Abstract
The mechanisms of idiosyncratic drug-induced liver injury (IDILI) are still a matter of dispute. Some of the characteristics of reactions that have been classed as metabolic idiosyncrasy could also be those of an immune-mediated reaction with an autoimmune component. Many auto-immune reactions appear to be mediated by TH17 cells, which are in part characterized by the production of interleukin (IL)-17. To test the involvement of TH17 cells in IDILI, we quantified a number of cytokines, chemokines, and autoantibodies in the serum of 39 patients with acute liver failure (ALF) due to IDILI and compared the values with those from 21 patients with acetaminophen-induced ALF and 10 patients with viral hepatitis-induced ALF. The IL-17 levels were elevated in 60% of patients with IDILI, but also in a similar number of patients with acetaminophen-induced ALF and occasionally in patients with viral hepatitis. Levels of other cytokines, such as IL-21, that are also produced by TH17 cells were higher in patients with IDILI, but again, there was overlap with acetaminophen DILI. Autoantibodies were more frequent in patients in the IDILI group but were absent in most patients. These data provide a picture of the cytokine/chemokine profile in patients with various types of ALF. The pattern varies from patient to patient and not specifically by etiology. This suggests that different underlying disease mechanisms may be at play in different individuals, even among those demonstrating injury from the same drug. Since cytokines may originate from more than one type of cell, interpretation of results of cytokine assays remains difficult in complex disease settings.
Introduction
Idiosyncratic drug-induced liver injury (IDILI) has been categorized as being due to either immune idiosyncrasy or metabolic idiosyncrasy. The designation of immune idiosyncrasy is based on the presence of fever, rash, eosinophilia, accelerated onset with re-challenge, and anti-drug antibodies (Zimmerman, Citation1999). The designation of metabolic idiosyncrasy is based on the event being rare, unpredictable, the lack of allergic features, and typically a long latency period, sometimes more than a year. Although genetic polymorphisms in a metabolic pathway have been suspected to be involved, there are no clearly defined cases where a polymorphism in a metabolic pathway is sufficient to explain the idiosyncratic nature of an IDILI reaction.
Certainly not all immune-mediated events have the characteristics that are used to classify IDILI as being immune idiosyncrasy. Some drugs that cause IDILI classified as metabolic idiosyncrasy, such as isoniazid, also cause a lupus-like autoimmune syndrome, and the autoimmune IDILI caused by minocycline is characterized by a relatively long latency period (Lawrenson et al., Citation2000). It is possible that many cases of IDILI classed as metabolic idiosyncrasy have an autoimmune component even though they are not associated with the classic autoantibodies associated with autoimmune hepatitis. Recently, a new subtype of helper T-cells, TH17 cells, has been identified and characterized by a set of pro-inflammatory cytokines, including interleukin (IL)-17, IL-21, and IL-22 (Dong, Citation2009; Korn et al., Citation2009). The IL-17 receptor is expressed on various epithelial tissues and TH17 cells are therefore considered a very crucial messenger between immune system and tissues (Ouyang et al., Citation2008; Shen and Gaffen, Citation2008). Growing evidence suggests that TH17 cells play a very important role in pathogenesis of many kinds of autoimmune syndromes, especially in organ specific autoimmunity (Aranami and Yamamura, Citation2008; Emamaullee et al., Citation2009; Nistala and Wedderburn, Citation2009; Peck and Mellins, Citation2009). Our recent studies also suggested the involvement of TH17 cells in the animal model of penicillamine-induced idiosyncratic autoimmunity, which includes hepatotoxicity (unpublished data). In the present study, we set out to investigate the cytokine pattern of patients with various forms of acute liver failure (ALF) to determine if different causes of ALF are associated with characteristic cytokine patterns, and in particular, if any have a TH17-related pattern that suggests an autoimmune component.
