Abstract
The intestinal environment is considered to play an important role both in colorectal tumor development and in the evolution and modulation of mucosal immunity. Studies in animals reared in germ-free (GF, without any intestinal microflora) versus conventional (CV, with regular microflora in bowel) conditions can aid in clarifying the influence of bacteria on carcinogenesis and anti-cancer immune responses in situ. The lower incidence of colon cancers and better immunological parameters in GF animals versus CV ones after chemically-induced carcinogenesis raises questions about specific characteristics of the immunological networks in each respective condition. Different levels of tolerance/regulatory mechanisms in the GF versus CV animals may influence the development of immune responses not only at the level of mucosal, but also at the systemic, immunity. We hypothesize that GF animals can better recognize and respond to evolving neoplasias in the bowel as a consequence of their less-tolerogenic immunity (i.e., due to their more limited exposure to antigens to become tolerated against at the intestinal level). In this paper, we review the role of bacteria in modulating gut environment and mucosal immunity, their importance in cancer development, and aspects of immune regulation (both at local and systemic level) that can be modified by bacterial microflora. Lastly, the use of GF animals in comparison with conventionally-raised animals is proposed as a suitable and potent model for understanding the inflammatory network and its effect on cancer immunity especially during colorectal cancer development.
Introduction
Colorectal carcinoma is a cancer still characterized by high incidence and mortality worldwide, especially in the Western world (extensively reviewed in Hewitson et al., Citation2007). In Europe alone, 412,900 cases were reported (12.9% of all cancers), along with 207,400 deaths in 2006 (Ferlay et al., Citation2006). In the United Kingdom, it is the second most commonly diagnosed cancer in females (34.8/100,000) and third in males (54.8/100,000). Similarly, it is the third most commonly diagnosed cancer in both males (62.7/100,000) and females (45.8/100,000) in the United States and the second most commonly diagnosed cancer for males (60.7/100,000) and females (52.1/100,000) in Australia.
An increase of colorectal cancer incidence in Asiatic countries has also been reported (i.e., it is now the third most common cancer). For example, in Japan and China (Hong Kong, specifically), incidence levels have been attained that were very similar to those in the United States (Sung et al., Citation2005). Investigators have indicated that most of this increase in incidence was very likely associated with the populations’ progressive adoption of a Western lifestyle and diet. Interestingly, in Africa, while the colorectal cancer incidence has been found to range from 3.7% to 10% of all diagnosed cancer, the cancers were seen to occur among a larger number of young patients (relative to the incidence in this population in Western countries) as well as among the increasingly urbanized populations (Abdulkareem et al. Citation2008). As a result, it has been suggested that general environmental factors (i.e., lifestyle, alimentation [diet]) were likely critical for enhanced carcinogenesis in the gut and colorectal carcinoma development, and these outcomes were being influenced through possible changes in the local intestinal environment (e.g., commensal microflora) that variously impacted upon the mucosal components, including immune system cells (Tlaskalova-Hogenova et al., Citation2004; McGarr et al., Citation2005).
The potential role of bacteria and inflammation in carcinogenesis and cancer progression has raised increasing interest among researchers over the last 15 years (Potter, Citation1995; Heavey and Rowland, Citation2004; Yu et al., Citation2009). A number of studies have shown inflammation to be a central event in various phases of cancer evolution due to the double-faceted activity of inflammatory cells (macrophages) and molecules (interleukin [IL]-1β, tumor necrosis factor [TNF]-α, transforming growth factor [TGF]-β, IL-4, metalloproteinases, nitric oxide [NO], etc.) in the cancer microenvironment (Balkwill and Mantovani, Citation2001; Lin and Karin, Citation2007; Borrello et al., Citation2008; Mantovani et al., Citation2008).
Intestinal bacterial flora has been described as displaying a double-edged activity, either assisting or preventing carcinogenesis, depending on the metabolic activities and degree of saprophytism of the bacterial species (e.g., Clostridia, Lactobacillus) (Hope et al., Citation2005; McGarr et al., Citation2005). The composition of the gut commensal microbiota and the prevalence of bacterial species are influenced by various factors during the lifetime of an individual (type of diet, traveling, use of antibiotics), with effects on the mucosal immune system of the gut and also the possible modulation of systemic immunity (Guarner and Malagelada, Citation2003; Cebra et al., Citation2005).
To study how microbiota, mucosal components, and immunity might cross-talk, comparative research has been developed using gnotobiologic animals versus individuals of the same strain reared under conventional conditions (i.e., with regular commensal microbiota in the gut). In fact, gnotobiologic animals can be either reared in GF conditions (with completely sterile gut) or be selectively contaminated with known bacteria. As it has been documented for more than 25 years, these animal models represent an extraordinary investigative tool for immunological and oncological studies on colorectal pathologies that occur in humans. (Readers are directed to papers from Tlaskalova-Hogenova et al., Citation1983; Stepankova, Citation1997 as good examples.)
Intestinal microbiota and local immunity
The intestinal environment is largely determined by the commensal microbiota normally present in the individual gut. The billions of bacteria forming the composite intestinal microflora start to colonize the gut after birth and undergo variations during their lifespan (Umesaki and Setoyama, Citation2000; Fanaro et al., Citation2003; Williams et al., Citation2006). Their establishment and different proportion of species can influence the development of local immunity (mucosal immunity), with concomitant effects that can extend to systemic immunity (see review of Smith et al., Citation2007). An example of this phenomenon is the induction of genes involved in innate immunity after co-colonization of the gut of gnotobiologic animals with Bifidobacterium longum and/or Bacterioides thetaiotaomicron (Turroni et al., 2008). Since only a small part of the intestinal bacterial species (≈500 species) can be cultured in vitro, there is still a lack of complete information about effects exerted by many species on the biology of the intestinal mucosa and, consequently, on mucosal and systemic immunity. Nevertheless, very recent “-omic” approaches (i.e., metagenomics, metabonomics) are trying to elucidate these aspects (Handelsman, Citation2004; Hord, Citation2008; Kinross et al., Citation2008; Tuohy et al., Citation2009).
In the bowel, especially the colon, the major microbiota (more than 90%) are obligate anaerobes, including Bacteroides, Eubacterium, Bifidobacterium. Both Escherichia coli and Lactobacillus are also present, but in more limited proportions. Many studies have shown that the balance between the bacterial populations can influence many carcinogenic processes, either by inducing tumor promotion (Clostridia) or inhibition (Lactobacillus) (Christensen et al., Citation2002; McGarr et al., Citation2005). The “eutrophism” of certain bacterial populations follows the dietary habits of the host, and the resulting metabolic products of the microbiota can possibly act as tumor promoting (e.g., nitrosamine, polyamines, etc.) or inhibiting (e.g., butyrate, etc.) factors (Olaya et al., Citation1999; McGarr et al., Citation2005).
The antigens derived from the various species of bacteria (e.g., lipopolysaccharides [LPS]), from the bacterial metabolism, and from the very large amount of ingested substances (from food, drugs, etc.), make essential the activation of immunological mechanisms permitting (oral) tolerance in order to avoid detrimental effects on the bowel in particular and the organism in general (i.e., chronic inflammatory diseases, alimentary intolerances, autoimmunity) (Tlaskalova-Hogenova et al., Citation2004; Dubois et al., Citation2005). Simultaneously while maintaining the symbiotic tolerance toward commensal microflora, the host’s immune system must still be able to counter any pathogenic bacteria assaulting the gut. The mechanisms underlying this “double play” approach are not yet completely elucidated. However, the possibility that efficient immune responses can be elicited at regional level in the bowel (involving mucosal cells, gut-associated lymphatic tissue [GALT], intraepithelial lymphocytes [IEL], dendritic cells [DC], and cells of the mesenteric lymph nodes [MLN]) (Macpherson and Harris, Citation2004; Macpherson and Uhr, Citation2004) has to be considered.
