Abstract
Continuous low-dose injection of d-galactose induces changes in mice that resemble accelerated aging. As such, these mice have been used as models to study mechanisms of aging. Here, we examined whether repeated (daily, for 60 days) subcutaneous injections (at 50 mg d-galactose/kg) into young adult (i.e., 2-month-old) mice induced changes in key immune system organs that were on par with those associated with aging. The results showed that galactose-treated mice develop histologic changes in their thymic cortical and medullary regions; immunohistochemical analysis revealed unorganized distributions of keratin-5 and keratin-8 proteins in the thymus of these hosts. These histological changes in the thymus of d-galactose-treated mice were also observed in the organs of aged (i.e., 24-month-old control mice); however, in this latter group, these changes were accompanied by a strong infiltration of adipose cells. Galactose-treated mice also evinced alterations within their splenic white and red pulp. Further, ultrastructural analyses of the thymus and spleen of the treated mice revealed increases in irregularly shaped lymphocytes bearing visible pyknosis. It was also seen that levels of autophagy within thymic epithelial cells were greatly decreased in the tissues of the galactose-treated mice, an outcome also seen in aged mice. Lastly, the level of memory T-lymphocytes and percentage of IgM-B220-B-lymphocytes in spleens of the galactose-treated mice were both increased (albeit insignificantly so) relative to values among splenocytes of age-matched control; however, these levels were not clealy as elevated as would be expected in “elderly” mice. Taken together, our results strongly suggest that d-galactose treatment can induce structural changes in the thymus and spleen, and some changes in organ-associated cell phenotypes, that are similar to several effects seen with aging. However, the fact that many endpoints do not appear to be truly reflective of what should be seen in immune system organs/cells of “elderly” mice now calls into question the appropriateness of the use of d-galactose (i.e., is it histologically/immunotoxicologically-proper?) to create age-mimicry in mice.
Introduction
d-Galactose a reducing sugar that readily reacts with the free amine groups of amino acids in proteins and peptides, both in vitro and in vivo, to form advanced glycation end-products (AGE) (Bucala and Cerami, Citation1992; Vlassara et al., Citation1994). The AGE represent a heterogeneous group of structures that form between the primary amino group of a protein and a carbohydrate-derived aldehyde group, and that have been implicated in aging and age-related diseases (Brownlee, Citation1995).
d-Galactose can cause accumulation of reactive oxygen species (ROS), or stimulate free radical production indirectly by the formation of AGE in vivo, ultimately resulting in oxidative stress (Xu, Citation1985; Zhang et al., Citation2005). Injection of d-galactose can induce neurological impairments in rodents (Zhang and Saito, Citation1986). Other studies showed that these changes included decreased neuromuscular activity, increased production of free radicals, decreased anti-oxidant enzyme activity and diminished immune responses (Gong and Xu, Citation1991). Because these changes resemble those observed during the normal aging process, d-galactose-treated mouse and rat models have been used widely for aging research and drug testing (Li et al., Citation1995).
A recent study showed that chronic systemic exposure of mice to d-galactose induced a spatial memory deficit, increases in cell karyopyknosis, apoptosis, and caspase-3 protein levels in hippocampal neurons, a decrease in the number of new neurons in the subgranular zone in the dentate gyrus (DG), reduced migration of neural progenitor cells, and increased cell death among newly formed neurons in the granular cell layer. d-Galactose exposure also induced an increase in peripheral oxidative stress, including an increase in malondialdehyde and decreases in total anti-oxidative capabilities (T-AOC), total superoxide dismutase (T-SOD), and glutathione peroxidase (GSH-PX) activities (Cui et al., Citation2006). Another study showed that d-galactose impaired neurogenesis in the DG in brain, which is similar to natural aging in mice. ROS accumulation as a result of d-galactose may be related to the decreased neurogenesis in the DG (Zhang et al., Citation2005). Serum AGE levels in d-galactose- and AGE-treated mice were increased significantly. The ultrastructure of the thymus and spleen of d-galactose- and AGE-treated mice provided evidence of regressive changes similar to those seen with organs from an aged control group. From an immunology-based functional point of view, ex vivo studies revealed that mitogenesis and interleukin (IL)-2 production by splenic lymphocytes were significantly decreased as a result of d-galactose exposures (Song et al., Citation1999; Lei et al., Citation2003; Deng et al., Citation2006).
Thymic epithelium can be divided phenotypically, morphologically, and topographically into two broad classes that form distinct compartments. Cortical thymic epithelial cells (TEC) are tightly packed with long, dendrite-like processes lying at the outer edges of the organ. At the center of the thymus are the medullary TEC, which are more loosely organized (Brekelmans and van Ewijk, Citation1990). The two compartments meet at the cortico-medullary junction (CMJ), which is the entry and exit point for developing thymocytes (Prockop and Petrie, Citation2000). Disruption of the CMJ and overall alterations to thymic architecture occurs during aging (Aw et al., Citation2008).