Patients and methods
Patients
The Acute Liver Failure Study Group (ALFSG) has studied prospectively more than 1400 cases of ALF over 11 years and obtained detailed data as well as serum and DNA samples on most of these patients, carefully stored at −80°C. ALF is defined as an acute hepatic illness that leads to coagulopathy with an international normalized ratio (INR) ≥ 1.5 accompanied by any degree of hepatic encephalopathy in less than 24 weeks. The focus in the present study was patient samples and data from the ALFSG registry that were diagnosed as having IDILI (n = 39) by the site investigator after a standard set of evaluations (CitationOstapowicz et al., 2002); patients with ALF caused by acetaminophen (n = 21) and viral hepatitis (either A or B: n = 10) were used for comparison purposes, as well as sera obtained from a cohort of 10 patients with chronic Hepatitis C as controls. Informed consent was obtained from next of kin because patients by definition had altered mentation. In addition to clinical samples, information on each case was available for review.
Determination of serum cytokine/chemokine profile by luminex or ELISA
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, and alkaline phosphatase were determined by the treating hospitals and recorded in the case report forms as noted above. Serum levels of 21 cytokines/chemokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p40), IL-13, IL-15, IL-17, interferon [IFN]-γ, eotaxin, GM-CSF, IFN-γ-induced protein 10 (IP-10), monocyte chemotactic protein [MCP]-1, macrophage inflammatory protein [MIP]-1α, and tumor necrosis factor [TNF]-α) were determined using a human cytokine/chemokine milliplex luminex kit (Millipore, St. Charles, MO). Serum concentrations of IL-21 were determined by ELISA kit according to the manufacturer’s instructions (eBioscience, San Diego, CA). Serum concentrations of B-cell activating factor (BAFF) were determined with an ELISA kit from R&D Systems (Minneapolis, MN).
Determination of serum autoantibodies
The BINDAZYME ANA screen enzyme immunoassay kit (Binding Site Ltd., Birmingham, UK) was used to collectively detect total anti-nuclear antibodies (ANAs) against dsDNA, histones, SSA/Ro (60 and 52 kDa), SSB/La, Sm, Sm/RNP, Scl-70, Jo-1, and centromeric antigens. This kit only determines the presence of these antibodies in aggregate; without further testing, it is impossible to know which of these specific autoantibody(s) is elevated. A human anti-MPO antibody ELISA kit (IMMCO Diagnostics Inc., Buffalo, NY) was used for semi-quantitation of antibodies to MPO following the protocol provided by the manufacturer.
Statistical analysis
In general, a t-test with Welch’s correction was used for the parametric comparison of means, while the Pearson’s correlation was used for the correlation test in . All the tests are two-tailed, with the confidence level of 95%.
Table 1. Correlation between IL-17 and other analytes in each patient category.
Results
Overall patient cohort
For this initial study, sera from a total of 70 ALF patients were utilized, obtained between Study Days 1 and 6. The values of ALT/AST, alkaline phosphatase, and bilirubin, provide an indication of the type and severity of the liver injury. Because we mainly wished to focus on drug-induced liver injury, sera were for study from patients enrolled in the overall registry based on availability of samples, but also within the following categories: 39 patients with IDILI, 21 with ALF caused by acetaminophen (APAP), and 5 each with Hepatitis A or Hepatitis B. Ten patients with chronic Hepatitis C, not receiving interferon treatment, were considered as a separate positive disease control group.
Biochemical parameters
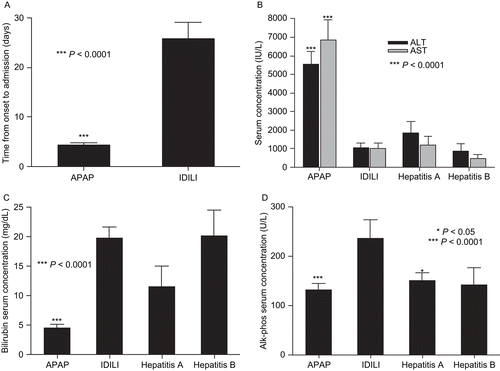
Duration of illness and patients’ biochemical parameters are summarized in . In the IDILI patients, a wide variety of medicines were involved, ranging from herbals to drugs such as diclofenac and troglitazone that are well recognized as causing IDILI.
