentiation of naive T-cells into T-helper (TH)17 cells (Oukka, Citation2008; Basso et al., Citation2009) that secrete the potent anti-tumor agent IL-21 (Spolski and Leonard, Citation2008a, Citation2008b). Thus, IL-6 plays a pivotal role in dictating the balance between the generation of regulatory T cells (Treg) and TH17 cells. Further study of the relationship between Treg and TH17 cells in the inflamed tissue and its role in prostate cancer is important for possible Treg cell-mediated therapeutic applications.
Preparations from Cannabis sativa have been used for millennia, and yet the chemical and biological bases of their pharmacological effects are still not fully understood. To date, about 60 different plant terpenophenols more or less structurally related to the main psychoactive ingredient of marijuana plant, δ9-Tetrahydrocannabinol (THC), have been isolated and defined as cannabinoids. In the past years, the interest in these compounds has been raised after the discovery of endogenous cannabinoids, such as anandamide, which are capable of mimicking the pharmacological actions of plant-derived ones (Di Marzo et al., Citation2005; Leggett, Citation2006). Both synthetic and naturally occurring cannabinoids produce their effects by acting mainly at two G-protein-coupled receptors named cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2). The CB1 receptor is expressed in high abundance within certain regions of the brain that correlate well with the observed central effects of cannabinoids, including impairments in cognition, memory, learning, and motor coordination. The CB2 receptor is mainly located in peripheral tissues such as the gut and spleen, as well as on immune cells where it is induced during inflammation (Cabral and Griffin-Thomas, Citation2009). Several endocannabinoids may also act through the cation channel TRPV1 that has been defined as the ionotropic cannabinoid receptor (Pertwee, Citation2006).
In recent years, cannabinoids and their derivatives have received much attention due to their pharmacological activities in a number of disorders (Pertwee, Citation2005, Citation2009; Hosking and Zajicek, Citation2008). Recent evidence suggests that cannabinoids are powerful regulators of cell growth and differentiation; in the past few years, the role of cannabinoids as anti-neoplastic agents have also been extensively studied. In particular, cannabinoids have been shown to exert anti-tumor effects by decreasing the viability, proliferation, adhesion, and migration upon a variety of cancer cells thereby suggesting the potential use of cannabinoids in the treatment of cancers (Bifulco and Di Marzo, Citation2002; Pisanti et al., Citation2009). On the other hand, it has been recently proposed that cannabinoids modulate the immune system by inducing both immunosuppressive and immunostimulatory effects (Di Marzo et al., Citation2000; Croxford and Yamamura, Citation2005). Nonetheless, the involvement of cannabinoid receptors and underlying mechanisms of cannabinoid action are still poorly understood.
Cannabinoid ligands, upon receptor binding, stimulate several kinase cascades involved in cell growth regulation, such as ERK, p38, and PI3K/AKT (Sanchez et al., Citation2003; Diaz-Laviada and Ruiz-Llorente, Citation2005). Several cannabinoids induce accumulation of the lipid ceramide that mediates cannabinoid-induced cell death (Velasco et al., Citation2005; Herrera et al., Citation2006). Ceramide is a membrane structural sphingolipid that has drawn attention as a bioactive signaling molecule involved in the regulation of cell growth, differentiation, senescence, and apoptosis (Saddoughi et al., Citation2008; Bartke and Hannun, Citation2009). It can be formed by de novo synthesis from serine and palmitoyl CoA (catalyzed by the enzyme serine palmitoyl transferase [SPT]) and further metabolism by a ceramide synthase. Ceramide can also be generated by hydrolysis of sphingomyelin catalyzed by sphingomyelinase. Ceramide can be metabolized to yield a variety of sphingolipid mediators with relevant roles as cell growth regulators. In particular, ceramide phosphorylation by a specific ceramide kinase (CERK) produces ceramide-P that has been shown to be involved in inflammatory responses (Lamour and Chalfant, Citation2005) and has become a new target for inflammation-related diseases (Saxena et al., Citation2008).
In the present study, we investigated the effect of the anandamide stable analogue R(+)methanandamide (MET) on IL-6 secretion by androgen-resistant tumorigenic PC3 cells, and explored the pathways involved. We found that MET induces IL-6 secretion by PC3 cells and accumulation of ceramide that is formed by de novo synthesis. The CB2 antagonist SR 144528 partially reversed both effects, suggesting a CB2-dependent mechanism. Pharmacological inhibition of ceramide synthesis did not influence the cannabinoid-induced IL-6 secretion. However, even an incomplete down-regulation of (i.e., not a total silencing of) CERK expression by siRNA inhibited the MET-induced increase in IL-6 secretion, suggesting that ceramide is further metabolized into C1P, and MET causes IL-6 secretion through ceramide phosphorylation.