Autophagy is a highly-regulated intracellular process that involves the turnover of most cellular constituents and maintains cellular homeostasis. It is well established that the basal autophagic activity of living cells decreases with age, thus contributing to the accumulation of damaged macromolecules during aging. Macro-autophagy is the major lysosomal pathway for the turnover of cytoplasmic components and will hereafter be referred to as autophagy. This process begins with the engulfment of cytoplasmic constituents by a membrane sac, called the isolation membrane. Then, this structure forms a double-membrane vesicle called the autophagosome that contains bulk portions of cytoplasm, and eventually fuses with the lysosome. Finally, the inner membrane of the autophagosome and its protein and organelle contents are degraded by lysosomal hydrolases and recycled.
Recently, several authors have proposed that the accumulation of cellular garbage associated with aging was primarily caused by an age-related decline of autophagic and lysosomal activities (Cuervo, Citation2004; Terman et al., Citation2007). In addition, it has been reported that the age-related decline of autophagy was caused by alterations in glucose metabolism and hormone levels, which are inherent to aging and whose onset is delayed by calorie restriction (Del Roso et al., Citation2003; Cuervo et al., Citation2005).
To date, details of the changes in immune status, architecture of the thymus, and levels of autophagy among the TEC of d-galactose-treated mice have not been fully examined. In the present study, we investigated structural and phenotypical changes in the thymus and spleen, and autophagy among the TEC, to establish whether d-galactose-treated mice might be a valid model system in studies of the effects of aging—from an immunotoxicological point of view.
Materials and methods
Animals and treatments
C57BL/6J female mice were purchased from SLC (Hamamatsu, Japan) and maintained at the Animal Research Facility at Nagoya University Graduate School of Medicine under specific pathogen-free conditions. Mice were given food and water ad libitum, and maintained on a 12 hr light/dark cycle. After 1 week of adaptation, the 2-month-old mice were given one of the following preparations daily (for 60 days) by subcutaneous injection: 0.4 mL phosphate-buffered saline (PBS, pH 7.2) as vehicle control for the control mice OR 0.4 mL of a solution of d-galactose in PBS (prepared based on weekly measures of animal body weight (BW)—to result in a dosage of 50 mg/kg body BW) for the treated mice. In the case of aged mice, C57BL/6J female mice were also purchased (as 2-month-olds) and housed/left untreated for 22-month for eventual use as comparative controls for several study endpoints. The day after the final treatment, all control, d-galactose-treated, and aged mice were sacrificed, and the thymus and spleen of each was immediately collected. Throughout these experiments, all mice were handled in a humane manner in accordance with the Nagoya University Guidelines for Animal Experiments.
Reagents and antibodies
d-Galactose was purchased from Sigma-Aldrich (Tokyo, Japan). Formaldehyde (37%) was obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). Mayer’s hematoxylin solution was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and eosin Y-solution (0.5% aqueous) from Merck (Darmstadt, Germany). Xylene was obtained from Katayama Chemical (Osaka) and malinol from Muto Pure Chemical Company (Tokyo). Mouse anti-keratin-5 (AF 138) polyclonal antibody was purchased from Covance (Emerville, CA) and biotin-conjugated anti-keratin-K8 from Progen Biotechnik Gmbh (Heidelbberg, Germany). Alexa Fluor-488 (AF488) goat anti-rabbit IgG (H+L) and fluorescent streptavidin conjugates were both obtained from Invitrogen (Tokyo). Fluorescent mounting medium and goat serum (normal) were each purchased from DakoCytomation/Dako (Carpinteria, CA/Denmark). Glutaraldehyde, DMP-30 (2,4,6-tri-dimethylaminomethyl phenol), DDSA EM (docenyl succinic anhydride), EPON 812 Resin, and MNA (methyl nadic anhydride) were obtained from TAAB (Berkshire, UK).
For the flow cytometric analysis, phycoerythrin (PE)-labeled rat anti-mouse CD4, fluorescein isothiocyanate (FITC)-labeled rat anti-mouse CD8a, FITC-labeled rat anti-mouse CD90.2, PE-labeled rat anti-mouse CD45RB, and FITC-labeled rat anti-mouse CD45R/B220 were purchased from BD Pharmingen (San Jose, CA). Allophycocyanin (APC)-labeled anti-mouse CD4 (L3T4), FITC-labeled anti-mouse CD44, and PE-labeled anti-mouse IgM were purchased from eBioscience (San Jose, CA).
Tissue processing for H&E and immunostaining
After euthanization, each mouse was perfused transcardially with PBS and then its thymus and spleen were surgically removed and embedded in Tissue-Tek OCT (optimum cutting temperature) compound (Sakura Finetek USA, Inc., Torrance, CA), frozen in liquid nitrogen, and then immediately stored at −30°C. When needed for analyses, the samples were then sectioned at 5-μm thickness using a Leica cryostat (CM3050 S; Leica Instrument Gmbh, Nussloch, Germany).