Figure 1. Biochemical parameters of liver failure patients. (A) Time from onset to hospitalization and serum levels of (B) AST and ALT, (C) bilirubin, and (D) alkaline phosphatase were compared between patients with acute liver failure of different etiologies. Statistical significance between IDILI patients (n = 39) and other patient groups (APAP, n = 21; Hepatitis A and B, n = 5 each) was determined by the t-test with Welch’s correction; levels of significance are indicated in the figure.
Serum cytokine profiles
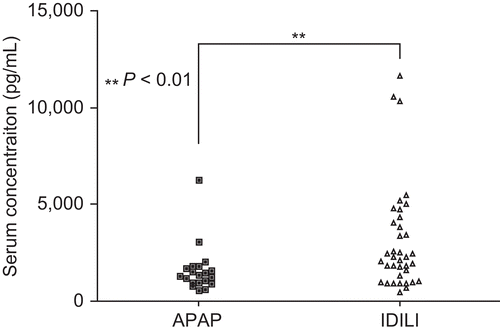
The lowest concentration of cytokine/chemokine for the standard curves was 3.2 pg/mL; any concentration lower than that was considered non-detectable. In normal individuals the levels of cytokines such as IL-4, IL-6, and IL-17 are less than 2 pg/mL. Thus, any detectable level may be considered abnormal. In contrast, normal serum levels for IL-21 are ~466 ± 90 pg/mL. A complete list of the cytokine/chemokine data in the current study is presented as Supplemental Data (available with on-line version of this paper). IL-17 was detectable in ~60% of APAP and IDILI patients and in a lower fraction in patients with viral hepatitis (). Serum levels of IL-6, a critical cytokine for the differentiation of naïve T-cells into TH17 cells, were elevated in most of the ALF patients but not in those with chronic hepatitis C (). The mean IL-21, a TH17 cytokine, and IL-1α levels were significantly higher in IDILI patients than other ALF patients (P = 0.013 and P= 0.008, respectively, ). Serum IP-10/CXCL10, reported to be dramatically elevated in both Type 1 diabetes and autoimmune liver diseases, was also highest in IDILI patients (P = 0.001, ). Serum levels of BAFF were also significantly higher in IDILI patients than in APAP patients (3106 ± 447.5 versus 1609 ± 276.8, P = 0.006, ). In contrast, the mean levels of MCP-1 and IL-15 were higher in the APAP group (). In the IDILI group, IL-17 levels significantly correlated with several other cytokines; in contrast, correlations with other cytokines in the APAP group were far fewer ().
Figure 2. TH17-related cytokine/chemokine comparisons between patient groups. Serum concentrations of TH17-related cytokines/chemokines (e.g., IL-17, IL-21, IL-6, and IL-1α) were measured by Luminex using a human multiplex kit. The statistical significance of any differences between the APAP (n = 21) and IDILI (n = 39) patients’ values were determined by the t-test with Welch’s correction; levels of significance are indicated in the figure. Data from patients in the Hepatitis A, B, and C virus groups are shown for comparison (n = 5, 5, and 10, respectively).
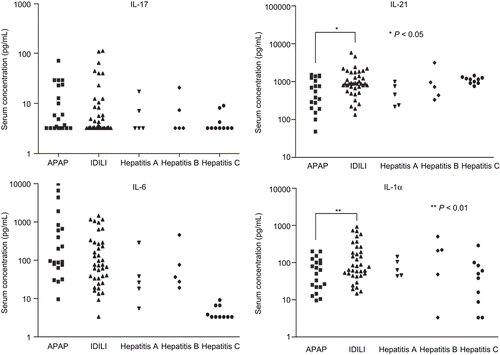
Figure 3. Innate cytokine/chemokine comparisons between patient groups. Serum concentrations of innate cytokines/chemokines (e.g., MCP-1, IL-15, IP-10, and IFNγ) were measured by Luminex using a human multiplex kit. The statistical significance of any differences between the APAP (n = 21) and IDILI (n = 39) patients’ values were determined by the t-test with Welch’s correction; levels of significance are indicated in the figure. Data from patients in the Hepatitis A, B, and C virus groups are shown for comparison (n = 5, 5, and 10, respectively).