Materials and methods
Reagents
R(+)methanandamide, capsazepine (CPZ), and Fumonisin B1 were purchased from Sigma (St. Louis, MO). SR 141716 (SR1; Rimonabant) and SR 144528 (SR2) were kindly provided by Sanofi Recherche (Montpelier, France). The inhibitor D609 and the enzyme diacylglycerol kinase were each obtained from Calbiochem (La Jolla, CA).
Cell lines and cell culture
Human PC3 cells were purchased from American Type Culture Collection (ATCC CRL-1435) (Rockville, MD) and cultivated as published previously (Sanchez et al., 2008). PC3 cells were routinely grown in RPMI 1640 medium supplemented with 100 IU penicillin G sodium/mL, 100 μg streptomycin sulfate/mL, and 0.25 μg amphotericin B/mL (Invitrogen, Paisley, UK). Cells were maintained at 37°C in the presence of 5% CO2. On the day before the experiments, the serum-containing medium was removed and a chemically defined medium consisting of RPMI 1640 supplemented with 5 μg insulin/mL, 5 μg transferrin/mL, and 5 ng sodium selenite/mL was added.
MTT colorimetric assay
Cells were seeded at a density of 10,000 cells/well into 24-well plates. After different treatments, MTT (5 mg/ mL) was added to the wells (10 μL/well). After 4 hr of incubation, the medium in each well was replaced with dimethyl sulfoxide (DMSO) (100 μL/well). The plates were then agitated at room temperature for 10 min and the absorbance in every well assessed at 570 nm in an ELISA plate reader (ELX 800 Biotech Instruments, Spain).
IL-6 quantification
Interleukin-6 secretion was measured in culture cell supernatants using an ELISA assay according to manufacturer’s recommendations (Diaclone Research, Besançon, France). Briefly, after the different treatments, cell supernatants were collected and incubated for 1 h in a 96-well plate that had its wells previously coated with a primary anti-human IL-6 antibody. After extensive washing with phosphate-buffered saline (PBS) containing 0.05% Tween 20, the plates were incubated with an anti-human IL-6 biotinylated antibody for 1 h, and then with streptavidin–horseradish peroxidase (HRP) for 20 min. The plates were then washed and incubated with the HRP susbstrate TMB (3,3′,5,5′-tetramethylbenzidine). Color was developed over a 15-min period, and then absorbance in each well was measured at 450 nm in the ELISA reader.
Measurement of ceramide levels
Ceramide quantification in cell lipid extracts was performed according to the method described by Bielawska et al. (Citation2001) for ceramide quantification in cultured cells. Briefly, cell pellets were suspended in 0.6 mL distilled water, and cells were disrupted at 4°C by sonication. Lipids were extracted with chloroform/methanol using the procedure of Bligh and Dyer (Citation1959) and ceramide content was determined by phosphorylation with Escherichia coli diacylglycerol kinase (Calbiochem) and [32P]-γ-ATP (6000 Ci/mmol; Perkin-Elmer, Barcelona, Spain). Products of this reaction were purified by thin layer chromatography (TLC) and quantified as previously reported (Malagarie-Cazenave et al., Citation2009).
siRNA tranfections
Cells were seeded at 2 × 105 cells/35 mm well the day before transfection. Cells were then transfected in 1 mL OPTIMEN containing 4 μg lipofectamine 2000 (Invitrogen, Carlsbad, CA) along with 100 nM CERK-specific small interfering RNA (siRNA) duplexes (5′-UGCCUGCUCUGUGCCUGUAdTdT-3′) (Applied Bio-systems, Foster City, CA) or control scrambled RNA for 72 h according to manufacturer’s protocols (Ambion/Applied Biosystems). At 24 h after transfection, the medium was removed and replaced by RPMI 1640 containing 10% fetal calf serum. At dedicated timepoints after the transfection, cells were used for IL-6 detection (ELISA) and in MTT cell viability assays.
Immunoblot (Western blot) analysis
Cultured cells were placed into a lysis buffer (50 mM Tris–HCl, [pH 7.4], 5 mM EDTA, 1 mM EGTA, and 10 mM 2-mercaptoethanol) containing 5 μg/mL leupeptin, 5 μg/ mL aprotinin, and 1 mM phenylmethylsulfonyl fluoride, and disrupted by sonication. Protein concentration in the lysates was then determined using a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Equivalent protein amounts of each sample were then separated over 10% SDS–PAGE gels and electrotransferred on to nitrocellulose transfer membranes. After blocking with 5% dried skim milk, immunoblot analysis was performed, followed by enhanced chemoluminescence detection; each of these protocols have been described in detail in previous publications from our laboratory (Sanchez et al., Citation2007). Primary antibodies anti-CERK and anti-tubulin used for the Western blots were purchased from Cell Signalling Technology (Danvers, MA).
