Abstract
1,2:5,6-Dibenzanthracene (DBA) is ubiquitous in our environment as a contaminant produced by incomplete combustion of organics from sources such as forest fires, cigarette smoke, and asphalt paving, and it is more immunosuppressive of the T-dependent antibody-forming cell (AFC) response than the well-studied polycyclic aromatic hydrocarbon, benzo(a)pyrene. The systemic immunosuppressive effects of DBA were investigated following a single pharyngeal aspiration (pa) in female B6C3F1 mice. The immunotoxic effects of DBA were evaluated using numerous assays of varying complexity to evaluate innate (natural killer [NK] cell activity), cell-mediated (T-lymphocyte proliferation, mixed leukocyte response [MLR], cytotoxic T-lymphocyte [CTL] activity, delayed-type hypersensitivity [DTH]), and humoral immunity (B-lymphocyte proliferation, T-dependent antibody responses). A single pa of DBA at doses up to 30 mg/kg had no effect on NK cell activity, anti-CD3 antibody-mediated T-lymphocyte proliferation, the MLR, or B-lymphocyte proliferation. DBA at 30 mg/kg suppressed Concanavalin A (ConA)-stimulated T-lymphocyte proliferation and the CTL response. DBA exposure reduced cytokine production in spleen cell culture supernatants after in vitro stimulation with ConA or lipopolysaccharide (LPS). Immunosuppression was observed at lower doses in the holistic assays. The DTH response to Candida albicans was significantly decreased at 3.0 mg/ kg DBA, while the AFC response was intermittently suppressed at 1.0 mg/kg, with no effect observed at 0.3 mg/kg. These results demonstrate that a single pa of DBA produces systemic immunotoxicity, and of the assays utilized, the holistic assays (i.e., DTH, AFC) appear to be most sensitive to the immunosuppressive effects of DBA.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) have been studied extensively over the last 30 years for their carcinogenic and immunotoxic effects. The vast majority of human PAH exposure occurs through the diet, via inhalation of polluted air from a large number of sources (including car exhaust fumes, forest fires, wood-burning stoves, smoking, charcoal grilling, asphalt paving, roofing, and coke production), or through occupational exposure. Occupational exposure to PAHs significantly increases the risk of developing lung cancer, particularly among roofers, smelters, asphalt workers, and coke oven workers (Talaska et al., Citation1996). Using urinary 1-hydroxypyrene (1-OHP) levels as a biomarker approximating PAH exposure, Chuang and Chang (Citation2007) demonstrated that occupational exposure of taxi drivers to exhaust fumes resulted in significantly higher 1-OHP levels as compared with office workers. In addition, these authors also demonstrated that smoking significantly increased 1-OHP levels in both taxi drivers and office workers.
1,2:5,6-Dibenzanthracene (DBA) has not been as extensively studied as other PAHs such as benzo(a)pyrene (B[a]P) or 7,12-dimethylbenzanthracene (7,12-DMBA). Although well-studied, 7,12-DMBA is of little relevance with regard to human health since it exists only as a laboratory compound and does not occur naturally. B[a]P, however, is ubiquitous as an environmental contaminant and is the most extensively studied non-substituted PAH.
DBA is reported to be a carcinogen with at least 10 times greater potency than B[a]P (Okona-Mensah et al., Citation2005) and may be > 100 times more potent than B[a]P (Nesnow et al., Citation1995). In fact, a number of PAHs have been identified as having higher carcinogenic potencies than B[a]P, including DBA, several dibenzopyrenes, and 5-methylchrysene (Pufulete et al., Citation2004), thus questioning the suitability of B[a]P as the “prototypic” PAH. It has been suggested that the relative contributions of these higher potency PAHs to the carcinogenic activity of PAH mixtures may be much greater than that of B[a]P (Okona-Mensah et al., Citation2005).
To the best of our knowledge, there are few published manuscripts related to the immunotoxicity of DBA. One study from our laboratory from over 20 years ago demonstrated DBA to be more potent than B[a]P (following subcutaneous [sc] exposure) in suppressing the T-dependent antibody response to sheep red blood cells (SRBC) (White et al., Citation1985). Recent work by Hernandez et al. (Citation2005) has demonstrated that DBA administered repeatedly by sc injection suppressed NK cell activity, T-lymphocyte proliferation, and the antibody-forming cell (AFC) response, in addition to decreasing delayed-type hypersensitivity (DTH) responses to Candida albicans. DBA was also shown to be immunosuppressive in in vitro human peripheral T-lymphocyte mitogenesis studies (Davila et al., Citation1996).