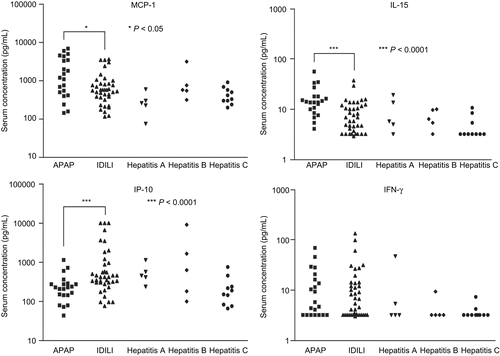
Figure 4. Serum levels of BAFF. Serum concentrations of BAFF were measured by ELISA. The statistical significance of any difference between the APAP (n = 21) and IDILI (n = 39) patients’ values was determined by the t-test with Welch’s correction by the t-test with Welch’s correction; level of significance is indicated in the figure.
Anti-nuclear autoantibodies and anti-MPO antibodies
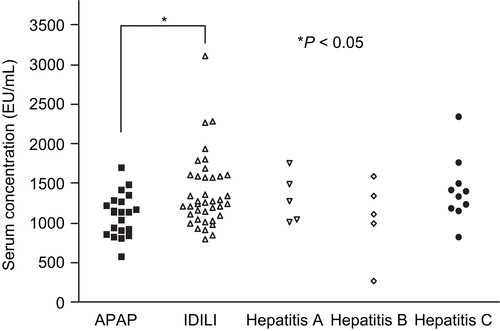
Anti-nuclear autoantibodies (ANA) were detectable in 14 out of 39 IDILI patients (33%) and markedly elevated in 3, but they were only elevated in 1 out of 21 APAP patients (). Three of 10 patients with viral hepatitis also had significant elevations of ANA. As for the serum level of anti-MPO autoantibodies, the mean was also significantly higher in DILI patients than APAP patients, but of all the patients only one IDILI patient had a marked elevation of anti-MPO antibodies (). Neither ANA nor anti-MPO antibodies correlated significantly with any serum cytokine.
Figure 5. Serum levels of ANA. Serum levels of ANA were measured using an ELISA kit that detects 9 different anti-nuclear antibodies. Serum samples were diluted in 1:100 and the manufacturer indicates that values greater than 10 should be considered positive. The statistical significance of any difference between the APAP (n = 21) and IDILI (n = 39) patients’ values was determined by the t-test with Welch’s correction; level of significance is indicated in the figure. Data from patients in the Hepatitis A, B, and C virus groups are shown for comparison (n = 5, 5, and 10, respectively).
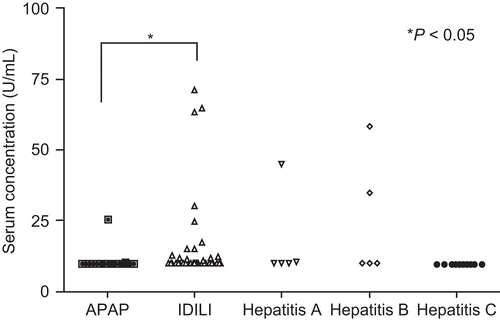
Figure 6. Serum levels of anti-MPO antibodies. Serum anti-MPO levels were determined (semi-quantitation) by an ELISA kit. Serum samples were diluted in 1:100. The statistical significance of any difference between the APAP (n = 21) and IDILI (n = 39) patients’ values was determined by the t-test with Welch’s correction; level of significance is indicated in the figure. Data from patients in the Hepatitis A, B, and C virus groups are shown for comparison (n = 5, 5, and 10, respectively).
Discussion
Acute liver failure represents the most severe form of liver injury and frequently results in a fatal outcome unless transplantation is performed in a timely fashion. The massive hepatic inflammation that results when severe necrosis occurs is a principal finding on biopsy in such patients, along with widespread destruction of hepatocytes. How inflammation is initiated and the sequence of events that triggers massive necrosis remains poorly understood. In the present study, we evaluated the serum levels of a variety of cytokines, chemokines, and autoantibodies in patients with idiosyncratic drug-induced ALF, comparing these values with those observed in patients with acetaminophen-induced ALF or fulminant or chronic viral hepatitis.