Statistical analysis
Data are presented as the mean ± SD of the number of experiments indicated. Statistical comparisons among groups were made with a Student’s t-test and the difference was considered to be statistically significant when the P-value was < 0.05.
Results
The cannabinoid MET reduces cell viability and induces IL-6 secretion in PC3 cells
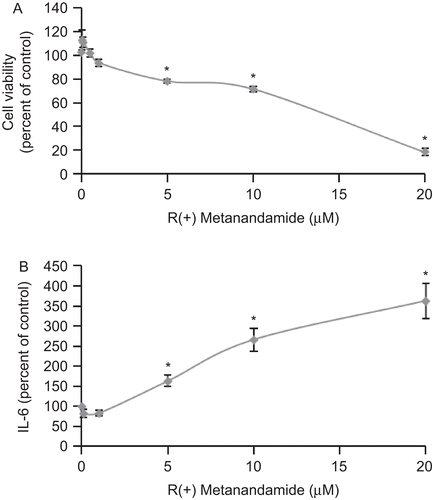
To study the effect of the cannabinoid MET on prostate cancer PC3 cells, we treated cells with different doses of MET for 48 h and then cell viability and IL-6 secretion were evaluated. Results in show that MET inhibited PC3 prostate cell growth and stimulated IL-6 secretion by prostate cells in a dose-dependent manner. We then performed a time-course study of MET action; under our conditions, the maximal effect was observed at 24–48 h (data not shown).
Figure 1. R(+)Methanandamide induces IL-6 secretion and reduces cell viability in tumor prostate PC3 cells. PC3 cells were incubated with different doses of R(+)methanandamide (MET) for 48 h after which cell viability was assayed by MTT (A) or IL-6 was measured using ELISA of the cell culture supernatant (B). Controls received medium/vehicle only. Data are the mean ± SE of three different experiments, each performed in triplicate. All values shown are based on relative value compared to control value (i.e., set at 100% for comparative purposes; cell viability control value = 0.249 absorbance units and IL-6 control value = 113 pg/mL). Statistical analysis was performed by Student’s t-test. (*P < 0.01 vs. control).
Our laboratory (Ruiz-Llorente et al., Citation2003; Sanchez et al., Citation2003) and others (Sarfaraz et al., Citation2005) have previously shown that PC3 cells express the cannabinoid receptors CB1 and CB2, as well as the TRPV1 receptor (Sanchez et al., Citation2005). To further investigate the role of the cannabinoid receptors CB1, CB2, and TRPV1 in this effect, the receptors were pharmacologically blocked with the selective Sanofi antagonists/inverse agonists Rimonabant (SR1) for CB1, SR 144528 (SR2) for CB2, and CPZ for TRPV1. As can be seen in , the CB1 receptor was not involved in the anti-proliferative effect or in the IL-6 induction exerted by MET (as inferred from the fact that the compound SR1 did not reverse any of the two effects). In contrast, the CB2 antagonist SR2 partially reversed the anti-proliferative effect of MET as well as the increase in the IL-6 secretion induced by MET on prostate cells, suggesting a CB2 receptor-dependent mechanism. The TRPV1 antagonist CPZ did not have any effect, suggesting that MET induced the antiproliferative effect as well as the IL-6 secretion independently of TRPV1 ().
Figure 2. MET induces IL-6 secretion and reduces cell viability: Involvement of CB2 receptor. PC3 cells were treated with 10 μM MET for 48 h in the presence of the CB1 antagonist SR 141716 (SR1) at 1 μM, the CB2 antagonist SR 144528 (SR2) at 2 μM, or the TRPV1 antagonist capsazepine (CPZ) at 1 μM and cell viability was assayed by MTT (A) or IL-6 was measured using ELISA in the cell supernatant (B). Data are the mean ± SE of three different experiments, each performed in triplicate. All values shown are based on relative value compared to control value (i.e., set at 100% for comparative purposes; cell viability control values [from left to right] were 0.323, 0.249, and 0.267 absorbance units and IL-6 control values [from left to right] were 145, 172, and 136 pg/mL). Statistical analysis was performed by Student’s t-test. (*P < 0.01 vs. control; #P < 0.01 vs. MET-treated cells).
