Abstract
The T-helper 2 (TH2) bias associated with pregnancy may predispose the pregnant mother to the development or exacerbation of allergic disease. To determine the effects of pregnancy on pre-existing maternal sensitization, we sensitized BALB/c mice before breeding by two intratracheal aspiration (IA) exposures to the fungal allergen, Metarhizium anisopliae crude antigen (MACA). Some mice also received three IA exposures to MACA on gestational days 11, 15, and 19. After weaning, all mice were challenged IA with MACA before killing. To determine the effects of pregnancy on susceptibility to future sensitization, naïve parous and nulliparous BALB/c mice were sensitized by three IA exposures to MACA or to Hank’s buffered salt solution vehicle control. Pregnancy did not have a significant effect on individual inflammatory parameters (airway responsiveness to methacholine, total serum and bronchoalveolar lavage fluid (BALF) IgE, BALF total protein, lactate dehydrogenase activity, and total and differential cell counts) following allergen challenge in sensitized mice, regardless of post-breeding allergen exposure. In conclusion there was a weak inhibition of the overall response in mice exposed to allergen during pregnancy compared to identically treated nulliparous mice. In contrast, parous mice that did not encounter allergen post-breeding tended to have exacerbated responses. Parity had no significant impact on future susceptibility to sensitization.
Introduction
Pregnancy represents a unique period of susceptibility for both mother and fetus. The gravid uterus is considered to be a T-helper 2 (TH2)-biased environment, as the local cytokine milieu is rich in TH2 type cytokines such as interleukin-4 (IL-4) (Lin et al., Citation1993; Blois et al., Citation2004). In contrast, only low levels of T-helper 1 (TH1) cytokines, such as interferon gamma (IFNγ) and IL-12, can be demonstrated through most of pregnancy (Lin et al., Citation1993). In addition, pregnancy is associated with increased local and systemic levels of immunosuppressive hormones, such as progesterone (Blois et al., Citation2004). These circumstances are necessary for maternal tolerance of the fetus (which may be considered an allograft).
Certain conditions of the local uterine environment also occur systemically in pregnant women, including depressed cell-mediated immunity (Yip et al., Citation2006). In the case of TH2 bias, these effects might predispose the pregnant mother to the development or exacerbation of allergic disease. However, little work has been performed to investigate the effects of pregnancy (with or without concurrent allergen exposure) on maternal sensitization susceptibility. In experimental scenarios, identification of any such effect may be complicated by the sensitization protocol itself. In many animal studies, maternal sensitization has been achieved before breeding by intraperitoneal (IP) and/or subcutaneous (SC) allergen injections, frequently in the presence of adjuvant. The response produced by these aggressive protocols might mask any effects of pregnancy on pre-existing maternal sensitization or skew future responses.
There is evidence of an association between the development of allergic asthma, a chronic inflammatory disease of the airways characterized by bronchial hyperresponsiveness, airway inflammation, mucus hypersecretion, and airway remodeling, with the presence of IgE antibodies (Type I hypersensitivity) to environmental allergens, such as pollens, house dust mites, and molds (Jaakkola et al., Citation2006). Certain allergens, such as house dust mite, have been linked to the development and exacerbation of asthma (Johnson et al., Citation2004). The role that mold allergens play in the induction of asthma is less clear. An Institute of Medicine report found sufficient evidence to conclude that asthmatic symptoms can be exacerbated by exposure to molds, but could not demonstrate a clear link between molds and the induction of asthma (Institute of Medicine, Citation2004).
Our laboratory has previously demonstrated that multiple exposures to extracts of the entomopathogenic fungus Metarhizium anisopliae can induce robust responses characteristic of human allergic asthma in BALB/c mice (Ward et al., Citation2000). These responses can be obtained following intratracheal aspiration (IA) exposure to M. anisopliae crude antigen (MACA). Neither IP priming nor adjuvant administration is required for sensitization (Ward et al., Citation2000).
We hypothesized that pregnancy would be associated with an enhanced maternal allergic response to M. anisopliae fungal extract. Our first objective was to determine whether full-term pregnancy was associated with an exacerbation of pre-existing maternal allergic respiratory disease. Our second objective was to determine whether pregnancy altered maternal susceptibility to future sensitization.
Materials and methods
Animals
Eight-week-old BALB/c mice were obtained from Charles River Laboratories (Raleigh, NC). Female mice were group housed before breeding with male BALB/c mice. Females were removed after a 48-hr co-habitation with males and housed singly until pup weaning, after which they were re-grouped. All studies conformed to the principles outlined by the Animal Welfare Act and the National Institutes of Health guidelines for the care and use of animals in biomedical research.