Within the gut, if antigens pass through the mucosal barrier and are directly presented to GALT (i.e., via a damaged lamina propria), they may escape the regulatory network and are then able to elicit a rapid and vigorous immune response. This response at the systemic level would give rise to the appearance of antigen-specific antibodies in the serum and, locally, an antigen-specific IgA response in the bile. Breaking the mucosal barrier and its tolerant environment, the GALT appears capable of active reactions to foreign antigens penetrating the gut wall (Nicklin and Miller, Citation1983). Because certain bacteria types (e.g., Escherichia coli, Klebsiella pneumoniae, Streptococcus viridians, Lactobacillus brevis) induce modification of the mucosal permeability, the balance among commensal microbiota species is of considerable importance (Umesaki and Setoyama, Citation2000, Christensen et al, Citation2002; Macpherson et al., Citation2005).
The GALT-articulated immunological barrier and, in particular, the IEL, DC, and macrophages, constitute a very reactive network. Because of the continuous attempts of bacteria to penetrate the mucosa, it is understandable that there must be a constant activation of the mucosal immunity to preserve mucosal integrity and immunological homeostasis. In general, this activated network has the characteristics of a “controlled” inflammatory environment, also referred to as a “physiological inflammation” (Gordon and Pesti, Citation1971; Mowat, Citation2003; Forchielli and Walker, Citation2005). The regulation of this inflammation within “physiological” limits involves secretion of at least three important cytokines: TGFβ, IL-4, and IL-10. In particular, these TH2 cytokines are (physiologically) involved in the termination of inflammatory responses and in the termination of local wound reparation processes (Hanada and Yoshimura, Citation2002; Liu et al., Citation2003).
Immune cells associated to the lamina propria along with intestinal epithelial cells (IEC) can collaborate in the production of TGFβ. There are several examples of this collaborative effect in the literature. For example, it is well known that IEC are able to secrete a variety of cytokines, permitting their active participation to the modulation of local immune responses. The IEC, because of their close proximity to B-lymphocytes in the lamina propria, may, and do, affect local antibody production (e.g., IgA, IgG, and IgM isoforms) via some of their cytokines, i.e., IL-6 and TGFβ (Goodrich and McGee, Citation1998). Smythies et al. (Citation2006) showed that IL-8 and TGFβ, that was being produced by gut epithelial cells and lamina propria mast cells, were critical factors in the regulation of mononuclear cell recruitment to both non-inflamed and inflamed intestinal mucosa. Lastly, even the mucosal cells can be harnessed by pathogens to help in thwarting the normal immune response required to encounter/remove the bacteria, in part, influencing of the cells’ abilities to release TGFβ. Specifically, Wu and colleagues recently showed that there were soluble proteins released by Helicobacter pylori that were able to preferentially induce TGFβ production by gastric epithelial cells (Wu et al., Citation2007).
Antigen tolerance, critical to maintain the symbiotic balance between mucosal cells and commensal microbiota, is enabled by the function of TGFβ-producing T-regulatory lymphocytes (CD4+CD25+Foxp3+) of the lamina propria and a special population of IL-10-producing DC. These DC are able to polarize naïve T-helper (TH0) lymphocytes to become regulatory cells producing IL-4, IL-10, and TGFβ and also stimulate B-lymphocytes to produce IgA (Makita et al., Citation2007; Tezuka et al., Citation2007). Furthermore, B-lymphocytes are also stimulated by BAFF/APRIL ligands released by IEC. The IL-10-producing DCs exert their function by migrating in the Peyer’s patches and in the MLN where the tolerance to the antigens originating from the alimentary tract is developed. In this way, these DC contribute to the control of the homeostasis in the bowel and permit the maintenance of a tolerant environment leading also to systemic tolerance and local IgA production (Bilsborough and Viney, Citation2004; Woof and Mestecky, Citation2005; Baba et al., Citation2008; ).
Figure 1. Interplay between microbiota and mucosal immunity in the gut. The figure shows a schematic view of the multiple relationships between commensal bacteria (cb), mucosal cells, and immune cells involved in the gut immune responses and homeostasis. Bacteria (b) on the mucosal surface are inhibited from invading the mucosa by both mechanical-anatomical barrier (mucus, tight intercellular junctions) and mucosal immunity activation. Immune cells involved in this network are mainly located in the lamina propria and within mucosal epithelial structures (intraepithelial lymphocytes, IEL). Innate immunity cells (e.g., macrophages, Mϕ; mast cells, MC; natural killer cells, NK) are involved (along with local dendritic cells [DC]) in recognition, killing, processing, and presentation of bacterial-/food-derived antigens to adaptive immunity T-cells (T) that are then primed and activated. These innate immunity cells, once having been activated following contact with the bacteria, generate pro-inflammatory molecules like TNFα, IL-1β, NO, and/or PGE2. If the antigens are processed passing through the lamina propria defense network, DC activation of T-helper cells in the mesenteric lymph nodes (MLN, cell maturation centers) will lead naïve (TH0) cells to develop into regulatory/tolerant (i.e., TH2, TH3, Treg) cells. The cytokines that these specific cell types produce (e.g., IL-4, IL-10, TGFβ) are essential in modulating the continuously activated mucosal immunity (“physiologic inflammation”) and for inducing IgA production by lamina propria-associated B-cells. These IgA are secreted in the mucosa (to protect it from the target microflora) and circulate systemically to induce/partake in general immunologic effects.
Part of the gut local immune response is an acute inflammatory response. This is elicited by direct contact of either bacteria or intestinal lumen antigens with immune cells “skipping” along the lamina propria barrier or via stimulation of these cells by bacterial products like lipopolysaccharides. In this case, the response is the maturation of TH1 cells, the release of TH1 cytokines (IL-2, IFNγ, TNFα), and the elicitation of cytotoxic activity. If these responses are not rigorously controlled and regularly terminated, damage to the mucosa can develop and the “physiological inflammation” can exceed the homeostatic limit and so become pathological. Activated (including tolerant) cells can pass into the lymphatics and blood vessels, entering the general circulation to impact upon systemic immune responses.