![Figure 2. MET induces IL-6 secretion and reduces cell viability: Involvement of CB2 receptor. PC3 cells were treated with 10 μM MET for 48 h in the presence of the CB1 antagonist SR 141716 (SR1) at 1 μM, the CB2 antagonist SR 144528 (SR2) at 2 μM, or the TRPV1 antagonist capsazepine (CPZ) at 1 μM and cell viability was assayed by MTT (A) or IL-6 was measured using ELISA in the cell supernatant (B). Data are the mean ± SE of three different experiments, each performed in triplicate. All values shown are based on relative value compared to control value (i.e., set at 100% for comparative purposes; cell viability control values [from left to right] were 0.323, 0.249, and 0.267 absorbance units and IL-6 control values [from left to right] were 145, 172, and 136 pg/mL). Statistical analysis was performed by Student’s t-test. (*P < 0.01 vs. control; #P < 0.01 vs. MET-treated cells).](/cms/asset/a76f681b-8ff5-44cf-91be-95c0f55a3a6b/iimt_a_424343_f0002_b.gif)
MET induces de novo biosynthesis of ceramide via CB2
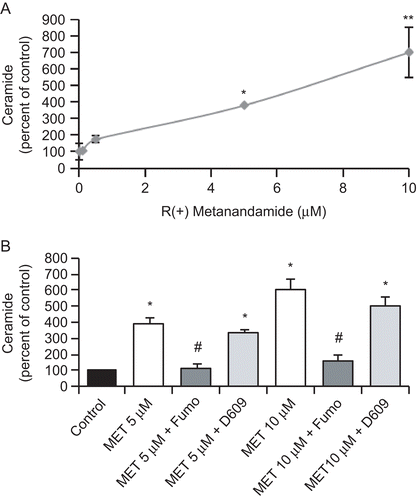
Ceramide has been described as an important intracellular mediator involved in the regulation of cell fate and metabolism. In recent years, it has garnered increasing attention for its role in modulating intracellular signaling events related to apoptosis. We thus investigated whether MET treatment-induced ceramide accumulation in prostate PC3 cells. Cells were treated with different doses of MET for 48 h and ceramide levels were then measured in cell extracts by the diacylglycerol kinase method. The results shown in indicate that MET dose-dependently induced an important ceramide accumulation (that was statistically significant starting at a 5 μM MET concentration).
To ascertain the source of ceramide production in PC3 cells, cells were pre-incubated with the ceramide synthase inhibitor Fumonisin B1, or with the sphingomyelinase inhibitor D609. As shown in , Fumonisin B1 reduced the ceramide increase in PC3 cells at both doses studied, whereas D609 did not have any effect. This result indicates that the ceramide accumulation induced by MET in PC3 cells was de novo synthesized.
Figure 3. MET induces ceramide biosynthesis in prostate PC3 cells. (A) PC3 cells were incubated with different doses of MET for 48 h and the intracellular content of ceramide was measured according to the Materials and Methods section. Controls received medium/vehicle only; control value = 960 pmol/mg protein. (B) PC3 cells were incubated with 5 μM or 10 μM MET in the presence of 50 μM Fumonisin B1 or 5 μM D609 and intracellular content of ceramide was measured. Controls received medium/vehicle only; control value = 1110 pmol/mg protein. Data are the mean ± SE of three different experiments, each performed in duplicate and are expressed as pmol ceramide/mg total protein. All values shown are based on relative value compared to control value (i.e., set at 100% for comparative purposes). Statistical analysis was performed by Student’s t-test. (*P < 0.01 vs. control; #P < 0.01 vs. MET-treated cells; **p<0.05 vs. control).
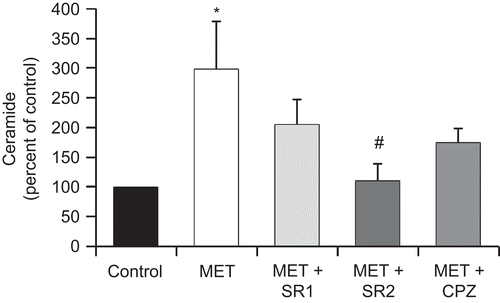
The involvement of cannabinoid receptors in the MET-induced ceramide increases was also analyzed. PC3 cells were incubated with 10 μM MET in the presence of the cannabinoid receptors antagonists SR1, SR2, and CPZ, and ceramide was then measured. As shown in , only the CB2 antagonist SR2 could significantly block the ceramide accumulation induced by MET. This result indicates that CB2 is involved in MET-induced ceramide production in prostate cells.
Figure 4. The cannabinoid receptor CB2 is involved in MET-induced ceramide accumulation. PC3 cells were incubated with 10 μM MET in the presence of the CB1 antagonist SR 141716 (SR1) at 1 μM, the CB2 antagonist SR 144528 (SR2) at 2 μM, or the TRPV1 antagonist capsazepine (CPZ) at 1 μM and intracellular content of ceramide was measured. Controls received medium/vehicle only; control value = 836 pmol/mg protein. Data are the mean ± SE of three different experiments performed in duplicate and are expressed as pmol ceramide/mg total protein. All values shown are based on relative value compared to control value (i.e., set at 100% for comparative purposes). Statistical analysis was performed by Student’s t-test. (*P < 0.01 vs. control; #P < 0.01 vs. MET-treated cells).