All mice were housed in polycarbonate cages with hardwood chip bedding in an environmentally-controlled Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited animal facility. Environmental enrichment was provided in the form of mouse nestlets (Ancare Corporation, Bellmore, NY) and mouse igloos (Bio-Serv, Frenchtown, NJ). Sentinel mice were monitored serologically and were found to be free of Sendai, mouse pneumonia, mouse hepatitis, other murine viruses, and mycoplasma. Mice also were monitored for, and found to be free of, ectoparasites and endoparasites. The animal holding room temperature (22.2°C ± 1.1°C) and relative humidity (50% ± 10%) were maintained at the recommended levels. Male mice received standard rodent chow (Lab Diet 5P00; Purina Mills, St. Louis, MO), while female mice were maintained on a diet suitable for use in pregnant mice (Lab Diet 5008; Purina) and water ad libitum. Mice were acclimated 1 week at this facility before the start of the experiment.
Fungal antigen preparation
Metarhizium anisopliae strain 1080 was obtained from USDA-ARS Entomopathogenic Fungus Collection (Ithaca, NY). Extracts of M. anisopliae mycelium and spores/conidia as well as inducible enzymes filtrate were produced as described previously (Ward et al., Citation1998). Proteins >3000 Da in molecular weight were concentrated using a stirred-cell concentrator with the help of a YM-3 (MWCO 3000) filter (Amicon, Beverly, MA). Concentrated extracts were assayed for total protein concentration as described later. These component extracts/filtrates were combined in equal protein amounts to form the MACA (Ward et al., Citation1998). Dosing aliquots were stored at −20°C until use.
Treatment administration
All treatments (sensitization and challenge) were administered by IA as described previously (Ward et al., Citation1998). Briefly, mice were anesthetized by inhalation of a mixture of isoflurane and oxygen, and then vertically suspended by their incisors to facilitate treatment administration. Swallowing was prevented by gently pulling the tongue out of the mouth in an upward direction. Antigen extract was then deposited into the oropharynx while the nose was briefly occluded, inducing aspiration of the extract.
Experimental design
Determination of the effects of pregnancy on pre-existing maternal allergic sensitization
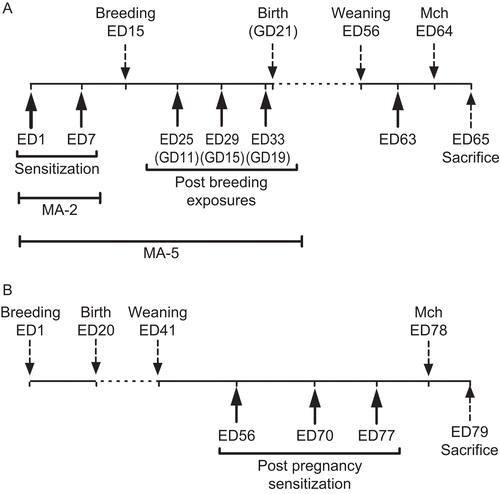
Prebreeding sensitization of female BALB/c mice was achieved by IA exposure to MACA (40 μg) in 50 μL of Hank’s buffered salt solution (HBSS) on experimental day 1 (ED1) and on ED7. Seven days after the last sensitizing exposure (ED15), mice were bred as described earlier. After breeding, some mice also received gestational (post-breeding) IA exposures to MACA on ED25, ED29, and ED33, which correspond to gestational days 11 (GD11), GD15, and GD19. These times correspond to the initial colonization of the fetal thymus with T-cell precursors, to initial T-cell responsiveness to mitogen stimulation, and to expression of surface-bound antibodies and mature T-cell receptors (TCR), respectively (Holsapple et al., Citation2003). Mice receiving only prebreeding sensitization were designated MA-2 mice, while mice that also received post-breeding exposures were designated MA-5 mice. Treatments were continued even if mice did not become pregnant. To determine whether the anesthesia protocol or the maternal sensitizing agent had a significant impact on pup viability, an analysis of pregnancy success and pup survival percentages relative to mice that had not received any treatments at all (i.e., Ctrl) was conducted.
Pups were weaned at 21 days of age (ED56). All mice received a final (challenge) exposure to MACA (40 μg in 50 μL HBSS) on ED63. Airway responsiveness to methacholine (Mch) was then evaluated the day after the final exposure (ED64); the mice were then killed 2 days after the final exposure (). Blood and bronchoalveolar lavage fluid (BALF) were obtained from each mouse for the evaluation of total serum and BALF IgE, BALF total protein, lactate dehydrogenase (LDH) activity, and both total and differential cell counts. Uteri were removed for examination to identify placental implantation sites.