Although there are numerous studies evaluating the toxic effects of intraperitoneal (ip) exposure to PAHs (Weston, Citation1967; Wojdani and Alfred, Citation1984; Wojdani et al., Citation1984; Galvan et al., Citation2006) and dermal exposure to PAHs (Parrot et al., Citation1989; CitationLlewellyn and White, 1998; Ullrich, Citation1999; Ullrich and Lyons, Citation2000), most of the immunotoxicological evaluations performed to date on PAHs have either been performed in vitro, or the PAHs have been administered sc or per os (po). While sc exposure is relatively easy to do in laboratory settings and gives reliable dose-responsive results, the method of administration is not biologically relevant with regard to human PAH exposure, which typically occurs via the diet or by inhalation. It has been well-established that the murine gastrointestinal tract has a remarkable ability to detoxify B[a]P following oral exposure (Uno et al., Citation2004, Citation2006, Citation2008); however, immunosuppression has been observed when B[a]P is administered orally in massive doses. In contrast, no currently published studies have evaluated the immunotoxic effects of DBA following respiratory exposure. Therefore, these studies were designed to evaluate the systemic effects of DBA following respiratory exposure. In addition to being costly, inhalation studies require specialized equipment and may result in unnecessary hazards to laboratory personnel; therefore, alternative respiratory exposure routes were considered.
Recently, intratracheal (it) installation has been increasingly utilized as a method of administration to model inhalation exposure. Driscoll et al. (Citation2000) concluded that it instillation yields qualitatively similar results to inhalation exposure for a diverse array of biological endpoints, including inflammation, fibrosis, infection, allergic sensitization, and lung cancer. In these studies, we used pharyngeal aspiration (pa), a technique similar to it exposure, with a few important distinctions previously discussed by Rao et al. (Citation2003). It has been demonstrated that this technique produces excellent deep lung distribution of administered particles and avoids the difficulties and trauma associated with it exposure (Woolhiser et al., Citation2000; Rao et al., Citation2003).
The purpose of this study was to characterize the systemic immunosuppressive effects of DBA following a single pa in B6C3F1 mice. Endpoints evaluated determined the immunocompetence of innate, humoral, and cell-mediated immunity (CMI) components of the immune response following DBA exposure. Based upon the work of Hernandez et al. (Citation2005, Citation2006), we hypothesized that a single pa of DBA would result in systemic immunotoxicity, manifesting primarily as suppression of the humoral immune response. Following a determination of the most sensitive component of the immune response adversely affected in these studies, comparisons were drawn between results presented herein with those obtained from previous studies evaluating DBA immunotoxicity.
Materials and methods
DBA
DBA (99.7% purity; Supelco, Bellefonte, PA) was prepared as suspension in phosphate-buffered saline (PBS; Sigma Chemical Co., St. Louis, MO) with 0.05% Tween 80 (Sigma-Aldrich, St. Louis, MO), and was constantly stirred using a Teflon-coated stir bar and magnetic stirrer during the dosing period. Initial studies in our laboratory (data not shown) demonstrated that this vehicle (VH) and route of administration did not affect immunological endpoints.
Anesthetic
Animals were anesthetized with a mixture of 8.9% ketamine (100 mg/mL stock; Phoenix Pharmaceutical, Inc., St. Joseph, MO), 3.3% xylazine (20 mg/mL stock; Burns Veterinary Supply, Westbury, NY), and 87.8% physiological saline given ip at ≈0.2 mL per each 20 g mouse. Animals were evaluated for loss of righting and toe pinch reflexes to demonstrate that anesthesia was deep enough to perform the pa procedure. Animals remained under anesthesia for 20–30 min and were monitored constantly until time of complete recovery (i.e., regaining of righting reflex).
Animals and animal exposure
Female B6C3F1 mice were obtained from Taconic Farms (Germantown, NY). Animals arrived at 9–10 weeks-of-age and were quarantined for at least 1 week prior to use. Mice were maintained on NTP 2000 Laboratory Diet and tap water ad libitum. Ambient temperatures were maintained at 21–24°C, relative humidity was maintained at 40–70%, and the light/ dark cycle was 12 h intervals. Prior to being placed on study, the mice were randomized and then assigned to respective treatment groups (8 mice/group). Mice were determined to be free of hepatitis and Sendai virus by serology testing. All animal procedures were conducted in an AAALAC-accredited facility under an animal protocol approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee (IACUC).