De novo synthesis of ceramide is not involved in R(+)methanadamide-induced IL-6 secretion
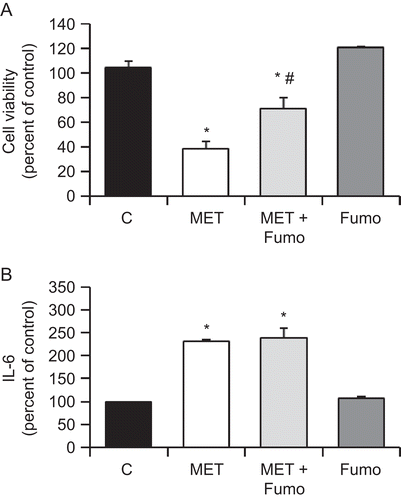
As the cannabinoid MET reduced cell viability and induced IL-6 secretion and ceramide accumulation in PC3 cells, we investigated whether those effects were related. Cells were treated with MET and the ceramide synthase inhibitor Fumonisin B1, and then viability and IL-6 secretion were measured. It was found that whereas Fumonisin B1 inhibited the cell viability decrease induced by MET in PC3 cells, it did not have any effect in the MET-induced IL-6 secretion (). This result indicated that ceramide biosynthesis was not involved in IL-6 induction by MET in prostate PC3 cells.
Figure 5. MET-induced ceramide biosynthesis is involved in the anti-proliferative effect but not in the induced secretion of IL-6. PC3 cells were incubated with 10 μM MET in the presence of 50 μM Fumonisin B1. Cell viability was then assayed by MTT (A) and IL-6 in the culture supernatant was measured using ELISA (B). Data are the mean ± SE of three different experiments, each performed in triplicate. All values shown are based on relative value compared to control value (i.e., set at 100% for comparative purposes; cell viability control value = 0.210 absorbance units and IL-6 control value = 119 pg/mL). Statistical analysis was performed by Student’s t-test. (*P < 0.01 vs. control; #P < 0.01 vs. MET-treated cells).
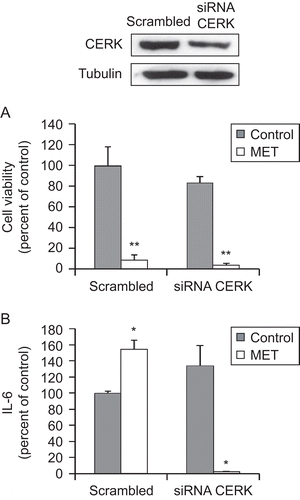
Intracellular ceramide can be metabolized into C1P by the enzyme CERK that has been shown to be involved in pro-inflammatory responses. We therefore investigated whether CERK was participating in IL-6 secretion induced by MET. CERK expression was downregulated with specific small interfering RNA (siRNA) for 72 h; thereafter, the cells were incubated with MET for 48 h and then IL-6 release was measured. The immunoblot data shown in qualitatively confirms that the siRNA employed for the knockdown was successful in causing a significant decrease (albeit, unfortunately, not a complete silencing) of CERK expression in the cells. The other results shown in indicate that even this degree of inhibition of CERK expression that was induced was sufficient to block any MET-induced IL-6 increase. This outcome suggests that ceramide must be phosphorylated to participate in the induction of IL-6 produced by MET in prostate PC3 cells.
Figure 6. Ceramide kinase is involved in the MET-induced IL-6 secretion by prostate PC3 cells. PC3 cells were transfected (for 72 h) with specific CERK siRNA or control scrambled RNA and then incubated with 10 μM MET for 48 h. Upper panel shows a representative immunoblot of the CERK expression in cell extracts after transfection of the PC3 cells with control scrambled RNA or specific CERK siRNA. Cell viability was then assayed by MTT (A) and IL-6 in the culture supernatant was measured using ELISA (B). Data are the mean ± SE of two different experiments, each performed in duplicate. All values shown are based on relative value compared to control value (i.e., set at 100% for comparative purposes; cell viability control value = 0.340 absorbance units and IL-6 control value = 210 pg/mL). Statistical analysis was performed by Student’s t-test. (*P < 0.01 vs. control; **P < 0.05 vs. control).
Discussion
It is now well accepted that uncontrolled cellular growth, which may be a result of defects in cell cycle and apoptotic machinery, is responsible for the development of most of the cancers including prostate cancer. In this regard, alterations in ceramide metabolism pathways are known to contribute to cancer cell resistance to apoptosis and overall malignancy (Pchejetski et al., Citation2008; Mahdy et al., Citation2009). Therefore, activation of cell death by the development of strategies that target ceramide pathways in androgen-independent prostate tumor cells may have significant therapeutic potential (Wang et al., Citation2004).