Figure 1. Experimental design. (A) Determination of the effects of pregnancy on pre-existing maternal allergy. Mice received 2 IA exposures to MACA before breeding. Some mice received additional exposures post-breeding (MA-5; n=15), while others did not (MA-2; n=12). (B) Determination of the effects of pregnancy on future sensitization. MACA-naïve female mice were bred by timed cohabitation with male mice. After pup weaning, female mice received 3 IA exposures to either MACA (n=28) or HBSS (n=28).
Determination of the effects of pregnancy on susceptibility to future sensitization
MACA-naïve female BALB/c mice were bred as described earlier. All treatment assignments were distributed as evenly as possible based on pregnancy success. Two weeks after pup weaning, parous and nulliparous mice received three IA exposures to either MACA (20 μg in 50 μL HBSS) or HBSS (50 μL) on ED56, ED70, and ED77. Airway responsiveness to Mch was evaluated the day after the final dose (ED78). Mice were killed 2 days after the final dose (). Samples were collected as described earlier.
Examination and ammonium sulfide staining of uteri
After killing, uteri from all bred mice were examined under a dissecting microscope to count placental implantation sites for determination of the frequency of complete or partial litter death. Subsequently, uteri were stained with ammonium sulfide to enhance the detection of fetal resorption sites (Narotsky et al., Citation2003). Briefly, uteri were placed into individual wells of a six-well tissue culture plate, each of which contained 5 mL of an ammonium sulfide solution (diluted to 2% in deionized water; Sigma-Aldrich, St. Louis, MO). After 1 hr uteri were re-examined and darkly stained implantation sites were counted.
The percentage of pregnancy success per group was calculated using the following formula: 100 * [(# mice bearing live pups)/(total # mice per group)]. The percentage of viable pups per litter was calculated using the following formula: 100 * [(# live pups)/(total # implantation sites)]. To determine whether maternal treatment had any adverse effects on pregnancy success or pup viability, the success and viability percentages were compared to those obtained from a group of female mice that were not treated either pre- or post-breeding (i.e., Ctrl).
Measurements of airway responsiveness
Airway responsiveness to Mch aerosol: Non-specific airway hyperreactivity and bronchoconstriction is a frequent symptom of asthma. The cholinergic agonist Mch has been widely used in studying non-specific bronchial reactivity. Airway responsiveness to Mch challenge was measured in unrestrained mice using whole body plethysmography (Buxco Electronics, Troy, NY). Briefly, Biosystem XA software (SFT3812, version 2.0.2.48, Buxco Electronics) was used to calculate enhanced pause (PenH), a unitless index of airway hyperreactivity that strongly correlates with lung resistance (Hamelmann et al., Citation1997). PenH is derived from whole body flow parameters [respiratory rate, tidal volume, inspiratory and expiratory times (Ti, Te), peak inspiratory and expiratory flows (PIF, PEF), and relaxation time (RT)]. PenH reflects changes in pulmonary resistance during bronchoconstriction according to the following equation: PenH = [(TE − RT) ÷ RT] × (PEF ÷ PIF). Baseline PenH was measured for 10 min, after which saline and then increasing concentrations of Mch (6.25, 12.5, and 25.0 mg/mL) were nebulized and delivered through an inlet of the chamber. Measurements of PenH were obtained for 10 min following each nebulization. “Adjusted” PenH values were obtained by subtracting baseline PenH results from subsequent postnebulization readings for each mouse.
Bronchoalveolar lavage and blood collection
Two days after the final exposure, blood and BALF samples were collected from all mice as described previously (Ward et al., Citation1998). Blood samples were collected by cardiac puncture and stored at room temperature for 30 min before centrifugation. Serum was stored at −20°C. The lungs were lavaged twice with 0.7 mL aliquots of HBSS. BALF aliquots for each animal were pooled and stored on ice. The BALF was centrifuged at 100 3 g for 15 min at 4°C. Aliquots of BALF supernatant were assayed for total protein and LDH activity (as described later), and the remainder was stored at −20°C for IgE quantification by ELISA. The cell pellet was resuspended in 1 mL HBSS and cytospin preparations of 200 μL BALF were made by centrifugation onto glass slides (200 rpm, 10 min on a Shandon Cytospin; Shandon Inc., Pittsburgh, PA). Following Wright-Giemsa staining (Fisher Scientific, Fairlawn, NJ), cells were differentially counted at 200 cells per slide (one slide per animal). Total BALF cell counts were obtained from the resuspended cells using a Coulter counter (Coulter Corp., Miami, FL).