H&E staining
Thymus and spleen cryosections (5 μm) were dried and fixed with 4% formaldehyde. The sections were then stained with hematoxylin for 2 min, washed in tap water for 5 min, then stained with eosin for 4 min, and washed in tap water for 5 min. After dehydration, the sections were mounted with mounting medium (Malinol), observed and then photographed using a Keyence BZ-8000 microscope (Osaka) and with an Olympus BX50F microscope fitted with an Olympus DP12-2 camera (Olympus Optical Co. Ltd, Tokyo).
Immunostaining
Frozen thymic tissue samples were sectioned at 5-μm thickness with the cryostat. The cryosections were then fixed in acetone and non-specific binding sites were blocked with 0.2% bovine serum albumin (BSA) and 1% goat serum in PBS. The sections were then incubated with optimal dilutions of rabbit anti-keratin-5 (1:1000) and biotin-conjugated anti-keratin-8 (1:30) antibodies. Immunoreactivity was ultimately detected with AF488-conjugated goat anti-rabbit IgG and PE-conjugated streptavidin, respectively. After dehydration, all slides were mounted with fluorescent mounting medium and viewed with a Nikon Eclipse E600 (Kawasaki, Japan) equipped with a Radience 2100 Model confocal scanning system (Bio-Rad, Hertfordshire, UK).
Ultrastructure analyses of thymus and spleen
The thymus and spleen of dedicated mice in each regimen were removed, cut into 1-mm3 pieces, immediately immersed in 2.5% glutaraldehyde in phosphate buffer (pH 7.2) for 1 hr, in osmium tetraoxide for 1 hr, and then dehydrated for 10 min in succession with 50%, 70%, 80%, 90%, and 100% ethyl alcohol. Thereafter, the samples were dehydrated three times with propylene oxide (for 10 min each), then infiltrated for 10 min with propylene oxide and epoxy resin (V/V=1:1), embedded with EPON 812 epoxy resin, DDSA, DMP-30, and MNA resin, and then aggregated for 24–48 hr at 60°C. After polymerization, 70-nm ultrathin sections were made with a diamond knife using Reichert-nissei ultracuts (Leica), and these were then stained with uranyl acetate and lead stain solution (Sigma Aldrich). The stained sections were then observed and photographed using a JEOL JEM-1400EX transmission electron microscope (TEM) (Tokyo). Using the TEM, autophagic vacuoles were counted in the vacuole-bearing TEC (in these studies, irrespective of epithelial cell type). All results were then reported as the average number of vacuoles/100 autophagic vacuole-bearing cells counted.
Flow cytometric analysis
Fluorescence-activated cell sorting (FACS) was performed using a FACSCalibur (Becton Dickinson, San Jose, CA). Spleen cells were triple-stained with APC-labeled anti-mouse CD4 (L3T4) (Clone RM4-5; eBioscience), FITC-labeled anti-mouse CD44 (Clone IM7; eBioscience) and PE-labeled rat anti-mouse CD45RB (Clone 16A: BD Pharmingen) to investigate the percentage of memory phenotype T-lymphocytes in the spleen. Spleen cells were also double-stained with PE-labeled anti-mouse IgM (Clone II/41: eBioscience) and FITC-labeled Rat anti-mouse CD45R/B220 (Clone RA3-6B2; BD Pharmingen) for the investigation of the B-lymphocyte populations. All FACS data were analyzed using CellQuest software (Becton Dickinson). Standard cytometry protocols were applied; all data was ultimately analyzed using FlowJo software (Tomy Digital Biology, Tokyo). A minimum of 10,000 events was recorded in each analytical run.
Statistical analysis
Results are expressed as means ± the standard deviation (SD). A Student’s t-test was used to compare experimental groups; P-values < 0.05 were considered significant.
Results
Histological changes in thymus and spleen
Hematoxylin and eosin (H&E) staining was used for preliminary observations of the changes in the thymus and spleen. This approach revealed that young control mice had distinct thymic cortical and of medullary compartments (‘C’ and ‘M’, respectively, in ), whereas d-galactose-treated mice showed marked changes in/between these regions of the thymus. Both the cortex and medulla in the d-galactose-treated mouse samples displayed a diffusely irregular histology—leading to a CMJ that was poorly defined. These histological changes in the thymuses of the d-galactose-treated mice were also observed in the organs from aged control mice; however, with this latter group, these changes were also accompanied by a strong infiltration of lipid-filled (adipose) cells into both regions.
Figure 1. Morphological changes in thymus and spleen. Frozen sections (5 μm) of (A) thymic and (B) splenic tissues from young mice that had been injected subcutaneously (SC, daily, for 60 days) with 0.4 mL PBS (control) or d-galactose [0.4 mL of solution (in PBS) prepared—based on weekly measures of animal body weight (BW)—to result in a dosage of 50 mg/kg body BW], as well as from aged (24-month-old) untreated C57BL/6J mice. Sections were stained with H&E (hematoxylin and eosin) and then examined under 100× and 200× magnifications for, respectively. the spleen and thymus samples. Images here are from a representative mouse from each regimen (5 mice/group). In the photos of the thymus sections, “M” and “C” indicate medulla and cortex, respectively; in the spleen section photos, “f “indicates a B-lymphocyte follicle.