In the present study, we have shown that the cannabinoid MET reduces cell viability and induces IL-6 secretion in the prostate cancer androgen-resistant PC3 cell line. The anti-proliferative effect of MET is elicited partially through CB2 receptor activation and ceramide de novo synthesis, whereas IL-6-induced secretion requires further phosphorylation of ceramide. Furthermore, even an “incomplete” reduction of CERK levels was still sufficient to almost totally block MET-induced IL-6 secretion; this observation suggested to us that CERK may be a critical regulator of IL-6 secretion, and was in agreement with previous reports establishing a pro-inflammatory role for CERK (Saxena et al., Citation2008). Many chemotherapeutic agents have been shown to mediate their apoptotic effects via regulation of ceramide production (Morales et al., Citation2007; Schenck et al., Citation2007). In line with these observations, our data implicate de novo synthesized ceramide in the anti-proliferative effect of MET. Our investigation is in agreement with recent studies showing that CB2 receptor activation induced apoptosis through ceramide de novo synthesis in colon, leukemia, and pancreatic cancer cells (Carracedo et al., Citation2006; Cianchi et al., Citation2008; Herrera et al., Citation2006) and reduced glioma cell invasion (Blazquez et al., Citation2008).
The fact that the CB2 receptor is involved in the anti-proliferative effects of cannabinoids in prostate cells has special relevance, since a normal prostate mainly expresses CB1 receptor (Ruiz-Llorente et al., Citation2003; Tokanovic et al., Citation2007) and CB2 expression levels are higher in prostate cancer cells than in normal cells (Sarfaraz et al., Citation2005). However, since the CB2 antagonist only partially prevented MET-induced cell death, it cannot be ruled out—and in fact is likely—that other mechanisms are most likely involved in the anti-proliferative effect observed from the treatment with MET. In this regard, it has been recently reported that cannabinoids may act as ligands of the PPAR family of nuclear receptors (Sun and Bennett, Citation2007). Moreover, recent data have shown that MET induces apoptosis in human cervical carcinoma cells via COX-2 and PPARγ receptor (Eichele et al., Citation2009). This potential involvement of other putative cannabinoid receptors or PPAR receptors deserves further investigation.
It is well accepted that tumor cells interact with their surrounding by the paracrine secretion of many local modulators which may promote growth and metastasis or by contrary can protect against tumor development to support the immune response that stimulate the infiltration of cytotoxic T-cells (CTL) and natural killer (NK) cells into the tumor microenvironment. It has been recently demonstrated that IL-6/other cytokines are required in the acute stages of inflammation due to their role in modulating the dynamics of innate immune cell recruitment and activation (McLoughlin et al., Citation2004; Sander et al., Citation2008). There is now a large body of data indicating that the cannabinoid receptor type 2 (CB2) is linked to a variety of immune responses in humans and animals, and more recently have been shown to modulate TH cell development, chemotaxis, and tumor development (Cabral and Griffin-Thomas, Citation2009). In the present study, we have shown that the cannabinoid MET dose-dependently induced IL-6 secretion by prostate epithelial cancer cells through CB2 activation and ceramide phosphorylation by CERK.
Results from this study show, for the first time, that cannabinoids induce IL-6 cytokine release by prostate epithelial cells. Our results suggest that these cells may play a significant role in sustaining and amplifying the inflammation process through local production of pro-inflammatory cytokines that, in turn, could promote recruitment and activation of additional immune cells in/to the prostate. The possibility that the CB2 receptor is linked to the cytokine production in vivo needs to be explored further.
Overall, our data reveal novel insights into the role of CB2 in the regulation of prostate tumor cell growth and in maintaining a homeostatic immune balance within the tumor microenvironment. Our findings might permit new perceptions to be reached as to the potential use of cannabinoid agents in prostate cancer immunotherapy. The involvement of this receptor in tumor cell death might be relevant for the design of potential cannabinoid-based anti-tumor therapies avoiding the psychoactive effects of cannabinoids, since those effects are mediated mainly by CB1.
Acknowledgements
This work was supported by Ministerio de Ciencia e Innovación (grant SAF2008-03220), Comunidad de Madrid (grants CAM/UAH CCG08-UAH/BIO-3914 and CAM S-SAL-0261-2006), and Comunidad Castilla-LaMancha (Grant PII1/09-0165-0822). SM-C receives a fellowship from the Juan de la Cierva programme from the Spanish MEC. NO-H and DV receive fellowships from the University of Alcalá.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bartke, N., and Hannun, Y.A. 2009. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 50:S91–S96.
- Basso, A.S., Cheroutre, H., and Mucida, D. 2009. More stories on Th17 cells. Cell Res. 19:399–411.
- Bielawska, A., Perry, D.K., and Hannun, Y.A. 2001. Determination of ceramides and diglycerides by the diglyceride kinase assay. Anal. Biochem. 298:141–150.
- Bifulco, M., and Di Marzo, V. 2002. Targeting the endocannabinoid system in cancer therapy: A call for further research. Nat Med. 8:547–550.