![Figure 1. Interplay between microbiota and mucosal immunity in the gut. The figure shows a schematic view of the multiple relationships between commensal bacteria (cb), mucosal cells, and immune cells involved in the gut immune responses and homeostasis. Bacteria (b) on the mucosal surface are inhibited from invading the mucosa by both mechanical-anatomical barrier (mucus, tight intercellular junctions) and mucosal immunity activation. Immune cells involved in this network are mainly located in the lamina propria and within mucosal epithelial structures (intraepithelial lymphocytes, IEL). Innate immunity cells (e.g., macrophages, Mϕ; mast cells, MC; natural killer cells, NK) are involved (along with local dendritic cells [DC]) in recognition, killing, processing, and presentation of bacterial-/food-derived antigens to adaptive immunity T-cells (T) that are then primed and activated. These innate immunity cells, once having been activated following contact with the bacteria, generate pro-inflammatory molecules like TNFα, IL-1β, NO, and/or PGE2. If the antigens are processed passing through the lamina propria defense network, DC activation of T-helper cells in the mesenteric lymph nodes (MLN, cell maturation centers) will lead naïve (TH0) cells to develop into regulatory/tolerant (i.e., TH2, TH3, Treg) cells. The cytokines that these specific cell types produce (e.g., IL-4, IL-10, TGFβ) are essential in modulating the continuously activated mucosal immunity (“physiologic inflammation”) and for inducing IgA production by lamina propria-associated B-cells. These IgA are secreted in the mucosa (to protect it from the target microflora) and circulate systemically to induce/partake in general immunologic effects.Part of the gut local immune response is an acute inflammatory response. This is elicited by direct contact of either bacteria or intestinal lumen antigens with immune cells “skipping” along the lamina propria barrier or via stimulation of these cells by bacterial products like lipopolysaccharides. In this case, the response is the maturation of TH1 cells, the release of TH1 cytokines (IL-2, IFNγ, TNFα), and the elicitation of cytotoxic activity. If these responses are not rigorously controlled and regularly terminated, damage to the mucosa can develop and the “physiological inflammation” can exceed the homeostatic limit and so become pathological. Activated (including tolerant) cells can pass into the lymphatics and blood vessels, entering the general circulation to impact upon systemic immune responses.](/cms/asset/fab57400-4cc3-453d-bb1c-2901241c2597/iimt_a_433612_f0001_b.gif)
It is also clear that the MLN represent a critical center both for the elimination of translocated bacteria and for processing of their products and other various antigens without direct challenge to the systemic immunity (Macpherson and Harris, Citation2004). In fact, the commensal bacteria are largely prevented from reaching the systemic immune compartment by efficient macrophage killing activity and by sequestering in DC at the MLN level. Intestinal inflammatory responses induced by pathogenic bacteria are driven by recognition of pathogen-associated molecular patterns (PAMPs) with involvement of Toll-like receptor (TLR) expressed on mucosal cells, mesenchymal cells, and mucosal immune cells. The pro-inflammatory cytokines (i.e., IL-1, IL-6, IL-8, and CCL5) collaborate with the activation of macrophages by lipopolysaccharides to stimulate DC (Macpherson et al., Citation2005; Baba et al., Citation2008). After uptake of the pathogenic antigens and migration in the MLN, the DC mature and produce IL-12. The primed naïve CD4+ T-helper (TH0) lymphocytes develop as TH1 and sustain the inflammatory process. The TH1 lymphocytes can express their function both locally and at the systemic level. Therefore, the tolerogenic response, produced by the DC and T-helper lymphocyte cross-talk with the epithelial cells and bacteria, induces modulation of the cytokine balance (TH1, TH2, and TH3 cytokines) as well as the inflammatory reaction elicited by TLR involvement, and, in the end, may produce effects that can influence systemic immunity (Steinman et al., Citation2003; Dubois et al., Citation2005).
Under these conditions, the highly efficient regulatory network generated through the chronic challenge of the mucosal immune cells by the commensal microbiota may affect the activation of mechanisms leading to the recognition of transformed cells (e.g., danger signals) (Gallucci and Matzinger, Citation2001). We can also hypothesize that the higher the number of antigens to be tolerated, the higher should be the possibility of cross-recognition of molecules that when expressed during the cancer cell transformation may mimic tolerated antigens.
Effects of intestinal microbial environment on cancer and natural immunity
Bacteria of different strains, including commensal microbiota, can support environmental conditions that can lead to mucosal cell transformation and/or the sustaining of tumor progression. Epidemiologic studies have estimated that ≈15% of the worldwide cancer incidence is attributable in a secondary manner to infectious agents (Kuper et al., Citation2000). In general, bacteria have been linked to cancer by two mechanisms: induction of chronic inflammation following bacterial infection and production of toxic bacterial metabolites. Some studies in human patients have revealed an interesting correlation between specific bacteria and gastrointestinal cancers (Swidsinski et al., Citation1998; Rowland, Citation2009). In 2004, Heavey and Rowland detailed some of the relationships between specific bacteria and gastrointestinal cancers, as well as pathologies (non-cancerous) that could give rise to enhanced bacterial contributions to the onset/development of neoplasias in the gut. For example, in patients with reduced gastric acid secretion (hypochlorhydria), the subjects often ultimately become achlorhydric. Hypochlorhydria is a condition that is common after gastric surgery and occurs with aging, diseases such as pernicious anaemia and hypogammaglobulinaemia, and, in patients with atrophic gastritis associated with chronic Helicobacter pylori (H. pylori) infection. The evolution to achlorhydria allows for diverse flora to establish in the stomach (i.e., usually species of salivary Neisseria, Streptococcus, and Staphylococcus, as well as Escherichia, Bacteroides, and Lactobacillus). This condition increases the probability of xenobiotic metabolism by bacteria, particularly since gastric emptying rates are often prolonged in these patients. It has been suggested (see Hill, 1988) that the increased gastric cancer risk seen in achlorhydric patients is linked, in part, to a now-increased formation of N-nitroso compounds by these gastric bacteria.
With respect to this one pathogen, both epidemiological and clinical evidence has indicated that H. pylori is associated with an increased risk of gastric carcinoma (see Kikuchi et al., Citation1995; Atherton, Citation1998). In fact, the International Agency for Research into Cancer (IARC) declared in 1997 “There is sufficient evidence in humans for the carcinogenicity of infection with Helicobacter pylori” (IARC, Citation1997), though similar conclusions in animal models were lacking. Furthermore, in developed countries, strains of H. pylori that carry the cag pathogenicity island are associated with an increased risk of adenocarcinoma compared with strains negative for cag. This effect appears to be dependent upon alterations in IL-4 balance and activity induced in the mucosa infected by cag island+ H. pylori, a status that could then promote a chronic inflammation and, ultimately, lead to metaplasia of the gastric mucosa (Moss, Citation1999; Asonuma et al., Citation2009). Even if it is generally accepted that H. pylori infection plays a significant role in the etiology of gastric cancer, however, the precise mechanisms have not yet been defined. Several proposed for H. pylori-associated carcinogenesis in humans have included: gastric epithelial cell cycle dysregulation, increased DNA adduct and free radical generation, altered growth factor secretion/cytokines, and effects that evolved secondarily to induced decreases in gastric secretion(s).
In their 2004 review, Heavey and Roland also postulated about the bacterial involvement in colorectal cancer. As they noted, most of the information on this phenomena was derived from animal work and some human studies and that evidence from a wide range of sources supported the view that the colonic microflora was involved in the etiology of local cancers. Among the major categorizations of evidence, it was indicated that: intestinal bacteria can produce, from dietary components, substances with genotoxic, carcinogenic, and tumor-promoting activities (Hambly et al., Citation1997); gut bacteria can activate pro-carcinogens to DNA-reactive forms; germ-free (GF) rats fed 1,2-dimethylhydrazine or fed human diets had a lower incidence of, respectively, colon tumors and of DNA adducts than similarly-treated rats with a normal microflora (Reddy et al., Citation1975; Rumney et al., Citation1993); and, that human fecal matter has been documented to contain mutagenic and genotoxic substances of bacterial origin (Venturi et al., Citation1997). Moreover, a direct effect of Enterococcus faecalis as an inducer of aneuploidy and tetraploidy was demonstrated in intestinal cell cultures (Wang et al., Citation2008). The DNA damage and instability was associated to the oxidative stress due to the production of extracellular superoxide, also by induction of macrophage COX-2, generating chromosomal instability. Antioxidants were able to attenuate this effect while it was worsened by the administration of inhibitors of glutathione synthase. Moreover, epithelial cells were induced to G2 cell cycle arrest by the direct contact with the bacteria (Wang and Huycke, Citation2007).