![Figure 1. Morphological changes in thymus and spleen. Frozen sections (5 μm) of (A) thymic and (B) splenic tissues from young mice that had been injected subcutaneously (SC, daily, for 60 days) with 0.4 mL PBS (control) or d-galactose [0.4 mL of solution (in PBS) prepared—based on weekly measures of animal body weight (BW)—to result in a dosage of 50 mg/kg body BW], as well as from aged (24-month-old) untreated C57BL/6J mice. Sections were stained with H&E (hematoxylin and eosin) and then examined under 100× and 200× magnifications for, respectively. the spleen and thymus samples. Images here are from a representative mouse from each regimen (5 mice/group). In the photos of the thymus sections, “M” and “C” indicate medulla and cortex, respectively; in the spleen section photos, “f “indicates a B-lymphocyte follicle.](/cms/asset/adcd66c1-eb91-45d1-91c8-bc9a62ac2cbd/iimt_a_451513_f0001_b.gif)
Control mice displayed a normal spleen histology, i.e., an organ with typically distributed areas of red and white pulp and healthy B-lymphocyte follicles (‘f’ in ). In contrast, the spleens of d-galactose-treated mice showed marked alterations within their white and red pulp; this included the finding that the structures of B-lymphocyte follicles were destroyed. As with the patterns noted earlier, the changes in the spleens of the d-galactose-treated mice were somewhat similar to those noted in the organs of the aged control mice—albeit that in this latter group, the follicles were not destroyed outright but now appeared irregular as compared to normal.
Structural changes in thymus in the d-galactose-treated mice
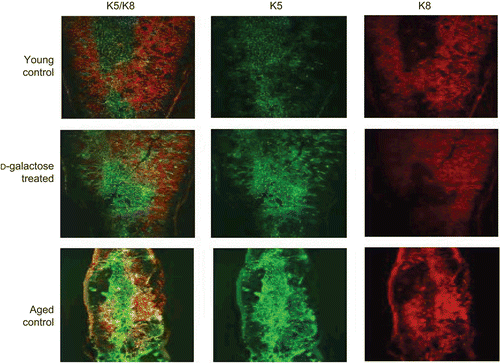
The general architecture of the thymus was assessed via staining of distinctive cellular niches and an examination of the distinctive boundary between its cortex and medulla (i.e., the CMJ). As shown in , cortical TEC were stained with anti-K8 (red) and medullary TEC with anti-K5 (green) antibodies. In the young control mouse thymus, the division between the cortex and medulla was distinct, with cortical TEC (stained red by K8 staining) demonstrating classical reticular staining, whereas TEC of the medulla were revealed by K5 immunostaining (upper panel). At the boundary, there were TEC (expressing both K5 and K8) thought to represent immature TEC. In contrast, in the tissues from the d-galactose-treated mice, this division became indistinct (middle panel); the K5 and K8 were not strictly localized in specific medulla or cortex regions—in fact, distribution was found throughout the entire thymus and there was almost no boundary between the two regions. These disruptions of the CMJ and overall alterations in thymic architecture in the d-galactose-treated mice were clearly not present in the normal young mouse tissues. The changes observed tended to reflect those taken to an extreme in the thymus samples from the aged mice. In the latter case: the organs had clearly lost any formal demarcation between the medulla or cortex regions; there was no definable CMJ; and, medullary TEC appeared to have spread over vast regions of the entire organ.
Figure 2. Architectural changes of TEC. Frozen sections (5 μm) of thymic tissues from young mice injected SC (daily, for 60 days) with PBS (control) or d-galactose (50 mg/kg BW; see legend for details), as well as from aged (24-month-old) untreated C57BL/6J mice were stained with rabbit anti-keratin-5 and biotin-conjugated anti-keratin-8 antibodies. Immunoreactivity was detected with AF488-conjugated goat anti-rabbit IgG and PE-conjugated streptavidin, respectively, and then observed using a confocal laser scanning microscope. Images are from representative mouse from each of the indicated regimens (5 mice/group).