Total IgE ELISA
The BALF and serum total IgE ELISAs were performed as described previously (Ward et al., Citation2000). Briefly, microtiter plates were coated with rat anti-mouse IgE (Pharmingen-BD, San Diego, CA) in phosphate-buffered saline (PBS, pH 7.3; Pharmingen-BD) and incubated overnight at 4°C. Following blocking with blocking buffer (PBS plus 1% bovine serum albumin [BSA]), serum samples and the mouse IgE standard (monoclonal anti-trinitrophenol (TNP); Pharmingen-BD)—ranging from 800 to 0.7 ng/mL diluted in blocking buffer—were applied to separate dedicated wells and incubated for 1 hr. The plates were then washed three times with wash buffer (PBS plus 0.05% Tween 20; Fisher Scientific), and biotinylated detection antibody (rat anti-mouse IgE; Pharmingen; diluted to 1.5 μg/ml in blocking buffer) was then added. The plates were incubated for 1 hr and then washed six times with wash buffer. Streptavidin-horseradish peroxidase (diluted 1:10,000 in blocking buffer; Zymed-Invitrogen, Carlsbad, CA) was then applied and the plates were incubated for 30 min. After this final incubation, the plates were washed six times with wash buffer, and then (3, 3’, 5, 5’-tetramethylbenzidine (TMB) peroxidase substrate solution (Dako, Carpinteria, CA) was then applied. Optical density was read on a Thermomax® Plate Reader (Molecular Devices Corp., Menlo Park, CA) at a wavelength of 650 nm. Softmax Pro® version 2.6.1 (Molecular Devices) software was used for data collection and conversion from optical density to protein concentrations. The limit of detection for this assay was 6.25 ng/mL.
Antigen-specific IgE assay
A rat basophilic leukemia (RBL) cell β-hexosaminidase release assay was performed as an indirect measure of antigen-specific IgE in serum. The procedure used has been previously described in Chung et al. (Citation2005). Briefly, RBL-2H3 cells (ATCC, Rockville, MD) were maintained in a growth medium [71% (v/v) Eagle’s minimal essential medium (EMEM; Gibco BRL, Gaithersburg, MD), 23% (v/v) RPMI-1640 (Gibco BRL), 5% (v/v) fetal calf serum (FCS; Gibco BRL), 100 U penicillin/mL, 100 μg streptomycin/mL (Gibco BRL), and 1% (v/v) 0.2 M l-glutamine] at 37°C in a 5% CO2 humidified incubator. Cells were detached and resuspended in assay medium [93% (v/v) EMEM, 5% (v/v) FCS, 100 U penicillin/mL, 100 μg streptomycin/mL, and 1% (v/v) 0.2 M l-glutamine] then placed in 96-well flat bottom tissue culture plates (105 cells/100 μL/well). After 18 hr cells were passively sensitized by 2 hr incubation with individual mouse sera (diluted 1:5 in assay medium). Control cells for spontaneous and total release were sensitized with normal BALB/c mouse serum.
Following incubation, cells were washed three times in Tyrode’s buffer (Sigma-Aldrich). Subsequently, allergen extract (100 μL; diluted to 10 μg/mL in Tyrode’s buffer) was added and incubated 1 hr at 37°C. Spontaneous release cells were incubated with Tyrode’s buffer alone. Total release cells were incubated with Tyrode’s buffer containing 1% (v/v) Triton X-100 (Sigma). Cell supernatant (40 μL) from each well was transferred to a fresh 96-well plate. β-Hexosaminidase activity was measured by adding 80 μL of substrate (p-nitrophenyl-N-acetyl β-d-glucosaminide 1.3 mg/mL in 0.1 M citric buffer [pH 4.5]) and incubating for 1 hr at room temperature. To detect endogenous β-hexosaminidase activity, fungal extract alone in Tyrode’s buffer was also incubated with substrate. The enzyme reaction was stopped by adding 100 μL of 0.2 M glycine. Absorbance at 405 nm was measured 30 min later using a SpectraMax 340 PC Plate Reader (Molecular Devices Corp.) and Softmax Pro® version 2.6.1 software (Molecular Devices). Data are presented as an adjusted percent total release following subtraction of spontaneous release, calculated using the following formula:
Total protein and lactate dehydrogenase (LDH) assays
Elevations in BALF total protein concentration are suggestive of pulmonary vascular leakage and edema. As such, the BALF samples were assayed for total protein using Pierce Coomassie Plus Protein Assay Reagent (Pierce/Thermo Fisher Scientific, Rockford, IL). Concentrations were determined from a standard curve using BSA standards obtained from Sigma Chemical Co. (St. Louis, MO).