The importance of specific bacterial strains in exerting pro-carcinogenic activities is demonstrated also by studies that utilized gnotobiological animals. For example, colonization with a single strain or a selected mix of Bacteroides and Clostridium species increased the incidence of chemically-induced intestinal cancers (Onoue et al., Citation1997; Horie et al., Citation1999). These Investigators noted that the cancer-promoting activity of bacteria like Mitsuokella multiacida, Clostridium butyricum, Bifidobacterium longum, C. paraputrificum, C. butyricum, and E. coli was related to the de-conjugation of biliary acids. The expansion of these particular species of bacteria in the colon can be related to dietary factors. In particular, the prevalence of fat and red meat in the diet appear to create a favorable substrate for the survival and proliferation of such bacteria. Their metabolic products can also be mutagenic, and the risk of colorectal cancer was enhanced by their formation of polyamines and the increased formation of secondary bile acids such as deoxy- and lithocholic acids (Mastromarino et al., Citation1976; Reddy et al., Citation1980; McGarr et al., Citation2005; Rohrmann et al., Citation2009). Moreover, it is possible that these (and other) products may modify the mucosal barrier, favoring antigen penetration, deregulation of the physiological inflammation, and oxidative stress, ultimately resulting in damage to and genetic instability within the mucosal cells. In turn, the subsequent elicitation of reparative responses can additionally lead to the increased production of vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), factors useful for sustaining tumor development (Cianchi et al., Citation2004).
On the other hand, probiotics like Lactobacillus rhamnosus GG, L. reuteri, bifidobacteria and certain strains of L. casei or the L. acidophilus group have been shown to have protective effect in both animal cancer models and in some clinical trials. However, their effectiveness in anti-cancer prevention is still under evaluation. Interestingly, not only the use of living probiotic bacteria but also of some of their components should be helpful in promoting possible anti-carcinogenic effects. Choi and colleagues (Citation2006) proved this concept by showing that both L. acidophilus 606 and the soluble polysaccharide components of this strain were able to exert anti-oxidative effects and to induce apoptosis in colon cancer cell lines.
Lactic acid bacteria were also found to significantly enhance natural killing activity of spleen cells in vivo. NK1.1 positive natural killer (NK) cells and natural killer-T (NKT) cells produced interferon (IFN)-γ after stimulation with this bacterium in vitro. The IFNγ-producing cells stimulation was secondary to IL-12 production by CD11c+ DC in a TLR2- and/or TLR4-dependent manner. A direct interaction between DC and NK1.1+ cells was necessary, confirming also in this context the importance of the DC-NK cell crosstalk (Koizumi et al., Citation2008). NK1.1+ T-lymphocytes in the liver are mostly CD1d-dependent, whereas in the large intestine they are mostly CD1d-independent. While Lactobacilli (L. casei, L. rhamnosus) administered to mice stimulated selective expansion and IFN-γ expression of this subpopulation of NK1.1+ T-lymphocytes in the colon, bacteria like Pseudomonas aeruginosa, E. coli, Staphylococcus aureus, or Lactobacillus gasseri elicited activation of CD1d-dependent NKT (Takahashi et al., Citation2006, Nieuwenhuis et al., Citation2009).
Some studies have also shown that the association of probiotics with prebiotics (e.g., inulin) can better stimulate NK cell activities. In the mouse, saccharides like inulin, oligofructose, and dextran administered together with Lactobacilli or Bifidobacterium lactis more effectively enhanced NK cytotoxicity and cytokine production of spleen derived mononuclear cells, indicating systemic effectiveness of environmental manipulations at the gut level. Applied in colon cancer models in vivo, they reduced tumor incidence (Roller et al., Citation2004; Watzl et al., Citation2005).
Some evidence was also found about the capability of NK cells to inhibit effectors CD4+CD45RBhigh T-lymphocytes (producing IL-2) by a perforin-dependent mechanism in a mouse model of colitis. Consequently, the bacterial modulation of NK cell activity should exert further effects, including regulation of effector T-lymphocytes in their responses to gut–bacteria interactions (Fort et al., Citation1998). Finally, the increase of NK cell activities related to administration of pro- and pre-biotics was also documented in humans (healthy volunteers) and related to an increase in IL-12, as was evidenced in murine models (Ogawa et al., Citation2006).
When the local conditions permit the predominance of bacterial strains able to modify the mucosal barrier, we can suppose a shift from the physiologically controlled inflammatory network toward a more aggressive inflammation. Both LPS and living bacteria can induce phosphorylation of STAT3 in the mucosa activating JAK/STAT pathway and leading to IL-1β and IL-6 production (Samavati et al., Citation2009). The binding of LPS to TLR4 receptor also induces Neu1 sialidase with following activation of NF-κB. The consequent macrophage production of nitric oxide and pro-inflammatory IL-6 and TNFα cytokines can further collaborate to sustain inflammation. Additional proliferative effects and resistance to apoptosis can be induced in autocrine manner by the IL-6 through STAT3, enabling the establishment of a cancer-promoting circle (Slattery et al., Citation2007; Amith et al., Citation2009; Bromberg and Wang, Citation2009).
Germ-free animals and cancer
The field of gnotobiology utilizes GF raised animals (without any microflora) that are maintained either in amicrobial conditions or conditions associated with one/multiple known or specified bacteria. These animal models are especially useful to generically study host–bacteria interactions but, critically, are also very useful in performing detailed mechanistic studies of the immune responses to specific pathogens (akin to how knockout mice are important for helping to define/predict very specific aspects of human immune responses to pathogens or environmental toxicants, pharmaceuticals, etc.). The advantages of this particular approach (i.e., use of gnotobiotic hosts) are clear, including: the possibility to evaluate and compare immune responses in normally contaminated hosts versus GF counterparts; to elucidate how immunity works as a result of either physiological or pathological stimulation(s); the possibility to colonize a sterile host with one/more select bacterial species and, thus, uniquely clarify specific interactions with the specific pathogen(s); and, the possibility of performing investigations using animals that possess a completely efficient immune system that arises from a “natural” exposure to a very limited antigenic challenge rather than via genetic engineering (knockout/knockdown/silenced models) (Gordon and Pesti, Citation1971; Trexler, Citation1978; Tlaskalova-Hogenova, Citation1997).
As underlined in two recent reviews on gut bacteria and their role in early human development (Tannock, 2007; Wilks, Citation2007), these animal models are consistent to enlightening the importance of the cross-talk between bacteria, mucosal immunity and gut structure also in relation to human conditions. As observed by the Authors, bacterial colonization of the gut appears to be able to shape human immunity and organ development. Similarities in immunological, biological, and pathological factors and mechanisms were found between gnoto-biotic animals and humans. In particular, the bowel environment of human neonates appears be mimicked by gnotobiotic animals. In fact, newborns are initially germ-free, then show mono-association with bifidobacteria. Later on, a progressively more complex panel of bacteria become associated to the gut reaching the maximal level of colonization ≈4 years after birth. Thus, during these first years of life, bacteria (together with food components) contribute to the progressive maturation of the immature immune system and tolerance to the gut environment-related antigenic challenge(s). This is a not yet fully clarified process; thus, the gnotobiologic animals can provide an important model especially for understanding the possible long-lasting biological and immunological effects from these early-life processes and, more generally, after contact with specific bacteria (Prescott and Holt, Citation1998; Boudry et al., Citation2004; Cario and Podolsky, Citation2005; Kelly et al., Citation2005; Macpherson et al., Citation2005).