Ultrastructures of lymphocytes in thymus and spleen
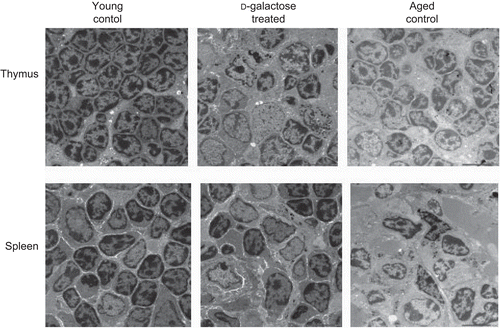
The ultrastructures of thymus glands recovered from the various hosts are shown in (upper panel). In the thymus of an age-matched young control mouse, lymphocytes appear as round with large nuclei with their mitochondrial structures clearly visible in the cytoplasm. In comparison, the lymphocytes in the tissues of aged mice display irregular shapes and signs of pyknosis. Thymuses of d-galactose-treated mouse revealed ultrastructural similarities with the organs from the aged mice, i.e., their lymphocytes were irregularly shaped with pyknotic phenomena. Ultrastructural analyses of the spleens also showed many of the same features as those above (; lower panel). The splenic lymphocytes in the samples from the young mice were uniformly distributed, with round, clear nucleoli. With the d-galactose-treated mice, splenic lymphocyte sizes were unequal and nuclei were irregular and pyknotic; these same observations were made with the splenic tissues obtained from the aged mice.
Figure 3. Ultrastructural analysis of thymus and spleen. Electron micrograph (1000×) of thymus (upper panel) and spleen (lower panel) from control C57BL/6J mice injected SC (daily, for 60 days) with PBS (n = 4) or d-galactose (50 mg/kg BW; see legend for details) (n = 4), and from aged (24-month-old) control mice (n = 3). 70-nm ultrathin sections of both thymus and spleen were stained with uranyl acetate and lead stain solution, observed with a TEM, and photographed at 1000×. The images here are from a representative mouse from each of the indicated regimens.
Distribution of autophagic vacuoles in TEC
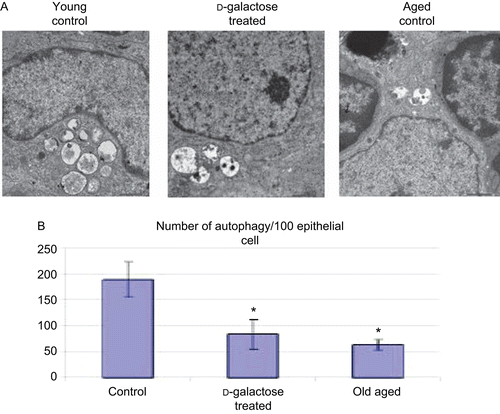
Autophagosomes within epithelial cells of the thymus gland from a representative young control, d-galactose-treated, and aged mouse are shown in . Using TEM, both the total number of autophagic vacuoles within vacuole-bearing TEC in the thymic ultrastructure were counted. Those analyses revealed that the number of autophagic vacuoles in young control mice was about 190/100 vacuole-bearing TEC (). This number was significantly decreased by more than 50% in the tissues from the d-galactose-treated mice. In the aged mice, the number of autophagic vacuoles/vacuole-bearing TEC was also decreased (relative to control values) to levels similar to (albeit slightly less than) those seen in the d-galactose-treated mice samples.
Figure 4. Distribution of autophagic vacuoles in TEC. (A) Electron micrograph (4000×) of thymus from C57BL/6J mice injected SC (daily, for 60 days) with PBS (control; n = 4) or d-galactose (50 mg/kg BW, n = 4; see legend for details), and from aged (24-month-old, n = 3) control mice. 70-nm ultrathin sections were stained with uranyl acetate and lead stain solution and examined using a TEM. An autophagic vacuole (arrow) in thymic epithelial cell is shown (at 4000×). The images here are from a representative mouse from each of the indicated regimens. (B) Total number of TEC in the thymic ultrastructure and autophagic vacuoles in TEC were counted; values shown are mean (± SD) number of autophagic vacuoles/100 TEC counted in samples from each indicated group of mice. *Value significantly different from that seen with the control young mice.
Flow cytometric analysis of lymphocyte populations in spleen
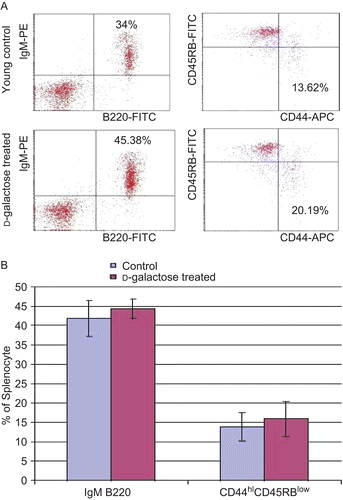
Compared to values associated with cells from the young control mice, the levels (percentages) of memory phenotype CD44hiCD45RBlow CD4+ T-lymphocytes were increased among the splenocytes recovered from the d-galactose-treated mice (, right panels) were increased. However, in this particular study, the change from the control levels failed to reach statistical significance. Similar results were usually observed in the analyses of cells from aged control mice (data not shown). In a similar manner, among the splenocytes from the d-galactose-treated mice, the percentages of IgM+B220+ double-positive cells were higher than those seen among cells of the age-matched young controls (, left panels). However, again, these differences were not significant. Furthermore, it was also clear that there were no significant differences between the percentages of single-positive IgM+ and B220+ cells between the two treatment groups’ populations of splenocytes.