Following anesthesia, mice were inclined on the pa platform, supported by a rubber band. Their tongues were gently extended to the side with forceps to prevent swallowing, and 0.1 mL of either VH (PBS with 0.05% Tween 80) or DBA (as a suspension in VH) was delivered directly into the oropharynx using a positive displacement pipette. In general, DBA treatment groups consisted of a single administration of 0.3, 3.0, or 30 mg/kg DBA. Treatment groups for the AFC assay also included groups exposed to 0.1, 1.0, or 10 mg/kg DBA. Unless otherwise indicated, systemic effects on the immune system in these studies were evaluated on Day 4, counting the day of the single pa of DBA as Day 0. On the assay date, mice were euthanized by cervical dislocation after anesthetizing with CO2.
When appropriate, cyclophosphamide (CPS; Sigma) was given as a positive control for the immunotoxicological assays, unless specifically noted. Positive control animals received 50 mg/kg CPS daily by ip injection (0.1 mL/10 g of body weight) for 4 days prior to euthanization. For the natural killer (NK) cell assay, positive control animals received 0.2 mL intravenously (i.v.) of rabbit anti-asialo GM1 antibody (AAGM1; Wako Pure Chemical Industries, Osaka, Japan) at a 1:10 dilution in sterile physiological saline 24 h prior to euthanization.
Toxicological parameters
Animal body weights were obtained prior to being placed on study and after euthanization to determine the effects of DBA on body and organ weights. Animals were examined for gross pathology, and the following organs were removed and weighed: thymus, liver, spleen, lungs, and kidneys with adrenals.
Spleen collection and single-cell suspension preparation
Spleens were collected aseptically and placed in 3 mL of Earle’s balanced salt solution (EBSS; Sigma) with 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; GibcoBRL, Grand Island, NY) and, in the case of assays requiring sterile conditions, with 50 μg/mL gentamicin (Invitrogen, Carlsbad, CA). Splenocyte suspensions were prepared by gently pressing the spleen between the frosted ends of two sterile microscope slides into a 30 × 15 mm Petri dish. The slides were washed with EBSS using a Pasteur pipette, and then single-cell suspensions were placed into 12 × 75 mm snap-cap polystyrene tubes. Splenocytes were then centrifuged at 300 g for 10 min and re-suspended in Roswell Park Memorial Institute (RPMI) 1640 media supplemented with 10% fetal bovine serum (FBS), 15 mM HEPES, 2 mM l-glutamine, 5% sodium bicarbonate (7.5% stock solution), 50 μg/mL gentamicin, and 10 μM 2-mercaptoethanol (2-ME) (complete media). For the AFC assay, cells were re-suspended in EBSS. Cell counts were determined using a ZBII Coulter counter in the presence of ZAP-OGLOBIN II lytic reagent (Beckman Coulter Corporation, Miami, FL).
Immunofluorescent staining and flow cytometric analysis
Flow cytometric analysis was conducted accordingly as previously described (Bradley et al., Citation1994), with minor modifications. Splenic populations evaluated included total B-lymphocytes (immunoglobulin [Ig+]), total T-lymphocytes (CD3+), T-helper lymphocytes (CD4+), cytotoxic T-lymphocytes (CTL; CD8+), macrophages (Mac-3+), and NK cells (NK1.1+). The antibodies utilized to identify these populations were obtained from BD Pharmingen (San Diego, CA). As a viability stain, propidium iodide (PI) solution was utilized following the initial antibody staining. Enumeration was performed on a Becton Dickinson FACScan Flow Cytometer in which log fluorescence intensity was read by setting a live gate on red fluorescence PI to eliminate nonviable cells. The data were analyzed using CellQuest software (Becton Dickinson, San Jose, CA). Five thousand cells were counted for each sample.
NK cell activity
NK cell activity was measured as described by Reynolds and Herberman (Citation1981) and modified according to Wilson et al. (Citation2001) with noted changes. After being radiolabeled by incubation with 500 μCi [51Cr] (as 51Na2CrO4; Perkin Elmer, Waltham, MA), YAC-1 cells (target cells; ATCC, Manassas, VA) were adjusted to a final concentration of 5 × 104 viable cells/mL and added to wells of a 96-well plate in a volume of 100 μL. Spleen cells (effector cells) were plated in duplicate in a volume of 100 μL at appropriate concentrations to obtain effector:target ratios of 200:1, 100:1, 50:1, 25:1, 12.5:1, and 6.25:1. Following the 4-h [51Cr] release assay, 100 μL of supernatant from each well was counted using an LKB γ-counter to obtain counts per minute (CPM), and results were expressed as percent cytotoxicity, calculated as (experimental release CPM − spontaneous release CPM)/(maximum release CPM − spontaneous release CPM). Maximum and spontaneous releases were determined by adding 0.1 mL of 0.1% Triton X-100 or complete RPMI media to each of 12 replicate cultures containing 0.1 mL of target cells.