GF animals present some anatomical differences of the gut in comparison to conventionally (CV)-reared animals. For example, they present a much larger cecum (3–4 times than in CV) and their intestinal walls appear subtler (). Furthermore, the mesenteric lymph nodes in these animals are less developed than in their CV counterparts. These features suggest that the presence of commensal microbiota (and the local stimulation by them) may assist in the modeling of gut structures and the stimulation of lymphatic organ(s) development.
Figure 2. Conventional and germ-free (GF) reared mice. (A) Conventionally reared mouse (CV); (B) GF reared mouse. The cecum of both mice is framed by the interrupted line; the continuous line indicates the diameter of the cecum in its middle part. Differences in dimension are clearly evident. The GF animal has a very large and smooth cecum; its dark color is due to visibility of fecal content through a very transparent wall, and also due to a biliary acid metabolism that differs from that in conventional mice. In addition, the small bowel appears larger than in the CV animal control.
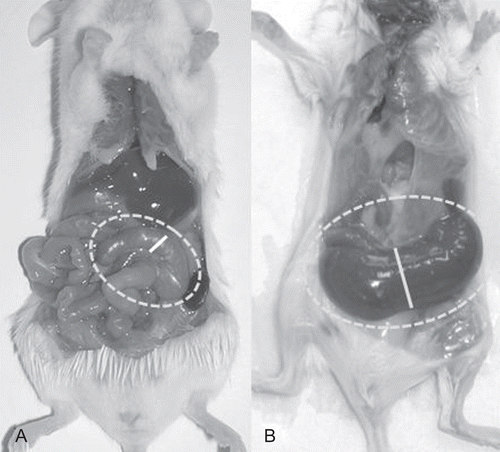
It is known that chronic inflammation, through the release of mediators, can induce hypertrophy/hyperplasia of smooth muscle cells in the gut as in other organs (e.g., airways). A similar mechanism, as a consequence of the “physiological” inflammation activated by the commensal microbiota, may also be suggested for the (re)modeling of intestinal wall structures (Blennerhassett et al., Citation1992; Tlaskalova-Hogenova, Citation1997; Blennerhassett et al., Citation1999; Chiappara et al., Citation2001; Tlaskalova-Hogenova et al., Citation2004).
In GF animals, the number of lamina propria cells (namely T-lymphocytes) is reduced. These cells appear also to be less sensitive to mitogen-induced proliferation. Same hypo-responsiveness was found in the splenocytes (Woolverton et al., Citation1992; Stepankova, Citation1998). We have also found higher numbers of T-lymphocytes in the spleen and blood of CV rats than in GF rats. An opposite situation was found with NK and NKT cells; in this case, these cell types were more numerous in GF than in CV rats (Vannucci et al., Citation2008).
After conventionalization of GF rats by gut colonization with regular commensal flora, the composition of intestinal intraepithelial lymphocyte subsets (IEL) showed an increase in, respectively, CD4+, CD8a+, CD8b+, TCRa/b+ cells. A “conventional” lymphocytic pattern was induced also in blood and spleen (Stepankova et al., Citation1998; Woolverton et al., Citation1992). These differences suggest that the relative prevalence of innate immunity cells versus adaptive immunity cells in the GF animals should be a consequence of the more limited antigenic challenges they have to sustain. This might be also a factor that is then able to influence the local immune responses toward newly expressed antigens, like cancer antigens (Vannucci et al, Citation2008).
More than 30 years ago, studies about the incidence of tumors in GF versus CV animals evidenced a reduced capability of GF animals to develop solid tumors. In 1976, Sacksteder wrote in his report on the spontaneous tumors occurring in GF rats that “for unknown reasons, significantly fewer solid tumors were observed in germfree than in conventional male rats” (Sacksteder, Citation1976). In the same and following years, Reddy et al. published various papers about the induction of colorectal cancers either with dimethylhydrazine (DMH) or azoxymethane (AOM; an active derivative of DMH) in CV and GF rats under various experimental conditions (i.e., using variations in route of administration, association of carcinogens, hyperlipidic alimentation, etc.) (Reddy and Ohmori, Citation1981; Reddy et al., Citation1975, 1976). These investigators confirmed the reduced occurrence of colon cancers in GF rats (20% incidence in GF versus 93% in CV groups).
In 2008, our own studies confirmed the lower incidence of colorectal tumors in cancer-induced GF rats, but also showed evidence of a different degree of immune reactivity between GF and CV hosts (Vannucci et al., Citation2008). This was documented at the systemic level (i.e., in the blood and spleen) by evaluating both the cytotoxic cell activity and proportions of lymphocyte subpopulations. Healthy GF and CV rats were, respectively, compared with rats that developed cancer or those that were resistant to developing cancer, after carcinogen administration. Interestingly, the cytotoxic activity in the GF rats was higher than that in CV rats, even under the “cancer conditions”. Levels of cytotoxic cells (NK and CD8+ T-lymphocytes [CTL]) were also significantly increased in the peripheral blood of cancer-resistant GF rats, both in comparison to healthy controls and CV rats with the same condition. It was noted that the colorectal cancers that developed in the GF rats were smaller than those in the CV rats, and tended to be singular entities rather than multiple tumors synchronously developed in various segment of the large bowel as was found in the CV animals (Vannucci et al., Citation2008). The different susceptibility to cancer development and the smaller tumor dimensions in the absence of bacteria can be explained in terms of various factors, both non-immunological and immunological. Specifically, the absence of bacteria would lead to a different pattern of metabolism of food components and biliary acids, thereby resulting in a more limited production of cholic acid, fatty acids, and polyamine molecules that could potentially be active pro-/co-carcinogens (Reddy et al., Citation1980; Wedeima et al., Citation1985; Olaya et al., Citation1999; Rao et al., Citation2001; Cianchi et al., Citation2004).
Still, from an immunological viewpoint, the absence of chronic pro-inflammatory environment, the reduced regulatory network in the lamina propria, the much lower amount of molecules to be tolerated, deriving from the bacterial metabolisms of alimentary products and from the same bacteria, can lead to a more naïve and plastic immunity (Vannucci et al., Citation2008; Hrncir et al., Citation2008). This should be indicated by the relatively lower proportion of T-lymphocytes versus the innate immunity cells in healthy conditions, the hypoplastic mesenteric lymph nodes, but also by a more valid immune reactivity against transformed cells, as described above. The absence of the pro-inflammatory background should permit a more prompt reaction to danger signals and the constitutive lower needs of regulatory molecules in the gut environment could permit more efficient anti-cancer responses. The earlier and more efficient immune response assumed to be elicited in these conditions should also lead to a more effective antigen presentation to DC, adaptive immunity priming and extension of the response from local also to systemic level.
Conclusions and perspectives
In conclusion, the balance between bacterial species of commensal microbiota is important for intestinal homeostasis. The mucosal homeostasis is maintained by a complex immunological network of activation-regulatory mechanisms with local and systemic effects. To dissect the role that environmental factors and bacterial balance can have on gut and organism, the GF animals represent a unique and suitable model. They aid in highlighting important mechanisms addressing local and systemic immune responses against pathological conditions, e.g., cancer. Comparative studies in CV and GF animals can efficaciously address the development of new possibilities of therapeutic interventions (e.g., manipulation of the intestinal microbiota by pre- and pro-biotics in immunomodulation or anti-cancer prevention), as well as the identification of immunological therapeutic targets and their degree of dependence on local environmental factors involved in colorectal cancer development. The comparison between GF with conventionally raised animals can also introduce new models for studying inflammatory network and its regulation.