Figure 5. Flow cytometric analysis of splenocytes. (A) Cell suspensions were obtained from the spleens of young mice (C57BL/6J) injected SC (daily, for 60 days) with PBS (control) or d-galactose (50 mg/kg BW; see legend for details). Cells were then double-stained with FITC-anti-B220 and PE-anti-IgM. The cells were gated on lymphocytes via their forward (FSC) and side-scatter (SSC) properties. Cells were also triple-stained with APC-anti-CD4, FITC-anti-CD44, and PE-anti-CD45RB. The cells were first gated on lymphocytes, and then gated on CD4+ cells to observe memory phenotype CD4+ T-lymphocytes. (B) Percentages of both B220+IgM+ double-positive mature B-lymphocytes and CD44hiCD45RBlow CD4+ memory phenotype T-lymphocytes from young control (n = 4) and d-galactose-treated (n = 5) mice. Values shown are the mean (± SD) percentages of each cell type within the examined populations of splenocytes from mice in each indicated treatment regimen.
Discussion
Here, it was shown that age-matched young control mice have organized distributions of keratin-5 and keratin-8 in their thymic medulla and cortex regions, whereas d-galactose-treated and aged mice showed unorganized distributions of both proteins. The thymus is a primary lymphoid organ that generates immunocompetent, self-tolerant, self-major histocompatibility complex (MHC)-restricted T-lymphocytes due to specialized microenvironments (Miller, Citation1961; Ritter and Palmer, Citation1999). TEC are the major subcomponent and direct T-lymphocyte development through a combination of cell-to-cell contacts and soluble factors (Boyd et al., Citation1993; Anderson and Jenkinson, Citation2001). Immunofluorescence staining with antibodies for cortical and medullary TEC revealed that aging is associated with a disorganized thymic architecture, with a poorly-defined boundary between the medulla and cortex (Gui et al., Citation2007).
It was also found that the lymphocytes in the thymus of d-galactose-treated mice and aged mice displayed irregular shapes. These abnormal appearances seemed to be related, in part, to increased evidence of pyknotic phenomena and condensed intranuclear chromatin in the cells. These results were similar to a previously published report (Deng et al., Citation2006) that also reported that d-galactose treatment led to aberrant lymphocyte functions. These included significant decreases in lymphocyte mitogenesis and IL-2 activity (each assessed using bioassays); the latter was subsequently found to correlate with decreases in the mRNA expression of the cytokine in the cells.
Changes within the lysosomes of senescent tissues and organs are common and have been used as biomarkers of aging. In general, the formation of autophagic vacuoles in cells decreases with age. Decreased formation of autophagic vacuoles and an even more striking delay of fusion of autophagic vacuoles with lysosomes usually occur in aged mice (Terman, Citation1995). Evidence in this study obtained using TEM showed that the numbers of autophagic vacuoles in TEC were significantly decreased in d-galactose-treated mice compared to young control mice. As expected, the aged mice also had the same tendency for low numbers of autophagic vacuoles within their TEC.
During autophagy, cytoplasmic constituents (including organelles like mitochondria) are enwrapped first by a membrane sac. Although the endoplasmic reticulum, golgi, or a less well-characterized membrane compartment called the phagophore, have been proposed as origins of autophagosome membranes, recent studies indicate that autophagic vesicles may be formed de novo through nucleation, assembly, and elongation of small membrane structures (Noda et al., Citation2002). Closure of these membranes results in the formation of double-membrane structures called autophagosomes. After a few steps, autolysosomes are generated by fusion of the outer membranes of the autophagosomes and late endosomes or lysosomes. Lysosomal hydrolases degrade the cytoplasm-included contents of the autophagosome along with its inner membrane (Gozuacik and Kimchi, Citation2004).
While use of TEM was of great value in allowing us to enumerate autophagic vacuoles in TEC, the downside to this approach was that it was extremely difficult to differentiate cortex-associated epithelial cells from medulla-associated epithelial cells. Thus, for the enumeration studies, all epithelial cells were counted together, irrespective of a particular cell type. Generally, TEC can be categorized according to a classification set forth by Nabarra et al. (Citation2001): (A) Type I epithelial cells are present in the cortex only and contain a large cytoplasm with tonofilaments and desmosomes; (B) Type II epithelial cells, present in the medulla only, are characterized by an intra-cytoplasmic alveolar network formed by association of vacuoles with an empty lumen bordered by few short microvilli; and, (C) Type III epithelial cells, present in the medulla only, display an intra-cytoplasmic cavity bordered by numerous microvilli and cilia; the cavity lumen sometimes appears full of dense material. Lastly, in these studies, vacuole counts were only performed with vacuole-bearing TEC (again, irrespective of cell type). In the period since the conclusion of the experiments, it became clear that there were other pieces of information that could be gleaned from the tissue samples. These included determinations as to whether the effects from d-galactose were only on the number of vacuoles per cell and/or upon the number of epithelial cells with vacuoles (as percentages of all cells present) as well? Similarly, the question remains as yet unanswered as to whether medullary epithelial cells also contained changes in numbers of cells with vacuoles and/or in the numbers of vacuoles/vacuole-bearing medulla cell? Because these remain important questions to address about the effects of the d-galactose treatments on immune system organs and their associated cells, ongoing studies are revisiting the slides that were generated to perform the noted comparisons of sizes of vacuole-bearing TEC populations overall (as well as locations in the tissue) as an effect of treatment/age.