We suspected that markers of activation of the adaptive immune system would be most evident. In particular, we sought to determine whether patients suffering from IDILI that has been thought to be secondary to metabolic idiosyncrasy might have evidence of immune activation or even an autoimmune component as evidenced by elevated IL-17 levels. IL-17 levels were indeed elevated in many IDILI patients; however, this was not uniformly present. In addition, IL-17 levels were also elevated in several patients with APAP-induced ALF, a surprising finding given that APAP is thought to induce direct cellular necrosis or apoptosis not involving activation of the adaptive immune system. However, more recently it was found that a variety of other cells can produce IL-17, including neutrophils, CD8+ T-cells, NKT cells, γδ T-cells, macrophages, and NK cells; therefore, serum IL-17 cannot be used as an unambiguous marker of TH17 cells (Song et al., Citation2008; Benghiat et al., Citation2009). Given the acute nature of APAP-induced ALF, it is unlikely that the source of IL-17 in these patients was TH17 cells, which usually require days or even weeks to differentiate and expand from naïve T-cells to pathogenic cells. High doses of APAP cause significant cell damage, which is likely to lead to an innate immune response to dispose of dead and dying cells. In contrast, IL-17 levels in IDILI patients appear to correlate with the levels of several other cytokines, possibly because IL-17 in this setting is likely produced mostly by TH17 cells.
Of the cytokines released by TH17 cells, IL-21 appears to be the most specific (Ettinger et al., Citation2008; Sutherland et al., Citation2009); therefore, it should be a better measure of TH17 cell-mediated autoimmunity. IL-21 was higher, on average, in the IDILI patients, but only markedly elevated in a few patients. However, even other types of cells can produce IL-21. The greatest elevation of IP-10, a cytokine associated with autoimmune liver diseases and Type I diabetes (Nishioji et al., Citation2001; Feferman et al., Citation2009), was also found in the IDILI patients. BAFF was also elevated in a significantly greater number of IDILI patients. BAFF is a critical factor for survival and maturation of B-cells and has been shown to be involved in the pathogenesis of many kinds of chronic autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and autoimmune hepatitis (Bosello et al., Citation2007; Matsushita et al., Citation2007; Migita et al., Citation2007). Furthermore, a recent study has shown that silencing BAFF gene by short/small hairpin RNA (shRNA) was able to suppress the generation of TH17 cells leading to amelioration of autoimmune arthritis (Lai Kwan Lam et al., Citation2008). Therefore, the significantly elevated serum levels of BAFF in IDILI patients provide additional evidence to support the autoimmune nature of some IDILI cases. In contrast, MCP-1 and IL-15 were elevated more in the APAP patients than other groups, presumably a reflection of innate immune system activation in response to liver damage (Matsukawa et al., Citation2000; Ohteki, Citation2002).
Another parameter that would suggest an autoimmune component is the presence of autoantibodies. However, part of the differential diagnosis for ALF is idiopathic autoimmune hepatitis, and therefore a screen for ANA and liver/kidney microsome type 1 antibody should be part of the workup of these patients although in general they do not have a classic picture of autoimmune hepatitis. In spite of this we found that a few of the IDILI patients had significant elevations of ANA or anti-MPO antibodies. The drugs in this study that were associated with marked elevations in ANA were propylthiouracil, co-trimoxazole, and isoniazid, drugs known to cause a lupus-like syndrome (). A marked elevation of anti-MPO antibodies was observed in a patient with diclofenac-induced ALF (). There is no way to know whether these autoantibodies played a role in the IDILI that occurred. Obviously, ANA and anti-MPO antibodies only represent a tiny fraction of the total range of possible autoantibodies that these patients might have, and we are working on a much more comprehensive screen for autoanti-bodies that may be useful in the future. In addition, there could be auto-reactive T-cells in the absence of autoantibodies.
Table 2. Levels of IL-17, IL-21, IL-6, IP-10 (Luminex assay), ANA, and anti-MPO (ELISA assay) in individual IDILI patients.