Acknowledgements
The work was funded by the Grant Agency of the Academy of Sciences of the Czech Republic (grants IAA500200509 and IAA500200917) and by Institutional Research Concept AV0Z50200510 (CZ), and by the Cristina and Ido Gragnani Fund.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdulkareem, F. B., Abudu, E. K., Awolola, N. A., Elesh,a S. O., Rotimi, O., Akinde, O. R., Atoyebi, A. O., Adesanya, A. A., Daramola, A. O., Banjo, A. A., and Anunobi, C. C. 2008. Colorectal carcinoma in Lagos and Sagamu, Southwest Nigeria: A histopathological review. World J. Gastroenterol. 14:6531–6535.
- Amith, S. R., Jayanth, P., Franchuk, S., Siddiqui, S., Seyrantepe, V., Gee, K., Basta, S., Beyaert, R.., Pshezhetsky, A. V., and Szewczuk, M. R. 2009. Dependence of pathogen molecule-induced Toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj. J. [Epub ahead of print].
- Asonuma, S., Imatani, A., Asano, N., Oikawa, T., Konishi, H., Iijima, K., Koike, T., Ohara, S., and Shimosegawa, T. 2009. Helicobacter pylori induces gastric mucosal intestinal metaplasia through the inhibition of interleukin-4-mediated HMG box protein Sox2 expression. Am. J. Physiol. 297:G312–G322.
- Atherton, J. C. 1998. Helicobacter pylori virulence factors. Br. Med. Bull. 54:105–120.
- Baba, N., Samson, S., Bourdet-Sicard, R., Rubio, M., and Sarfati, M. 2008. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T-cells. J. Leukocyte Biol. 84:468–476.
- Balkwill, F., and Mantovani, A. 2001. Inflammation and cancer: Back to Virchow? Lancet 357:539–545.
- Bilsborough, J., and Viney, J. L. 2004. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology 127:300–309.
- Blennerhassett, M. G., Vignjevic, P., Vermillion, D. L., and Collins, S. M. 1992. Inflammation causes hyperplasia and hypertrophy in smooth muscle of rat small intestine. Am. J. Physiol. 262:G1041–1046.
- Blennerhassett, M. G., Bovell, F. M., Lourenssen, S., and McHugh, K. M. 1999. Characteristics of inflammation-induced hypertrophy of rat intestinal smooth muscle cell. Dig. Dis. Sci. 44:1265–1272.
- Borrello, M. G., Degl’Innocenti, D., and Pierotti, M. A. 2008. Inflammation and cancer: The oncogene-driven connection. Cancer Lett. 267:262–270.
- Boudry, G., Peron, V., Le Huerou-Luron, I., Lalles, J. P., and Seve, B. 2004. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 134:2256–2262.
- Bromberg, J., and Wang, T. C. 2009. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 15:79–80.
- Cario, E., and Podolsky, D. K. 2005. Intestinal epithelial TOLLerance versus inTOLLerance of commensals. Mol. Immunol. 42:887–893.
- Cebra, J. J., Jiang, H. Q., Boiko, N., and Tlaskalova-Hogenova, H. 2005. The role of mucosal microbiota in the development, maintenance, and pathologies of the mucosal immune system. In: Mucosal Immunology. 3rd Edition (Mestecky, J., Bienenstock, J., Lamm, M. E., Mayer, L., McGhee, J. R., and Strober, W., Eds), Amsterdam: Elsevier/Academic Press, pp. 335–368.
- Chiappara, G., Gagliardo, R., Siena, A., Bonsignore, M. R., Bousquet, J., Bonsignore, G., and Vignola, A. M. 2001. Airway remodeling in the pathogenesis of asthma. Curr. Opin. Allergy Clin. Immunol. 1:85–93.
- Choi, S. S., Kim, Y., Han, K. S., You, S., Oh, S., and Kim, S. H. 2006. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 42:452–458.
- Christensen, H. R., Froekiaer, H., and Pestka, J. J. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168:171–178.
- Cinchi, F., Cortesini, C., Fantappie, O., Messerini, L., Sardi, I., Lasagna, N., Perna, F., Fabbroni, V., Di Felice, A., Perigli, G., Mazzanti, R., and Masini, E. 2004. Cycoloygenase-2 activation mediates the pro-angiogenic effect of nitric oxide in colorectal cancer. Clin. Cancer Res. 10:2694–2704.
- Dubois, B., Goubier, A., Joubert, G., and Kaiserlian, D. 2005. Oral tolerance and regulation of mucosal immunity. Cell. Mol. Life Sci. 62:1322–1332.
- Fanaro, S., Chierici, R., Guerrini, P., and Vigi, V. 2003. Intestinal microflora in early infancy: Composition and development. Acta Paediatr. Suppl. 91:48–55.
- Ferlay, J., Autier, P., Boniol, M., Heanue, M., Colombat, M. and Boyle P., 2006. Estimates of cancer incidence and mortality in Europe in 2006. Ann. Oncol. 18:581–592.
- Forchielli, M. L., and Walker, W. A. 2005. The role of gut-associated lymphoid tissues and mucosal defence. Br. J. Nutr. 93(S1):41–48.
- Fort, M. M., Leach, M. W., and Rennick, D. M. 1998. A role for NK cells as regulators of CD4+ T-cells in a transfer model of colitis. J. Immunol. 161:3256–3261.
- Gallucci, S., and Matzinger, P. 2001. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 13:114–119.
- Goodrich, M. E., and McGee, D. W. 1998. Regulation of mucosal B-cell immunoglobulin secretion by intestinal epithelial cell-derived cytokines. Cytokine 10:948–955.
- Gordon, H. A., and Pesti, L. 1971. The gnotobiotic animal as a tool in the study of host-microbial relationships. Bacteriol. Rev. 35:390–429.
- Guarner, F., and Malagelada, J. R. 2003. Gut flora in health and disease. Lancet 361:512–519.
- Hambly, R. J., Rumney, C. J., Cunninghame, M., Fletcher, J. M., Rijken, P. J., and Rowland, I. R. 1997. Influence of diets containing high and low risk factors for colon cancer on early stages of carcinogenesis in human flora-associated (HFA) rats. Carcinogenesis 18:1535–1539.
- Hanada, T, and Yoshimura, A. 2002. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 13:413–421.
- Handelsman, J. 2004. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 68:669–685.
- Heavey, P. M., and Rowland, I. R. 2004. Microbial–gut interactions in health and disease. Gastrointestinal cancer. Best Practice Res. Clin. Gastroenterol. 18:323–e36.
- Hewitson, P., Glasziou, P., Irwig, L., Towler, B., and Watson, E. 2007. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. Cochrane Database System. Rev. (1):CD001216.
- Hope, M. E., Hold, G. L., Kain, R., and El-Omar, E. M. 2005. Sporadic colorectal cancer—role of the commensal microbiota. FEMS Microbiol. Lett. 244:1–7.
- Hord, N. G. 2008. Eukaryotic-microbiota cross-talk: Potential mechanisms for health benefits of prebiotics and probiotics. Annu. Rev. Nutr. 28:215–231.
- Horie, H., Kanazawa, K., Okada, M., Narushima, S., Itoh, K., and Terada, A. 1999. Effects of intestinal bacteria on the development of colonic neoplasm: An experimental study. Eur. J. Cancer Prev. 8:237–245.
- Hrncir, T., Stepankova, R., Kozakova, H., Hudcovic, T., and Tlaskalova-Hogenova, H. 2008. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T-cells: Studies in germ-free mice. BMC Immunol. 6;9:65.