LDH activity in BALF can be used as an indication of non-specific cellular damage. Thus, the BALF samples were assayed for LDH activity using a commercially-prepared kit and controls from Sigma Chemical Co. The assays were modified for use on a KONELAB 30 clinical chemistry spectrophotometer analyzer (Thermo Clinical Labsystems, Espoo, Finland).
Statistical analysis
The statistical analyses for all the data in this study were performed using SAS version 9.1.3 software (SAS Institute Inc., Cary, NC) to examine the main effects of each model (maternal exposure regimen) and the effects of pregnancy success on exposure outcomes. All biochemical and differential cell count data were analyzed using an analysis of variance (Factorial ANOVA) procedure. In the case of Mch hyperresponsiveness, Factorial ANOVA with repeated measures analysis was performed. Both analyses were performed using a linear mixed-model with restricted maximum-likelihood estimation analysis and least-squares means. Significant main effects were analyzed using a post-hoc (ital) test (Tukey’s) to determine statistical differences between individual treatment groups and to adjust significance levels to account for multiple comparisons. Reported values represent means ± standard error (SE) and P < 0.05 was considered as statistically significant.
Results
The effects of pregnancy on pre-existing maternal allergic sensitization
Pregnancy success
Pregnancy is associated with profound alterations in maternal respiratory function, such as decreased pulmonary reserve and functional residual capacity (Gluck et al., Citation2006). Although these alterations are normally well compensated for, additional stressors such as general anesthesia have the potential to tip the balance toward hypoxia, especially for asthmatic patients. These conditions may result in increased fetal stress and possibly death. We conducted an analysis of pregnancy success and pup survival percentages to determine whether our anesthesia protocol or our maternal sensitizing agent had a significant impact on pup viability. No significant decrease in pregnancy success or pup viability (relative to untreated control mice; Ctrl) was seen, regardless of maternal treatment ().
Table 1. Effects of maternal treatment on pregnancy success and pup viability.
BALF analysis
Overall, the inflammatory response of sensitized mice that had been exposed to MACA after breeding (MA-5) and identically-sensitized mice without post-breeding allergen exposure (MA-2) was remarkably similar (; cohort averages). When the two groups were compared as a whole, no significant difference was observed for BALF LDH activity, total protein concentration, or total and differential cell counts (; cohort averages).
Figure 2. BALF characteristics. BALF LDH activity, total protein concentration, and total and differential cell counts in mice sensitized before breeding with or without exposure during pregnancy. Data shown are expressed as mean ± SE. Parous MA-2 n = 6; Nulliparous MA-2 n = 6; Parous MA-5 n = 5; Nulliparous MA-5 n = 10.
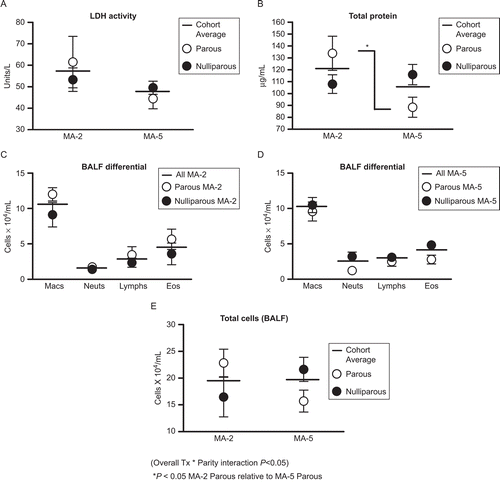
The two groups were then evaluated to determine the effects of pregnancy on the inflammatory response. No significant differences were observed between mice with successful pregnancies (parous) and mice that had not given birth (nulliparous or nonparous) for most parameters, either within or between treatment groups. One exception to this was BALF total protein, in which significantly higher levels were demonstrated in parous MA-2 mice relative to parous MA-5 mice (). There was also a significant interaction between treatment and parity for total BALF cell counts. For this parameter, parity was associated with higher cell counts in MA-2 mice and lower cell counts in MA-5 mice, as compared to identically treated nulliparous mice.
Parity was not associated with any other significant effects on BALF inflammation. However, when the response was viewed as a whole, we noted a possible trend toward a more pronounced inflammatory response in parous MA-2 mice compared to that seen in identically-treated nulliparous mice (). In contrast, the responses of parous MA-5 mice appeared to be somewhat less than that of their nulliparous treatment cohorts.