Splenocyte proliferative responses to Concanavalin A, anti-CD3 antibody, lipopolysaccharide, and anti-IgM F(ab′)2 plus IL-4
T-Lymphocyte proliferation in response to Concanavalin A (ConA) and B-lymphocyte proliferation in response to lipopolysaccharide (LPS) or anti-IgM F(ab′)2 antibody fragments plus IL-4 were performed as described by CitationGuo et al. (2000) with noted exceptions. The four concentrations of ConA utilized were 10, 5, 2.5, and 1.25 µg/mL. LPS (derived from Salmonella typhosa; Sigma) was utilized at 100 μg/mL, while F(ab′)2 concentrations of 0 and 5 μg/mL (Accurate Chemical, Westbury, NY) and IL-4 concentrations of 0, 10, and 100 ng/mL (R&D Systems, Minneapolis, MN) were employed.
Anti-CD3 (αCD3) antibody-mediated T-lymphocyte proliferation was performed as previously described (CitationGuo et al., 2001) with the following noted change. Splenocytes were added to wells of BD BioCoat T-Cell Activation (96-well) plates either coated with anti-mouse CD3 or uncoated (BD Biosciences, San Jose, CA) for T-lymphocyte proliferation or background unstimulated controls, respectively.
In all proliferation assays, plates were incubated at 37°C, 5% CO2, and 95% humidity. On Day 3, 18–24 h prior to harvest, cells were pulsed with [3H]-thymidine (50 µCi/mL [Perkin Elmer, Waltham, MA]; 20 µL/well). Cells were collected with a Tomtec Harvester 96 Mach IIIM (Tomtec, Hamden, CT) onto Wallac filtermats (Wallac, Turku, Finland) and counted using a 1450 Microbeta Trilux liquid scintillation and luminescence counter (Perkin Elmer, Gaithersburg, MD). The incorporation of [3H]-thymidine into the proliferating cells was used as the endpoint of the assay, and the data were expressed as CPM/2 × 105 cells.
One-way mixed leukocyte response to DBA/2 spleen cells
The proliferation of T-lymphocytes in recognition of an allogeneic stimulus provided by DBA/2 splenocytes was measured utilizing the one-way mixed leukocyte response (MLR) as described by CitationGuo et al. (2000).
CTL assay
Cell-mediated cytotoxicity of P815 tumor cells (target cells; ATCC) by CTLs (effector cells) was evaluated in two phases as previously described by Bradley et al. (Citation1994), in which splenocytes and P815 cells were co-cultured for 5 days (induction phase), harvested, and then cultured with radiolabeled P815 cells in a 4-h [51Cr] release assay (effector phase). Results were expressed as % cytotoxicity, calculated as described above for the NK cell assay.
DTH response to C. albicans
The DTH response to Candida albicans was measured using a modified DTH assay of Nghiem et al. (Citation2002). In brief, 10 days prior to sacrifice, mice were sensitized with 0.1 mL of formalin-fixed C. albicans (Alerchek, Inc., Portland, ME) by sc injection into the right flank within 2 h after pa of DBA. Eight days later, a 40 µL challenge of the C. albicans antigen chitosan (Alerchek) was injected into the right footpad after measuring and recording the pre-thickness of the footpad with a digital micrometer (Mitutoyo, Tokyo, Japan). Twenty-four- and 48-h post-challenge, the thickness of the right footpad was measured, and the footpad swelling for each mouse was calculated (post-challenge thickness − pre-challenge thickness). The background footpad swelling (negative control) was determined in a group of mice that were challenged but not sensitized (CO; challenge only). Data were reported as (post-challenge thickness − pre-challenge thickness) × 100.
LPS-stimulated IgM and IgG production
Polyclonal IgM and IgG antibody enzyme-linked immunosorbent assays (ELISA) were performed following a single pa of DBA. The antibody levels were quantified from the supernatants of splenocytes cultured for 24 h in the presence of LPS and assayed using a mouse IgM or IgG matched pairs antibody kit (Bethyl Laboratories, Montgomery, TX). The supernatants (neat) were aliquoted (100 μL) and serially diluted 1:2 across the plate in 96-well microtiter plates previously coated with goat anti-mouse IgM(G) capture antibody (10 μg/mL) in 0.05 M carbonate–bicarbonate coating buffer and blocked with blocking solution (50 mM Tris, 0.14 M NaCl, 1% BSA). After 1 h, plates were washed five times and 100 μL of goat anti-mouse IgM(G) antibody conjugated to horseradish peroxidase (HRP) was added to the plates for 1 h at room temperature (RT). This was followed by washing five times with wash buffer and addition of 100 μL of tetramethylbenzidine (TMB; BD Biosciences) substrate for 30 min at RT in the dark. Stop solution (2 N H2SO4) was then added to each well and the absorbance was read at 450 nm on a Thermomax microplate reader (Molecular Devices Corp., Sunnyvale, CA). Results were obtained using Softmax (v.2.32; Molecular Devices) and quantified against an IgM(G) standard and expressed as total IgM(G) (μg/mL).