- IARC (International Agency for Research on Cancer). 1997. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 61. Schistosomes, Liver Flukes and Helicobacter pylori. Geneva: World Health Organization.
- Kelly, D., Conway, S., and Aminov, R. 2005. Commensal gut bacteria: Mechanisms of immune modulation. Trends Immunol. 26:326–333.
- Kikuchi, S., Wada, O., Nakajaima, T., Nishi, T., Kobayashi, O., Konishi, T., and Inaba, Y. 1995. Serum anti-Helicobacter pylori antibody and gastric carcinoma among young adults. Research group on prevention of gastric carcinoma among young adults. Cancer 75:2789–2793.
- Kinross, J. M., von Roon, A. C., Holmes, E., Darzi, A., and Nicholson, J. K. 2008. The human gut microbiome: implications for future health care. Curr. Gastroenterol. Rep. 10:396–403.
- Koizumi, S., Wakita, D., Sato, T., Mitamura, R., Izumo, T., Shibata, H., Kiso, Y., Chamoto, K., Togashi, Y., Kitamura, H., and Nishimura, T. 2008. Essential role of Toll-like receptors for dendritic cell and NK1.1+ cell-dependent activation of Type 1 immunity by Lactobacillus pentosus strain S-PT84. Immunol. Lett. 120:14–19.
- Kuper, H., Adami, H. O., and Tricopoulos, D. 2000. Infections as a major preventable cause of human cancer. J. Intern. Med. 248:171–183.
- Lin, W.W., and Karin, M. 2007. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 117:1175–1183.
- Liu, X., Conner, H., Kobayashi, T., Abe, S., Fang, Q., Wen, F. Q., and Rennard, S. I. 2003. Synergetic effect of interleukin-4 and transforming growth factor-β1 on type I collagen gel contraction and degradation by HFL-1 cells: Implication in tissue remodeling. Chest 123(S3):427S–428S.
- McGarr, S. E., Ridlon, J. M., and Hylemon, P. B. 2005. Diet, anaerobic bacterial metabolism, and colon cancer: A review of the literature. J. Clin. Gastroenterol. 39:98–109.
- Macpherson, A.J., and Harris, N. L. 2004. Opinion: Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4:478–485.
- Macpherson, A. J., and Uhr, T. 2004. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann. N.Y. Acad. Sci. 1029:36–43.
- Macpherson, A. J., Geuking, M. B., and McCoy, K. D. 2005. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology 115: 153–162.
- Makita, S., Kanai, T., Nemoto, Y., Totsuka, T., Okamoto, R., Tsuchiya, K., Yamamoto, M., Kimono, H., and Watanabe M. 2007. Intestinal lamina propria retaining CD4+CD25+ regulatory T-cells is a suppressive site of intestinal inflammation. J. Immunol. 178:4937–4946.
- Mantovani, A., Allavena, P., Sica, A., and Balkwill, F. 2008. Cancer-related inflammation. Nature 454:436–444.
- Mastromarino, A., Reddy, B. S., and Wynder, E. L. 1976. Metabolic epidemiology of colon cancer: Enzymic activity of fecal flora. Am. J. Clin. Nutr. 29:1455–1460.
- Moss, S. F. 1999. The carcinogenic effect of H. pylori on the gastric epithelial cell. J. Physiol. Pharmacol. 50:847–856.
- Mowat, A. M. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3:331–341.
- Nicklin, S., and Miller, K. 1983. Local and systemic immune responses to intestinally-presented antigen. Int. Arch. Allergy Appl. Immunol. 72:87–90.
- Nieuwenhuis, E. E., Matsumoto, T., Lindenbergh, D., Willemsen, R., Kaser, A., Simons-Oosterhuis, Y., Brugman, S., Yamaguchi, K., Ishikawa, H., Aiba, Y., Koga, Y., Samsom, J. N., Oshima, K.,Kikuchi, M., Escher, J. C., Hattori, M., Onderdonk, A. B., and Blumberg, R. S. 2009. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J. Clin. Invest. 119:1241–1250.
- Ogawa, T., Asai, Y., Tamai, R., Makimura, Y., Sakamoto, H., Hashikawa, S., and Yasuda, K. 2006. Natural killer cell activities of synbiotic Lactobacillus casei ssp. casei in conjunction with dextran. Clin. Exp. Immunol. 143:103–109.
- Olaya, J., Neopikhanov, V., and Uribe, A. 1999. Lipopolysaccharide of Escherichia coli, polyamines, and acetic acid stimulate cell proliferation in intestinal epithelial cells. In Vitro Cell. Dev. Biol. Anim. 35:43–48.
- Onoue, M., Kado, S., Sakaitani, Y., Uchida, K., and Morotomi, M. 1997. Specific species of intestinal bacteria influence the induction of aberrant crypt foci by 1,2-dimethylhydrazine in rats. Cancer Lett. 113:179–186.
- Potter, J. D. 1995. Risk factors for colon neoplasia - epidemiology and biology. Eur. J. Cancer 31A:1033–-1038.
- Prescott, S. L., and Holt, P. G. 1998. Abnormalities in cord blood mononuclear cytokine production as a predictor of later atopic disease in childhood. Clin. Exp. Allergy 28:1313–1316.
- Rao, C. V., Hirose, Y., Indranie, C., and Reddy, B. S. 2001. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res. 61:1927–1933.
- Reddy, B. S., Narisawa, T., Maronpot, R., Weisburger, J. H., and Wynder, E. L. 1975. Animal models for the study of dietary factors and cancer of the large bowel. Cancer Res. 35:3421–3426.
- Reddy, B. S., Narisawa, T., Wright, P., Vukusich, D., Weisburger, J. H., and Wynder, E. L. 1975. Colon carcinogenesis with azoxymethane and dimethylhydrazine in germ-free rats. Cancer Res. 35:287–290.
- Reddy, B. S., Narisawa, T., and Weisburger, J. H. 1976. Colon carcinogenesis in germ-free rats with intra-rectal 1,2-dimethylhydrazine and subcutaneous azoxymethane. Cancer Res. 36:2874–2876.
- Reddy, B. S., Sharma, C., Darby, L., Laakso, K., and Wynder, E. L. 1980. Metabolic epidemiology of large bowel cancer. Fecal mutagens in high- and low-risk population for colon cancer. A preliminary report. Mutat. Res. 72:511–522.
- Reddy, B. S., and Ohmori, T. 1981. Effect of intestinal microflora and dietary fat on 3,2′-dimethyl-4-aminobiphenyl-induced colon carcinogenesis in F344 rats. Cancer Res. 41:1363–1367.
- Rohrmann, S., Hermann, S., and Linseisen, J. 2009. Heterocyclic aromatic amine intake increases colorectal adenoma risk: Findings from a prospective European cohort study. Am. J. Clin. Nutr. 89:1418–1424.
- Roller, M., Pietro Femia, A., Caderni, G., Rechkemmer, G., and Watzl, B. 2004. Intestinal immunity of rats with colon cancer is modulated by oligofructose-enriched inulin combined with Lactobacillus rhamnosus and Bifidobacterium lactis. Br. J. Nutr. 92:931–938.
- Rowland, I. R. 2009. The role of the gastrointestinal microbiota in colorectal cancer. Curr. Pharm. Des. 15:1524–1527.
- Rumney, C. J., Rowland, I. R., Coutts, T. M., Randerath, K., Reddy, R., Shah, A. B., Ellul, A., and O’Neill, I. K. 1993. Effects of risk-associated human dietary macrocomponents on processes related to carcinogenesis in human flora-associated (HFA) rats. Carcinogenesis 14:79–84.