- Blazquez, C., Salazar, M., Carracedo, A., Lorente, M., Egia, A., Gonzalez-Feria, L., Haro, A., Velasco, G., and Guzman, M. 2008. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res. 68:1945–1952.
- Bligh, E.G., and Dyer, W.J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917.
- Cabral, G.A., and Griffin-Thomas, L. 2009. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 11:e3.
- Carracedo, A., Gironella, M., Lorente, M., Garcia, S., Guzman, M., Velasco, G., and Iovanna, J.L. 2006. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 66:6748–6755.
- Cianchi, F., Papucci, L., Schiavone, N., Lulli, M., Magnelli, L., Vinci, M.C., Messerini, L., Manera, C., Ronconi, E., Romagnani, P., Donnini, M., Perigli, G., Trallori, G., Tanganelli, E., Capaccioli, S., and Masini, E. 2008. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor alpha-mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res. 14:7691–7700.
- Crawford, E.D. 2009. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology 73:S4–S10.
- Croxford, J.L., and Yamamura, T. 2005. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J. Neuroimmunol. 166:3–18.
- Di Marzo, V., Melck, D., De Petrocellis, L., and Bisogno, T. 2000. Cannabimimetic fatty acid derivatives in cancer and inflammation. Prostaglandins Lipid Med. 61:43–61.
- Di Marzo, V., De Petrocellis, L., and Bisogno, T. 2005. The biosynthesis, fate, and pharmacological properties of endocannabinoids. In: Cannabinoids [Handbook Exp. Pharmacol., Vol. 168 (Pertwee, R., Ed.), Berlin: Springer-Verlag, pp. 147–185.
- Diaz-Laviada, I., and Ruiz-Llorente, L. 2005. Signal transduction activated by cannabinoid receptors. Mini Rev. Med. Chem. 5:619–630.
- Eichele, K., Ramer, R., and Hinz, B. 2009. R(+)-methanandamide-induced apoptosis of human cervical carcinoma cells involves a cyclooxygenase-2-dependent pathway. Pharm. Res. 26:346–355.
- Germano, G., Allavena, P., and Mantovani, A. 2008. Cytokines as a key component of cancer-related inflammation. Cytokine 43:374–379.
- Herrera, B., Carracedo, A., Diez-Zaera, M., Gomez del Pulgar, T., Guzman, M., and Velasco, G. 2006. The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp. Cell Res. 312:2121–2131.
- Hosking, R.D., and Zajicek, J.P. 2008. Therapeutic potential of cannabis in pain medicine. Br. J. Anesth. 101:59–68.
- Jemal, A., Siegel, R., Ward, E., Hao, Y., Xu, J., Murray, T., and Thun, M.J. 2008. Cancer statistics, 2008. CA Cancer J. Clin. 58(2):71–96.
- Lamour, N.F., and Chalfant, C.E. 2005. Ceramide-1-phosphate: The “missing” link in eicosanoid biosynthesis and inflammation. Mol. Interv. 5:358–367.
- Leggett, T. 2006. A review of the world cannabis situation. Bull. Narc. 58:1–155.
- Mahdy, A.E., Cheng, J.C., Li, J., Elojeimy, S., Meacham, W.D., Turner, L.S., Bai, A., Gault, C.R., McPherson, A.S., Garcia, N., Beckham, T.H., Saad, A., Bielawska, A., Bielawski, J., Hannun, Y.A., Keane, T.E., Taha, M.I., Hammouda, H.M., Norris, J.S., and Liu, X. 2009. Acid ceramidase up-regulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Mol. Ther. 17:430–438.
- Malagarie-Cazenave, S., Olea-Herrero, N., Vara, D., and Diaz-Laviada, I. 2009. Capsaicin, a component of red peppers, induces expression of androgen receptor via PI3K and MAPK pathways in prostate LNCaP cells. FEBS Lett. 583:141–147.
- McLoughlin, R.M., Hurst, S.M., Nowell, M.A., Harris, D.A., Horiuchi, S., Morgan, L.W., Wilkinson, T.S., Yamamoto, N., Topley, N., and Jones, S.A. 2004. Differential regulation of neutrophil-activating chemokines by IL-6 and its soluble receptor isoforms. J. Immunol. 172:5676–5683.
- Mechergui, Y.B., Ben Jemaa, A., Mezigh, C., Fraile, B., Ben Rais, N., Paniagua, R., Royuela, M., and Oueslati, R. 2009. The profile of prostate epithelial cytokines and its impact on sera prostate-specific antigen levels. Inflammation 32:202–210.
- Morales, A., Lee, H., Goni, F.M., Kolesnick, R., and Fernandez-Checa, J.C. 2007. Sphingolipids and cell death. Apoptosis 12:923–939.
- Oukka, M. 2008. Th17 cells in immunity and autoimmunity. Ann. Rheum. Dis. 67(S3):26–29.