TH1/TH2 cytokine production
Four days after administration of a single pa of DBA, splenocytes were prepared and subsequently stimulated for 24 and 48 h with LPS or ConA. Supernatants from cultures were analyzed for cytokine levels using mouse FlowCytomix kits from Bender MedSystems (Burlingame, CA). Cytokines measured included interleukin (IL)-1, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-17, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF). Sample preparation was performed according to kit instructions, and flow cytometric analysis was performed using a Beckman Coulter Cytomics FC500 (Miami, FL). Results were obtained by analyzing standards and quantifying samples against the standard curves using Bender MedSystems software (v.2.2). For the ConA-stimulated experiments, animals received either VH or DBA at 0.3, 3, or 30 mg/kg, while for the LPS-stimulated experiments, animals received either VH or DBA at 30 mg/kg only. GM-CSF was not evaluated following ConA stimulation. IL-12 levels following LPS stimulation were measured after 24-h culture only; a mouse IL-12 ELISA kit (BD Biosciences) was utilized (according to kit directions), and absorbance was read at 450 nm on a Thermomax microplate reader (Molecular Devices). Results of the ELISA were obtained using Softmax (v.2.32; Molecular Devices). Each sample absorbance was quantified against an IL-12 standard and expressed as mean IL-12 (pg/mL).
T-Dependent antigen (keyhole limpet hemocyanin)-specific IgM antibody production
An ELISA was used to determine the antigen-specific primary IgM antibody response to keyhole limpet hemocyanin (KLH), a soluble T-dependent antigen, according to White et al. (Citation2007). Each animal received 2 mg KLH in 0.2 mL 0.9% saline i.v. within 2 h after DBA pa 5 days prior to euthanization. Results were obtained using Softmax (v.2.32; Molecular Devices). The sample absorbances were quantified against an anti-KLH IgM standard (BD Pharmingen) and expressed as mean IgM (μg/mL).
T-Dependent antigen (SRBC)-specific IgM antibody in serum
An ELISA system as described by Temple et al. (Citation1993) was used to determine the serum titers of the primary IgM response to SRBC, a particulate T-dependent antigen. Five days prior to sacrifice, mice were sensitized i.v. with 7.5 × 107 SRBC (Lampire, Pipersville, PA) within 2 h after the single pa of DBA. Results were obtained using Softmax (v.2.32; Molecular Devices). The mean absorbency values were calculated using Softmax program in which the linear portion of the curve was identified using a procedure as previously described (Kawabata et al., Citation1995). Interpolation at 0.5 optical density (OD) was calculated by Softmax and the value recorded in whole units. The “titer” is defined as the reciprocal of the serum dilution that has an OD value of 0.5.
Spleen IgM AFC response to the T-dependent antigen SRBC
Using the hemolytic plaque assay of Jerne et al. (Citation1963) with modifications as described (White et al., Citation2010), the primary IgM AFC response to SRBC was measured. Four days prior to sacrifice, mice were sensitized i.v. with 7.5 × 107 SRBC (Lampire, Pipersville, PA) within 2 h after the single pa of DBA. Plaques were enumerated using a Bellco plaque viewer, and experimental results were expressed as specific activity (AFC/106 spleen cells) and total spleen activity (AFC/spleen).
Statistical analysis
Results represent the mean ± standard error. Statistical analysis of all data was performed using the JMP™ 5.0 statistical software package (SAS Institute, Inc., Cary, NC) by first using Bartlett’s test for homogeneity of variances, followed by an analysis of variance (ANOVA) (parametric or non-parametric as necessary). Following a significant ANOVA result, further ad hoc comparisons between control and treatment groups were completed using Dunnett’s test for parametric data and the Wilcoxon rank sum test for non-parametric data. The positive or negative controls were compared with the VH using the Student’s t-test. In all evaluations, p < 0.05 indicated a statistically significant difference.