- Sacksteder, M. R. 1976. Occurrence of spontaneous tumors in the germ-free F344 rat. J. Natl. Cancer Inst. 57:1371–1373.
- Samavati, L., Rastogi, R., Du, W., Hüttemann, M., Fite, A., and Franchi, L. 2009. STAT3 tyrosine phosphorylation is critical for interleukin-1β and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol. Immunol. 46:1867–1877.
- Slattery, M. L., Wolff, R. K., Herrick, J. S., Caan, B. J., and Potter, J. D. 2007. IL-6 genotypes and colon and rectal cancer. Cancer Causes Control 18:1095–1105.
- Smith, K., McCoy, K. D., and Macpherson, A. J. 2007. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 19:59–69.
- Smythies, L. E., Maheshwari, A., Clements, R., Eckhoff, D., Novak, L., Vu, H. L., Mosteller-Barnum, L. M., Sellers, M., and Smith, P. D. 2006. Mucosal IL-8 and TGFβ recruit blood monocytes: Evidence for cross-talk between the lamina propria stroma and myeloid cells. J. Leukocyte Biol. 80:492–499.
- Steinman, R. M., Hawiger, D., and Nussenzweig, M. C. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711.
- Stepankova, R. 1997. Rearing germ-free rats, mice, and rabbits. In: Immunology Methods Manual, Vol. 3. Gnotobiologic Models (Lefkovits, I., Tlaskalova, H., and Sterzl, J., Eds). New York: Academy Press, pp. 1537–1542.
- Stepankova, R., Sinkora, J., Hudcovic, T., Kozakova, H., and Tlaskalova-Hogenova, H. 1998. Differences in development of lymphocyte subpopulations from gut-associated lymphatic tissue (GALT) of germfree and conventional rats: effect of aging. Folia Microbiol (Praha) 43:531–534.
- Sung, J. J., Lau, J. Y., Goh, K. L., Leung, W. K., and the Asia Pacific Working Group on Colorectal Cancer. 2005. Increasing incidence of colorectal cancer in Asia: Implications for screening. Lancet Oncol. 6:871–876.
- Swidsinski, A., Khilkin, M., Kerjaschki, D., Schreiber, S., Ortner, M., Weber, J., and Lochs, H. 1998. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology 115:281–286.
- Takahashi, S., Kawamura, T., Kanda, Y., Taniguchi, T., Nishizawa, T., Iiai, T., Hatakeyama, K., and Abo, T. 2006. Activation of CD1d-independent NK1.1+ T-cells in the large intestine by Lactobacilli. Immunol. Lett. 102:74–78.
- Tannock, G. W. 2007. What immunologists should know about bacterial communities of the human bowel. Sem.Immunol. 19:94–105.
- Tezuka, H., Abe, Y., Iwata, M., Takeuchi, H., Ishikawa, H., Matsushita, M., Shiohara, T., Akira, S., and Ohteki, T. 2007. Regulation of IgA production by naturally-occurring TNF-/iNOS-producing dendritic cells. Nature 448:929–933.
- Tlaskalova-Hogenova, H., Sterzl, J., Stepankova, R., Dlabac, V., Vetvicka, V., Rossmann, P., Mandel, L., and Rejnek, J. 1983. Development of immunological capacity under germfree and conventional conditions. Ann. N.Y. Acad. Sci. 409:96–113.
- Tlaskalova-Hogenova H. 1997. Gnotobiology as a tool - An introduction. In: Immunology Methods Manual, Vol. 3. Gnotobiologic Models (Lefkovits, I., Tlaskalova, H., and Sterzl, J., Eds). New York: Academic Press, pp. 1524–1529.
- Tlaskalova-Hogenova, H., Stepankova, R., Hudcovic, T., Tuckova, L., Cukrowska, B., Lodinova-Zadnikova, R., Kozakova, H., Rossmann, P., Bartova, J., Sokol, D., Funda, D. P., Borovska, D., Rehakova, Z., Sinkora, J., Hofman, J., Drastich, P., and Kokesova, A. 2004. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 93:97–108.
- Trexler, P. C. 1978. A rationale for the development of gnotobiotics. Lab. Anim. 12:257–262.
- Tuohy K. M., Gougoulias C., Shen Q., Walton G., Fava F., and Ramnani P. 2009. Studying the human gut microbiota in the trans-omics era - focus on metagenomics and metabonomics. Curr. Pharm. Des. 15:1415–1427.
- Umesaki, Y., and Setoyama, H. 2000. Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microb. Infect. 2:1343–1351.
- Vannucci, L., Fiserova, A., Horvath, O., Rossmann, P., Mosca, F., and Pospisil, M. 2004. Cancer evolution and immunity in a rat colorectal carcinogenesis model. Int. J. Oncol. 25:973–981.
- Vannucci, L., Stepankova, R., Kozakova, H., Fiserova, A., Rossmann, P., and Tlaskalova-Hogenova H. 2008. Colorectal carcinogenesis in germ-free and conventionally-reared rats: Different intestinal environments affect the systemic immunity. Int. J. Oncol. 32:609–617.
- Venturi, M., Hambly, R. J., Glinghammar, B., Rafter, J. J., and Rowland, I. R. 1997. Genotoxic activity in human fecal water and the role of bile acids: A study using the alkaline comet assay. Carcinogenesis 18:2353–2359.
- Wang, X., and Huycke, M. M. 2007. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology 132:551–561.
- Wang, X., Allen, T. D., May, R. J., Lightfoot, S., Houchen, C. W., and Huycke, M. M. 2008. Enterococcus faecalis induces aneuploidy and tetraploidy in colonic epithelial cells through a bystander effect. Cancer Res. 68:9909–9917.
- Watzl, B., Girrbach, S., and Roller M. 2005. Inulin, oligofructose, and immunomodulation. Br. J. Nutr. 93(S1):S49–S55.
- Wedeima, W. F., Deschner, E. E., Cohen, B. I., and DeCosse, J. J. 1985. Acute effects of dietary cholic acid and methylazoxymethanol acetate on colon epithelial cell proliferation; Metabolism of bile salts and neutral sterols in conventional and germ-free SD rats. J. Natl. Cancer Inst. 74:665–670.
- Williams, A. M., Probert, C. S., Stepankova, R., Tlaskalova-Hogenova, H., Phillips, A., and Bland, P. W. 2006. Effects of microflora on the neonatal development of gut mucosal T-cells and myeloid cells in the mouse. Immunology 119:470–478.
- Wilks, M. 2007. Bacteria and early human development. Early Hum. Dev. 83:165–170.
- Woof, J. M., and Mestecky, J. 2005. Mucosal immunoglobulins. Immunol. Rev. 206:64–82.
- Woolverton, C. J., Holt, L. C., Mitchell, D., and Sartor, R. B. 1992. Identification and characterization of rat intestinal lamina propria cells: Consequences of microbial colonization. Vet. Immunol. Immunopathol. 34:127–138.
- Wu, M. S., Lin, J. T., Hsu, P. N., Lin, C. Y., Hsieh, Y. T., Chiu, Y. H., Hsueh, P. R., and Liao, K. W. 2007. Preferential induction of transforming growth factor-beta production in gastric epithelial cells and monocytes by Helicobacter pylori soluble proteins. J. Infect. Dis. 196:1386–1393.
- Yu, L, Wang, L, and Chen, S. 2009. Toll-like receptors, inflammation and tumor in the human female reproductive tract. Am. J. Reprod. Immunol. 62:1–8.