Age-related immune deficiency is associated with a decrease in the proportions of naive T-lymphocytes in the spleen, which respond normally to non-specific mitogens, and with an increase in the proportions of memory T-lymphocytes, which respond poorly to mitogens. Aging is also associated with declines in proportions of peripheral blood T-lymphocytes. Mouse CD4+ T-lymphocytes are subdivided into two subsets by cell surface markers: CD44loCD45RBhi (naive) and CD44hiCD45RBlo (memory) T-lymphocytes. Levels of the CD44loCD45RBhi T-lymphocytes (predominant in young mice) decrease with age, while those of CD44hiCD45RBlo T-lymphocytes increase (Kurashima and Utsuyama, Citation1997). In the studies here, it was found that the percentages of memory phenotype T-lymphocytes in d-galactose-treated mice increased similar to that of levels in aged mice (see ).
Aging is also accompanied by greatly reduced B-lymphocyte production in the bone marrow, yet peripheral B-lymphocyte numbers do not decline (Johnson et al., Citation2002). The populations of both mature and immature splenic B-lymphocytes remain relatively constant as mice age (Stephan et al., Citation1996). B-Lymphocyte number is strictly regulated and, despite the decreased output of B-lymphocytes by the bone marrow, does not decline during aging due to self-renewal of peripheral B-lymphocytes (Weksler and Szabo, Citation2000). Furthermore, the responsiveness of these cells in aged mice remains relatively intact. In a similar manner, the population of mature splenic B-lymphocytes is maintained by their increased longevity (Klinman and Kline, Citation1997). From the experiments performed here, it was determined that the percentage of IgM+B220+ double-positive mature B-lymphocytes increased in the d-galactose-treated mice compared to that in the young control mice, although this increase was not statistically significant. These results indicate that mature B-lymphocytes tend to proliferate (in a homeostatic manner) despite the decrease in levels of naïve B-lymphocytes in d-galactose-treated mice.
From these experiments, two major findings can be reported. (1) There was a disruption of the CMJ that was accompanied by a disorganized distribution of K5 (present in medullary TEC) and K8 (present in cortical TEC) proteins in the thymus organs of d-galactose-treated mice as compared to in the those of control mice; these changes were akin (but not completely similar) to those seen in the organs of aged mice. (2) Autophagy within TEC (irrespective of specific TEC type) was greatly decreased in TEC of d-galactose-treated mice compared to levels in TEC of young control mice; specifically, levels of autophagic vacuoles/TEC in the d-galactose-treated hosts approached values only slightly greater than among TEC of aged mice.
In conclusion, there remains a paucity of information about both changes induced in the immune system of d-galactose-exposed hosts and regarding functional changes in the immune system cells of these hosts. Among the few studies that have been reported, the results indicated that ex vivo mitogenesis and IL-2 production were decreased by T-lymphocytes recovered from d-galactose-treated mice (see above). Based on the findings reported here, and because d-galactose-treated mouse and rat models have been used widely for research into the effects of aging, further studies are warranted to evaluate more fully the immunomodulatory effects of d-galactose treatment and whether its use to create age-mimicry is toxicologically-appropriate.
Acknowledgements
The Authors wish to thank Minoru Tanaka for technical assistance with the flow cytometry.
Declaration of interest
This work was supported by grants-in-aid from the Ministry of Education, Sports, and Culture of Japan.
References
- Anderson, G., and Jenkinson, E. J. 2001. Lymphostromal interactions in thymic development and function. Nat. Rev. 1:31–40.
- Aw, D., Silva, A. B., Maddick, M., von Zglinicki T., and Palmer, D. B. 2008. Architectural changes in the thymus of aging mice. Aging Cell 7:158–167.
- Boyd, R. L., Tucek, C. L., Godfrey, D. I., Izon, D. J., Wilson, T. J., Davidson, N. J., Bean, A. G., Ladyman, H. M., Ritter, M. A., and Hugo, P. 1993. The thymic microenvironment. Immunol. Today 14:445–459.
- Brekelmans, P., and van Ewijk, W. 1990. Phenotypic characterization of murine thymic microenvironments. Semin. Immunol 2:13–24.
- Brownlee, M. 1995. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 46:223–234.
- Bucala, R., and Cerami, A. 1992. Advanced glycosylation: Chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 23:1–34.