Results
Standard toxicological parameters
Pharyngeal aspiration of DBA was well-tolerated by B6C3F1 mice. While there were no significant differences in terminal body weights, a decrease in weight was observed in all animals, including controls, over the course of these studies. When compared with the VH control animals, a statistically significant decrease in percent change in body weight was observed in the high dose animals (−8.83% versus −3.36% for VH control animals). An increase (15%) in relative liver weight at the 30 mg/kg dose was the only statistically significant organ weight change observed (data not shown). This increase was not unexpected due to the well-published increases in enzyme activity following exposure to PAHs such as DBA. Spleen, thymus, lung, and kidneys (with adrenals) weights were also evaluated but were no different than those of the VH controls when evaluated as either absolute weight or relative weight.
Immunotoxicological parameters
A statistically significant decrease (17%) in total splenocyte number was observed for the high DBA dose group as compared with VH control (). Phenotypic analysis of spleen cell populations showed a significant decrease (25%) in the absolute number of B-lymphocytes at the high dose. No effects were observed on the absolute cell numbers of T-lymphocyte subsets or macrophages. At the high dose, a 17% decrease in absolute CD3+ cells was observed; however, this difference failed to reach the level of statistical significance (p = 0.054). A significant increase of 19% in the absolute number of NK cells was observed at the 3 mg/ kg dose level, although the effect was not dose-responsive. Statistically significant increases were also observed for NK cells at all DBA exposure levels when evaluated as percent values (18, 33, and 20% for the low, middle, and high dose groups, respectively). The percent of CD4+ T-lymphocytes was also significantly increased by 15 and 20% for the 0.3 and 3.0 mg/kg DBA exposure groups, respectively; however, these differences did not translate into differences in absolute CD4+ cell numbers due to decreasing total splenocyte number. The positive controls CPS and AAGM1 produced the anticipated changes in absolute and percent values.
Table 1. Flow cytometric data obtained for the B6C3F1 mice following a single pharyngeal aspiration of 1,2:5,6-dibenzanthracene.
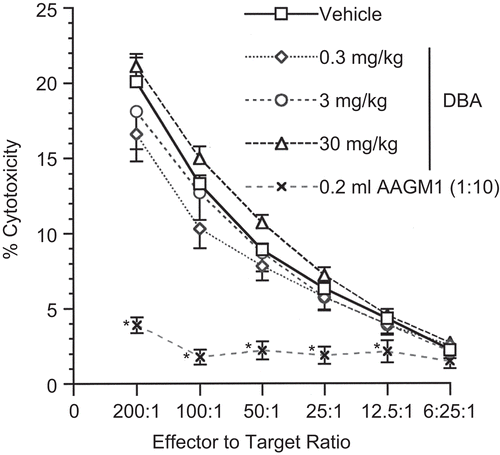
The effect of DBA on innate immunity was evaluated using the natural killer (NK) cell assay. NK activity was not affected following exposure to DBA (). As anticipated, the positive control AAGM1 significantly reduced NK cell activity.
Figure 1. Natural killer (NK) cell activity following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). NK activity expressed as % cytotoxicity after a single exposure to either DBA (0.3, 3, or 30 mg/kg) or vehicle (VH) in a single pa. Values represent the mean (± SE) derived from eight animals; *p < 0.05.
The effects of DBA on CMI were evaluated in several assays of varying complexity. First, T-lymphocyte mitogenesis in response to ConA was evaluated after in vivo pa with DBA at 0.3, 3, and 30 mg/kg. Results indicated a statistically significant decrease in the peak response (72%) in T-lymphocyte proliferation (measured by [3H]-thymidine incorporation) at the high dose (30 mg/kg; ). Interestingly, spleen cell cultures incubated with αCD3 after in vivo exposure to the same dose regimen showed no effect (). αCD3 bound to the plate provided the primary signal, and the co-stimulatory signal for CD28 (necessary for increased IL-2 production and T-lymphocyte proliferation) was provided by B7-expressing antigen-presenting cells present in the splenocyte suspensions. The MLR assay () also showed no dose-dependent effects of DBA on proliferation in recognition of an allogeneic stimulus. In contrast, the high dose of DBA (30 mg/kg) was immunosuppressive in the CTL assay, with decreases at the three highest effector-to-target ratios of 27, 42, and 55%, respectively (). CPS exposure produced the anticipated effects in each of the assays.