IgE analysis
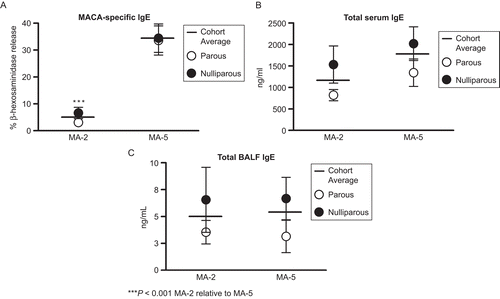
As expected, serum MACA-specific IgE levels were significantly lower in MA-2 mice as compared to MA-5 mice, which had received three more exposures to allergen (). However, no significant differences were detected between the two groups with regard to total serum or BALF IgE (). IgE levels were lower in parous versus nonparous mice of both groups (with the exception of MACA-specific IgE in MA-5 mice), but these differences were not significant.
Mch response
Non-specific airway hyperreactivity and bronchoconstriction is a frequent symptom of asthma. The cholinergic agonist Mch has been widely used in studying nonspecific bronchial reactivity. Administration of increasing doses of Mch by aerosol nebulization induced an increase in airway-enhanced pause (PenH) in all mice (). However, few differences were noted in the overall PenH response when MA-2 and MA-5 animals were compared, and no pregnancy-related effects on PenH response were seen regardless of treatment group ().
Table 2. Respiratory responses to increasing concentrations of methacholine aerosol in sensitized mice with or without post-breeding allergen exposure.
The effects of pregnancy on susceptibility to future sensitization
BALF and IgE analysis
Naïve female BALB/c mice, bred as described earlier, were treated with MACA or HBSS starting 2 weeks after their pups were weaned. As expected, MACA-treated mice demonstrated significant increases in most inflammatory parameters relative to HBSS-treated mice (–). Higher serum total and MACA-specific IgE levels were seen in MACA-treated mice, but these differences were not significant ( and ). BALF IgE concentrations did not differ appreciably (data not shown). It must be noted that all of the IgE levels were considerably lower than those usually obtained following similar exposure protocols in our laboratory (Ward et al., Citation2009), despite the otherwise robust inflammatory response. The reason for this discrepancy is unknown, but may reflect batch-dependent differences in IgE-inducing allergen components.
Figure 4. BALF characteristics. BALF LDH activity, total protein concentration, total and differential cell counts, and IgE levels in mice bred before sensitization. The assay limit of detection was 6.25 ng/mL for serum total IgE. Data shown are expressed as mean ± SE. *P < 0.05, ***P < 0.001 for MACA cohort average compared to HBSS cohort average. Parous n = 11 and Nulliparous n = 17 for both MACA and HBSS treatment groups.
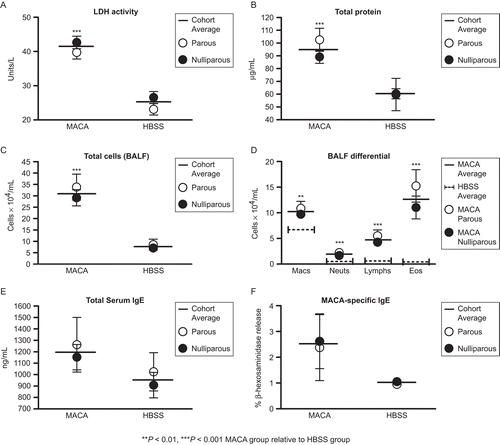
MACA- and HBSS-treated mice were then evaluated to determine the effects of prior successful pregnancy on the subsequent susceptibility to sensitization. Parity had no clear effect on the inflammatory response in either group of mice. There was a tendency for a greater response in parous relative to nulliparous MACA-treated mice for most parameters, but these differences were neither consistent nor significant ().
Mch response
Administration of increasing doses of Mch by aerosol nebulization induced an increase in airway-enhanced pause (PenH) in all mice (). However, few differences were noted in the overall PenH response when MACA and HBSS animals were compared, and no significant pregnancy-related effects on PenH response were seen regardless of treatment group ().
Table 3. Respiratory responses to increasing concentrations of methacholine aerosol in MACA and HBSS exposed mice.
Discussion and conclusions
In the present study, we examined the effects of pregnancy upon allergic responsiveness to challenge in mice sensitized to M. anisopliae fungal extract with or without post-breeding allergen exposure. With the exception of MACA-specific IgE, the inflammatory response following allergen challenge differed little between mice regardless of whether they had been exposed to allergen after breeding.