Even though there were differences in the parameters measured between the different categories of ALF, there was a large degree of overlap, and there is no simple parameter that could be used diagnostically or that would provide solid evidence that specific cases of IDILI have an autoimmune component. Even patients exposed to the same drug showed highly variable cytokine results. An important limitation of the data is that the patients were not part of a controlled study, and therefore sera were obtained at different time points in the course of the disease and the severity of liver injury was different in different patients. In addition, it is likely that there are significant differences in the mechanism of IDILI in different patients, and it is striking that the precise profile appeared different in each individual patient. However, these data do provide a picture of the cytokine profile of patients with different types of ALF.
Supplementary Material
Download PDF (1.9 MB)Acknowledgements
The continued support of the coordinators and investigators participating in the ALF Study Group (ALFSG 1998–2006) has been essential to this work and is gratefully acknowledged: William M. Lee (PI), Julie Polson, Carla Pezzia, Ezmina Lalani, Linda S. Hynan, and Joan S. Reisch, University of Texas Southwestern Medical Center, Dallas, TX; Anne M. Larson, University of Washington, Seattle, WA; Jeffrey S. Crippin, Washington University School of Medicine, St. Louis, MO; Timothy J. Davern, University of California at San Francisco, CA; Sukru Emre, Mt Sinai Medical Center, New York, NY; Timothy M. McCashland, University of Nebraska, Omaha, NE; J. Eileen Hay, Mayo Clinic, Rochester, MN; Natalie Murray, Baylor University Medical Center, Dallas, TX; A. Obaid Shakil, University of Pittsburgh Medical Center, Pittsburgh, PA; Andres T. Blei, Northwestern University Medical School, Chicago, IL; Atif Zaman, Jonathan Schwartz, Oregon Health Sciences University, Portland, OR; Steven Han, University of California at Los Angeles, Los Angeles, CA; Robert J. Fontana, University of Michigan Medical Center, Ann Arbor, MI; Brendan McGuire, University of Alabama, Birmingham, AL; Raymond Chung, Massachusetts General Hospital, Boston, MA; Michael Schilsky, Columbia-Presbyterian Medical Center/Cornell-New York Hospital, New York, NY; M. Edwyn Harrison, Mayo Clinic, Scottsdale, Scottsdale, AZ; Adrian Reuben, Medical University of South Carolina, Charleston, SC; Santiago Munoz, Albert Einstein Medical Center, Philadelphia, PA; Todd Stravitz, Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, University of California at Davis, Sacramento, CA; Raj Satyanarayana, Mayo Clinic, Jacksonville, FL; Raj Reddy, University of Pennsylvania, Philadelphia, PA; Tarek Hassanein, University of California at San Diego, San Diego, CA; and, Alistair Smith, Durham, NC.
Declaration of interest
J.U. holds a Canada Research Chair in Adverse Drug Reactions. The sample analysis was funded by grants from the Canadian Institutes of Health. Samples, clinical laboratory data, and information were obtained through the ALFSG which is funded by NIH Grant #DK U01-58369.
References
- Aranami, T., and Yamamura, T. 2008. TH17 cells and autoimmune encephalomyelitis (EAE/MS). Allergol. Int. 57:115–120.
- Benghiat, F. S., Charbonnier, L. M., Vokaer, B., De Wilde, V., and Le Moine, A. 2009. Interleukin 17-producing T-helper cells in alloimmunity. Transplant. Rev. (Orlando) 23:11–18.
- Bosello, S., Pers, J. O., Rochas, C., Devauchelle, V., De Santis, M., Daridon, C., Saraux, A., Ferraccioli, G. F., and Youinou, P. 2007. BAFF and rheumatic autoimmune disorders: Implications for disease management and therapy. Int. J. Immunopathol. Pharmacol. 20:1–8.
- Dong, C. 2009. Differentiation and function of pro-inflammatory TH17 cells. Microbes Infect. 11:584–588.
- Emamaullee, J. A., Davis, J., Merani, S., Toso, C., Elliott, J. F., Thiesen, A., and Shapiro, A. M. 2009. Inhibition of TH17 cells regulates autoimmune diabetes in NOD mice. Diabetes 58:1302–1311.