Figure 2. ConA- and anti-CD3-stimulated proliferation of T-lymphocytes following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). Response of splenocytes (A) co-cultured with or without ConA (0, 1.25, 2.5, 5, or 10 μg/mL) and (B) cultured in BD BioCoat T-cell activation plates either pre-coated with anti-CD3 (αCD3) antibody or in uncoated wells. Mice received either DBA (0.3, 3, or 30 mg/kg) or vehicle (VH) in a single pa. Values represent the mean (± SE) derived from eight animals; *p < 0.05; **p < 0.01.
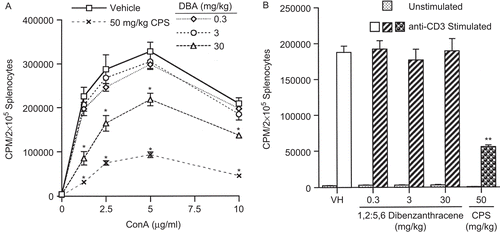
Figure 3. Mixed leukocyte response following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). Mice received either DBA (0.3, 3, or 30 mg/kg) or vehicle (VH) in a single pa and on the day of sacrifice, spleen cells were co-cultured with DBA/2 (allogeneic) spleen cells. Values represent the mean (± SE) derived from eight animals; **p < 0.01.
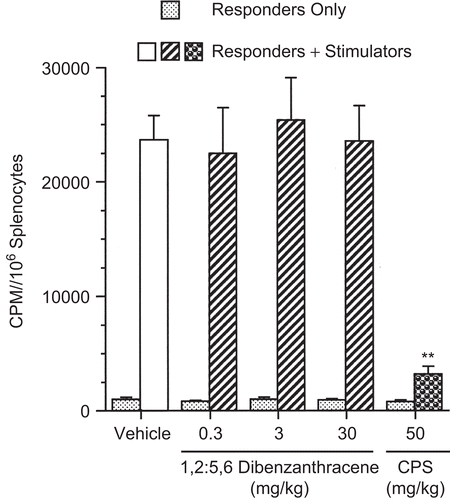
Figure 4. Activity of cytotoxic T-lymphocytes following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). Mice received either DBA (0.3, 3, or 30 mg/kg) or vehicle (VH) in a single pa. Splenocytes were cultured with P815 mastocytoma cells for 5 days followed by a 4-h [51Cr] release assay with six ratios of effector-to-target cells to assess cytotoxic T-lymphocyte (CTL) activity. Results are presented as % cytotoxicity. Values represent the mean (± SE) derived from eight animals; *p < 0.05.
![Figure 4. Activity of cytotoxic T-lymphocytes following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). Mice received either DBA (0.3, 3, or 30 mg/kg) or vehicle (VH) in a single pa. Splenocytes were cultured with P815 mastocytoma cells for 5 days followed by a 4-h [51Cr] release assay with six ratios of effector-to-target cells to assess cytotoxic T-lymphocyte (CTL) activity. Results are presented as % cytotoxicity. Values represent the mean (± SE) derived from eight animals; *p < 0.05.](/cms/asset/cc3dfe1d-165c-4a76-a992-64d92f6cd12c/iimt_a_487193_f0004_b.gif)
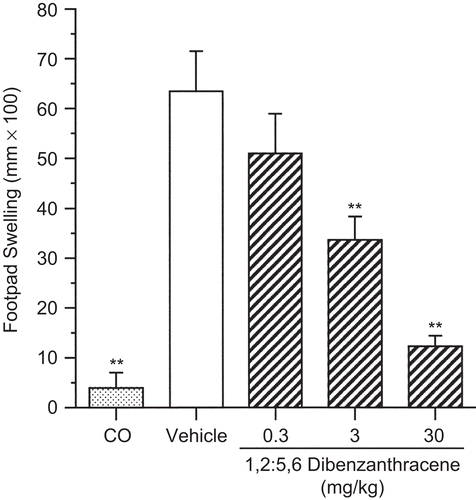
In a holistic approach to the evaluation of CMI, the effect of DBA on the DTH response was evaluated. Results after a single pa of DBA showed a dose-related decrease in the DTH response to C. albicans (). Unlike the CTL assay, where the effects were only observed at the high dose, a dose-dependent decrease was observed, with suppression reaching the level of statistical significance for the 3 and 30 mg/kg dose groups. There was a 47% decrease in the response at the 3 mg/kg dose and an 81% decrease in the response at the high dose (30 mg/kg). As expected, the CO group showed minimal footpad swelling, a result of the footpad trauma associated with the challenge injection.
Figure 5. Suppression of the delayed-type hypersensitivity response following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). Mice received either DBA (0.3, 3, or 30 mg/kg) or vehicle (VH) in a single pa, followed by sensitization to C. albicans with subsequent challenge to chitosan antigen 8 days later. One group of mice remained unsensitized but received the challenge with the remaining groups (CO = challenge only). Results shown depict footpad swelling measured 24 h following challenge. Values represent the mean (± SE) derived from eight animals; **p < 0.01.