- Pchejetski, D., Doumerc, N., Golzio, M., Naymark, M., Teissie, J., Kohama, T., Waxman, J., Malavaud, B., and Cuvillier, O. 2008. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Mol. Cancer Ther. 7:1836–1845.
- Pertwee, R.G. 2005. Pharmacological actions of cannabinoids. In: Cannabinoids [Handbook Exp. Pharmacol., Vol. 168 (Pertwee, R., Ed.), Berlin: Springer-Verlag, pp. 1–51.
- Pertwee, R.G. 2006. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obesity (London) 30(Suppl. 1):S13–S18.
- Pertwee, R.G. 2009. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 156:397–411.
- Pisanti, S., Malfitano, A.M., Grimaldi, C., Santoro, A., Gazzerro, P., Laezza, C., and Bifulco, M. 2009. Use of cannabinoid receptor agonists in cancer therapy as palliative and curative agents. Best Pract. Res. Clin. Endocrinol. Metab. 23:117–131.
- Ruiz-Llorente, L., Sanchez, M.G., Carmena, M.J., Prieto, J.C., Sanchez-Chapado, M., Izquierdo, A., and Diaz-Laviada, I. 2003. Expression of functionally-active cannabinoid receptor CB1 in the human prostate gland. Prostate 54:95–102.
- Saddoughi, S.A., Song, P., and Ogretmen, B. 2008. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell. Biochem. 49:413–440.
- Sanchez, M.G., Ruiz-Llorente, L., Sanchez, A.M., and Diaz-Laviada, I. 2003. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1) and CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell Signal 15:851–869.
- Sanchez, M.G., Sanchez, A.M., Collado, B., Malagarie-Cazenave, S., Olea, N., Carmena, M.J., Prieto, J.C., and Diaz-Laviada, I.I. 2005. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur. J. Pharmacol. 515:20–27.
- Sanchez, A.M., Malagarie-Cazenave, S., Olea, N., Vara, D., Chiloeches, A., and Diaz-Laviada, I. 2007. Apoptosis induced by capsaicin in prostate PC-3 cells involves ceramide accumulation, neutral sphingomyelinase, and JNK activation. Apoptosis 12:2013–2024.
- Sanchez, A.M., Martinez-Botas, J., Malagarie-Cazenave, S., Olea, N., Vara, D., Lasuncion, M.A., and Diaz-Laviada, I. 2008. Induction of the endoplasmic reticulum stress protein GADD153/CHOP by capsaicin in prostate PC-3 cells: A microarray study. Biochem. Biophys. Res. Commun. 372:785–791.
- Sander, L.E., Obermeier, F., Dierssen, U., Kroy, D.C., Singh, A.K., Seidler, U., Streetz, K.L., Lutz, H.H., Muller, W., Tacke, F., and Trautwein, C. 2008. Gp130 signalling promotes development of acute experimental colitis by facilitating early neutrophil/macrophage recruitment and activation. J. Immunol. 181:3586–3594.
- Sarfaraz, S., Afaq, F., Adhami, V.M., and Mukhtar, H. 2005. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 65:1635–1641.
- Saxena, S., Banerjee, M., Shirumalla, R.K., and Ray, A. 2008. Ceramide kinase: A potential anti-inflammatory target? Curr. Opin. Invest. Drugs 9:455–462.
- Schenck, M., Carpinteiro, A., Grassme, H., Lang, F., and Gulbins, E. 2007. Ceramide: Physiological and pathophysiological aspects. Arch. Biochem. Biophys. 462:171–175.
- Simmons, M.N., and Klein, E.A. 2009. Combined androgen blockade revisited: Emerging options for the treatment of castration-resistant prostate cancer. Urology 73:697–705.
- Spolski, R., and Leonard, W.J. 2008a. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26:57–79.
- Spolski, R., and Leonard, W.J. 2008b. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr. Opin. Immunol. 20:295–301.
- Sun, Y., and Bennett, A. 2007. Cannabinoids: A new group of agonists of PPARs. PPAR Res. 23513 [Epub ahead of print]..
- Tokanovic, S., Malone, D.T., and Ventura, S. 2007. Stimulation of epithelial CB1 receptors inhibits contractions of the rat prostate gland. Br. J. Pharmacol. 150:227–234.
- Velasco, G., Galve-Roperh, I., Sanchez, C., Blazquez, C., Haro, A., and Guzman, M. 2005. Cannabinoids and ceramide: Two lipids acting hand-by-hand. Life Sci. 77:1723–1731.
- Wang, G., Reed, E., and Li, Q.Q. 2004. Apoptosis in prostate cancer: Progressive and therapeutic implications. Int. J. Mol. Med. 14:23–34.
- Yuan, X., and Balk, S.P. 2009. Mechanisms mediating androgen receptor reactivation after castration. Urol. Oncol. 27:36–41.