- Ettinger, R., Kuchen, S., and Lipsky, P. E. 2008. Interleukin 21 as a target of intervention in autoimmune disease. Ann. Rheum. Dis. 67(S3):83–86.
- Feferman, T., Aricha, R., Mizrachi, K., Geron, E., Alon, R., Souroujon, M. C., and Fuchs, S. 2009. Suppression of experimental autoimmune myasthenia gravis by inhibiting the signaling between IFNγ-inducible protein 10 (IP-10) and its receptor CXCR3. J. Neuroimmunol. 209:87–95.
- Korn, T., Bettelli, E., Oukka, M., and Kuchroo, V. K. 2009. IL-17 and TH17 cells. Annu. Rev. Immunol. 27:485–517.
- Lai Kwan Lam, Q. King, Hung Ko, O., Zheng, B. J., and Lu, L. 2008. Local BAFF gene silencing suppresses TH17-cell generation and ameliorates autoimmune arthritis. Proc. Natl. Acad. Sci. USA 105:14993–14998.
- Lawrenson, R. A., Seaman, H. E., Sundstrom, A., Williams, T. J., and Farmer, R. D. 2000. Liver damage associated with minocycline use in acne: A systematic review of the published literature and pharmacovigilance data. Drug Safety 23:333–349.
- Matsukawa, A., Hogaboam, C. M., Lukacs, N. W., and Kunkel, S. L. 2000. Chemokines and innate immunity. Rev. Immunogenet. 2:339–358.
- Matsushita, T., Hasegawa, M., Matsushita, Y., Echigo, T., Wayaku, T., Horikawa, M., Ogawa, F., Takehara, K., and Sato, S. 2007. Elevated serum BAFF levels in patients with localized scleroderma in contrast to other organ-specific autoimmune diseases. Exp. Dermatol. 16:87–93.
- Migita, K., Abiru, S., Maeda, Y., Nakamura, M., Komori, A., Ito, M., Fujiwara, S., Yano, K., Yatsuhashi, H., Eguchi, K., and Ishibashi, H. 2007. Elevated serum BAFF levels in patients with autoimmune hepatitis. Hum. Immunol. 68:586–591.
- Nishioji, K., Okanoue, T., Itoh, Y., Narumi, S., Sakamoto, M., Nakamura, H., Morita, A., and Kashima, K. 2001. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin. Exp. Immunol. 123:271–279.
- Nistala, K., and Wedderburn, L. R. 2009. TH17 and regulatory T-cells: Rebalancing pro- and anti-inflammatory forces in autoimmune arthritis. Rheumatology (Oxford) 48(6):602–606.
- Ohteki, T. 2002. Critical role for IL-15 in innate immunity. Curr. Mol. Med. 2:371–380.
- Ostapowicz, G., Fontana, R. J., Schiodt, F. V., Larson, A., Davern, T. J., Han, S. H., McCashland, T. M., Shakil, A. O., Hay, J. E., Hynan, L., Crippin, J. S., Blei, A. T., Samuel, G., Reisch, J., and Lee, W. M. (2002). Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 137:947–954.
- Ouyang, W., Kolls, J. K., and Zheng, Y. 2008. The biological functions of T-helper 17 cell effector cytokines in inflammation. Immunity 28:454–467.
- Peck, A., and Mellins, E. D. 2009. Breaking old paradigms: TH17 cells in autoimmune arthritis. Clin Immunol. 132(3):295–304.
- Shen, F., and Gaffen, S. L. 2008. Structure–function relationships in the IL-17 receptor: Implications for signal transduction and therapy. Cytokine 41:92–104.
- Song, C., Luo, L., Lei, Z., Li, B., Liang, Z., Liu, G., Li, D., Zhang, G., Huang, B., and Feng, Z. H. 2008. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J. Immunol. 181:6117–6124.
- Sutherland, A. P., Van Belle, T., Wurster, A. L., Suto, A., Michaud, M., Zhang, D., Grusby, M. J., and von Herrath, M. 2009. Interleukin-21 is required for the development of Type 1 diabetes in NOD mice. Diabetes 58:1144–1155.
- Zimmerman, H. (Ed.). 1999. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd ed. Philadelphia: Lippincott Williams & Wilkins.