- Cuervo, A. M. 2004. Autophagy: In sickness and in health. Trends. Cell. Biol. 14:70–77.
- Cuervo, A. M., Bergamini, E., Brunk, U. T., Droge, W., Ffrench, M., and Terman, A. 2005. Autophagy and aging: the importance of maintaining ‘clean’ cells. Autophagy 1:131–140.
- Cui, X., Zuo, P., Zhang, Q., Li, X., Hu, Y., Long, J., Packer, L., and Liu, J. 2006. Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of [R]-α-lipoic acid. J. Neurosci. Res. 83:1584–1590.
- Del Roso A., Vittorini S., Cavallini G., Donati A., Gori Z., Masini M., Pollera M., and Bergamini E. 2003. Aging-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp. Gerontol. 38:519–527.
- Deng, H., Cheng, C., Cui, D., Li, D., Cui, L., and Cai, N. 2006. Structural and functional changes of immune system in aging mouse induced by d-galactose. Biomed. Environ. Sci. 19:432–438.
- Gong, G. Q., and Xu, F. B. 1991. Study of aging model in mice. J. China Pharm. Univ. 22:101–103.
- Gozuacik, D., and Kimchi, A. 2004. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23:2891–2906.
- Gui, J., Zhu, X., Dohkan, J., Cheng, L., Barnes, P. F., and Su, D. M. 2007. The aged thymus shows normal recruitment of lympho-hematopoietic progenitors but has defects in thymic epithelial cells. Int. Immunol. 19:1201–1211.
- Johnson, S. A., Rozzo, S. J., and Cambier, J. C. 2002. Aging-dependent exclusion of antigen-inexperienced cells from the peripheral B-cell repertoire. J. Immunol. 168:5014–5023.
- Klinman, N. R., and Kline, G. H. 1997. The B-cell biology of aging. Immunol. Rev. 160:103–114.
- Kurashima, C., and Utsuyama, M. 1997. Age-related changes of cytokine production by murine helper T-cell subpopulations. Pathobiology 65:155–162.
- Lei, H., Wang, B., Li, W. P., Yang, Y., Zhou, A. W., and Chen, M. Z. 2003. Anti-aging effect of astragalosides and its mechanism of action. Acta Pharmacol. Sin. 24:230–234.
- Li, W. B., Wei, F., Fan, M., Zhan, J. L., Zhang, B. L., Ma, X. C., Yang, W. P., and Wei, W. 1995. Mimetic brain aging effect induced by d-galactose in mice. Chinese J. Pharm. Toxicol. 9:93–95.
- Miller, J. F. 1961. Immunological function of the thymus. Lancet 2:748–749.
- Nabarra, B., Mulotte, M., Casanova, M., Godard, C., and London, J. 2001. Ultrastructural study of the FVB/N mouse thymus: Presence of an immature epithelial cell in the medulla and premature involution. Dev. Comp. Immunol. 25:231–243. Erratum in 25:539–543.
- Noda, T., Suzuki, K., and Ohsumi, Y. 2002. Yeast autophagosomes: De novo formation of a membrane structure. Trends Cell. Biol. 12:231–235.
- Prockop, S., and Petrie, H. T. 2000. Cell migration and the anatomic control of thymocyte precursor differentiation. Semin. Immunol. 12:435–444.
- Ritter, M. A., and Palmer, D. B. 1999. The human thymic microenvironment: New approaches to functional analysis. Semin. Immunol. 11:13–21.
- Song, X., S., Bao, M., Li, D., and Li, Y. M. 1999. Advanced glycation in d-galactose-induced mouse aging model. Mech. Age. Devel. 108:239–251.
- Stephan, R. P., Sanders, V. M., and Witte, P. L. 1996. Stage-specific alterations in murine B-lymphopoiesis with age. Int. Immunol. 8:509.
- Terman, A. 1995. The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology 41:319–325.
- Terman, A., Gustafsson, B., and Brunk, U. T. 2007. Autophagy, organelles and ageing. J. Pathol. 211:134–143.
- Vlassara, H., Bucala, R., and Striker, L. 1994. Pathogenic effects of advanced glycation: Biochemical, biologic, and clinical implication for diabetes and aging. Lab. Invest. 70:138–141.
- Weksler, M. E., and Szabo, P. 2000. The effect of age on the B-cell repertoire. J. Clin. Immunol. 20:240–249.
- Xu, F. B. 1985. Sub-acute toxicity of d-galactose. Proceedings of Second National Conference on Aging Research, Herbin, China – 1985.
- Zhang, J. T., and Saito, H. 1986. Studies of susceptibilities to the amnestic effects of twelve chemicals on passive avoidance responses in mice: Comparison between step-down and step-through tests. Acta. Pharm. Sin. 21:12–19.
- Zhang, Q., Li, X., Cui, Xu., and Zuo, P. 2005. d-Galactose-injured neurogenesis in the hippocampus of adult mice. Neurolog. Res. 5:552–556.