With the exceptions of BALF total protein and total cell counts, we were unable to demonstrate any significant impact of pregnancy on allergen challenge responses in sensitized mice, regardless of post-breeding allergen exposure. Nevertheless, when the inflammatory responses were evaluated as a whole, two trends were observed. First, there was a tendency for greater BALF inflammatory responses in parous MA-2 mice compared to identically treated nulliparous mice. In contrast, the reverse effect was seen in MA-5 mice, in which parous mice tended to have a lesser response than nulliparous treatment cohorts.
The biological relevance of these findings remains unclear. Although these differences did not reach statistical significance, it is tempting to speculate that pregnancy may differentially affect pre-existing allergic disease depending on whether or not the subject encounters allergen during that period. If a sensitized individual is exposed to homologous allergen while pregnant, subsequent inflammatory responses to that allergen may be inhibited. In this situation, the immunosuppressive or tolerizing aspects of pregnancy would predominate, as occurs during normal pregnancy. Antigen presentation by tolerogenic dendritic cells, generated in the face of systemic immunosuppression, to allergen-specific lymphocytes could mediate this effect. Alternately, if a sensitized subject becomes pregnant but avoids homologous allergen during pregnancy, subsequent responses may actually be exacerbated. In this case, the effects of lymphocyte exposure to an environment rich in TH2 cytokines (i.e., pregnancy) would not be ameliorated by concurrent tolerogenic dendritic cell regulation. Hormonal alterations associated with pregnancy and lactation may also play a role in mediating these effects. Progesterone is typically associated with a decrease in elicitation of allergic symptoms, whereas estrogen is more commonly associated with symptom exacerbation (Chen et al., Citation2008). Clearly, further studies are needed to replicate and provide statistical confirmation of the results of the current study. Confirmation of any such effect is particularly relevant in light of recent efforts to minimize the impact of asthma during pregnancy by decreasing environmental allergen exposure (Blaiss, Citation2004).
Parity did not appear to have an effect on future susceptibility to sensitization. Although we were able to demonstrate significant differences for most parameters in the inflammatory profile of mice sensitized and exposed to MACA compared to HBSS, we were unable to demonstrate any clear effect of parity. It is possible that any immunological perturbations associated with pregnancy do not persist long enough to significantly impact the development of postlactational maternal sensitization. A different response may have been seen if sensitization had begun either immediately postpartum or shortly thereafter.
Finally, it must be emphasized that the present study was designed to evaluate the response to a single allergen source, and did not address the potential impact of exposure to multiple allergen sources and/or to non-allergen adjuvants (such as diesel exhaust particles) on maternal allergy. In “real-world” scenarios, an expectant mother would be co-exposed to many environmental allergens and irritants. Several studies have demonstrated an enhancement of airway inflammation (including allergic responses) following coexposure to multiple allergens or to allergen and adjuvants such as diesel particles, ozone, or tobacco smoke (Wagner et al. Citation2002; Min et al., Citation2007; Inoue et al., Citation2008; Goplen et al., Citation2009). Furthermore, a study by Fedulov et al. (Citation2008) demonstrated that intranasal administration of either diesel or “inert” titanium dioxide particles was associated with a robust airway inflammatory response in pregnant (but not nonpregnant) BALB/c mice. However, it remains unclear as to whether such exposures would influence maternal response to coadministered allergen.
In summary, our results do not demonstrate a clear effect of pregnancy on pre-existing allergic sensitization or future susceptibility to sensitization. We did observe a minor inhibition of allergen challenge responses in mice exposed to allergen during pregnancy, as compared to identically treated nulliparous mice. In contrast, parous sensitized individuals that did not encounter homologous allergen during pregnancy tended to have exacerbated challenge responses.
Acknowledgments
The authors would like to thank Debora Andrews, Elizabeth Boykin, James Lehmann, Judy Richards, Dr. Yong Joo Chung, and Dr. Michael Narotsky of U.S. EPA for technical and intellectual assistance. In addition, we would like to thank Drs. Robert Luebke, MaryJane Selgrade, and Christal Bowman for their critical review of the manuscript. This work was supported by UNC/EPA training agreement CR83323701.
Disclaimer: This research paper has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Blaiss, M. S. 2004. Management of asthma during pregnancy. Allergy Asthma Proc. 25:375–379.
- Blois, S. M., Joachim, R., Kandil, J., Margni, R., Tometten, M., Klapp, B. F., and Arck, P. C. 2004. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the TH1/TH2 cytokine profile. J. Immunol. 172:5893–5899.
- Chen, W., Mempel, M., Schober, W., Behrendt, H., and Ring, J. 2008. Gender difference, sex hormones, and immediate-type hypersensitivity reactions. Allergy 63:1418–1427.
- Chung, Y. J., Coates, N. H., Viana, M. E., Copeland, L., Vesper, S. J., Selgrade, M. K., and Ward, M. D. 2005. Dose-dependent allergic responses to an extract of Penicillium chrysogenum in BALB/c mice. Toxicology 209:77–89.
- Fedulov, A. V., Leme, A., Yang, Z., Dahl, M., Lim, R., Mariani, T. J., and Kobzik, L. 2008. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am. J. Respir. Cell Mol. Biol. 38:57–67.
- Gluck, J. C., and Gluck, P. A. 2006. The effect of pregnancy on the course of asthma. Immunol. Allergy Clin. North Am. 26:63–80.
- Goplen, N., Karim, M. Z., Liang, Q., Gorska, M. M., Rozario, S., Guo, L., and Alam, R. 2009. Combined sensitization of mice to extracts of dust mite, ragweed, and Aspergillus species breaks through tolerance and establishes chronic features of asthma. J. Allergy Clin. Immunol. 123:925–932.
- Hamelmann, E., Schwarze, J., Takeda, K., Oshiba, A., Larsen, G. L., Irvin, C. G., and Gelfand, E. W. 1997. Non-invasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156:766–775.
- Holsapple, M. P., West, L. J., and Landreth, K. S. 2003. Species comparison of anatomical and functional immune system development. Birth Defects Res. B Dev. Reprod. Toxicol. 68:321–334.
- Inoue, K., Koike, E., Yanagisawa, R., and Takano, H. 2008. Impact of diesel exhaust particles on TH2 response in the lung in asthmatic mice. J. Clin. Biochem. Nutr. 43:199–200.
- Institute of Medicine. 2004. Damp Indoor Spaces and Health. Washington, D.C.: National Academies Press.
- Jaakkola, M. S., Ieromnimon, A., and Jaakkola, J. J. 2006. Are atopy and specific IgE to mites and molds important for adult asthma? J. Allergy Clin. Immunol. 117:642–648.
- Johnson, J. R., Wiley, R. E., Fattouh, R., Swirski, F. K., Gajewska, B. U., Coyle, A. J., Gutierrez-Ramos, J.-C., Ellis, R., Inman, M. D., and Jordana, M. 2004. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 169:378–385.
- Lin, H., Mosmann, T. R., Guilbert, L., Tuntipopipat, S., and Wegmann, T. G. 1993. Synthesis of T-helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151:4562–4573.
- Min, M. G., Song, D. J., Miller, M., Cho, J. Y., McElwain, S., Ferguson, P., and Broide, D. H. 2007. Co-exposure to environmental tobacco smoke increases levels of allergen-induced airway remodeling in mice. J. Immunol. 178:5321–5328.
- Narotsky, M. G., and Kavlock, R. J. 2003. In vivo assessment of prenatal developmental toxicity in rodents. In: Current Protocols in Toxicology (Bus, J. S., Costa, L. G., Hodgson, E., Lawrence, D. A., and Reed, D. J., Eds.), Hoboken, New Jersey: John Wiley & Sons, Inc., pp. 13.15.11–13.15.25.
- Wagner, J. G., Hotchkiss, J. A., and Harkema, J. R. 2002. Enhancement of nasal inflammatory and epithelial responses after ozone and allergen co-exposure in Brown–Norway rats. Toxicol. Sci. 67:284–294.
- Ward, M. D. W., Chung, Y. J., Haykal-Coates, N., and Copeland, L. 2009. Differential allergy responses to Metarhizium anisopliae fungal component extracts in BALB/c mice. J. Immunotoxicol. 6:62–73.
- Ward, M. D. W., Madison, S. L., Andrews, D. L., Sailstad, D. M., Gavett, S. H., and Selgrade, M. J. 2000. Comparison of respiratory responses to Metarhizium anisopliae extract using two different sensitization protocols. Toxicology 147:133–145.
- Ward, M. D. W., Sailstad, D. M., and Selgrade, M. K. 1998. Allergic responses to the biopesticide Metarhizium anisopliae in Balb/c Mice. Toxicol. Sci. 45:195–203.
- Yip, L., McCluskey, J., and Sinclair, R. 2006. Immunological aspects of pregnancy. Clin. Dermatol. 24:84–87.