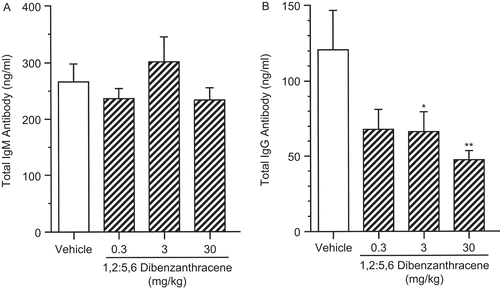
As shown in and , DBA exerted little to no effect on B-lymphocyte proliferation when stimulated by either LPS or F(ab′)2 antibody with IL-4. Total supernatant polyclonal IgM protein levels were evaluated, and no effect was observed at any dose level (). However, total IgG antibody in supernatants of LPS-stimulated cell cultures was significantly reduced (). There were reductions in IgG antibody production in all dose groups, reaching the level of statistical significance for the 3 and 30 mg/kg dose groups. A reduction of 45% was observed in the 3 mg/kg group while the 30 mg/kg group was reduced by 62%.
Figure 6. Lipopolysaccharide (LPS)-stimulated proliferation of B-lymphocytes following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). Mice received either DBA (0.3, 3, or 30 mg/kg) or vehicle (VH) in a single pa. On the day of euthanization, splenocytes were co-cultured in microtiter wells with LPS (100 μg/mL) or in wells without LPS at 37°C. The data were expressed as CPM/2 × 105 splenocytes. Values represent the mean (± SE) derived from eight animals; **p < 0.01.
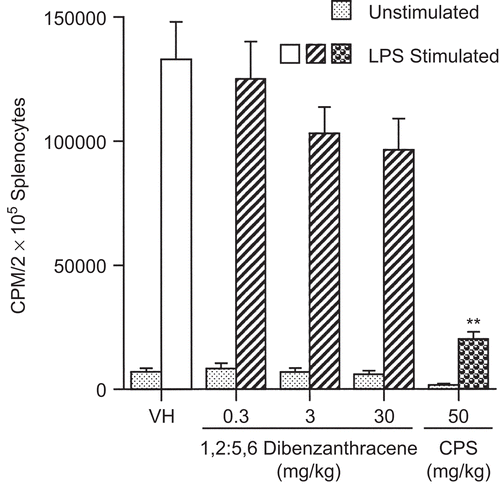
Figure 7. F(ab′)2 + interleukin 4 (IL-4)-stimulated proliferation of B-lymphocytes following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). Mice received either DBA (0.3, 3, or 30 mg/kg) or vehicle (VH) in a single pa. On the day of euthanization, splenocytes were co-cultured in microtiter wells with F(ab′)2 (5 μg/mL) and IL-4 (10 or 100 ng/ mL) or in untreated wells at 37°C. The data were expressed as CPM/2 × 105 splenocytes. Values represent the mean (± SE) derived from eight animals; **p < 0.01.
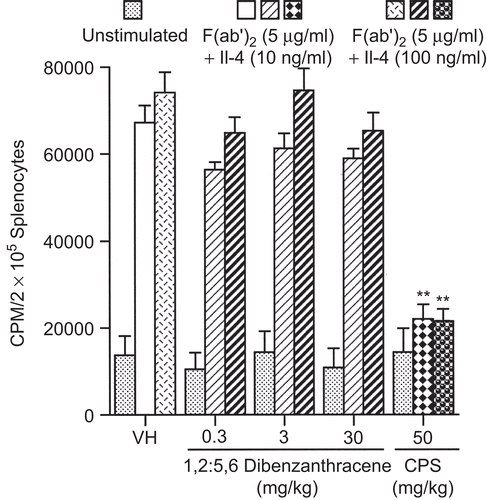
Figure 8. Lipopolysaccharide (LPS) stimulated IgM and IgG production by splenocytes following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). Mice received DBA (0.3–30.0 mg/kg) or vehicle (VH) in a single pa. On the day of euthanization, splenocytes were co-cultured in microtiter wells with 100 μg/mL LPS at 37°C for 24 h. Supernatant was evaluated for IgM and IgG protein levels. The data are expressed as (A) mean IgM (ng/mL) and (B) mean IgG (ng/mL). Values represent the mean (± SE) derived from n = 8 animals except in the 30 mg/kg in (B), where n = 7; *p < 0.05, **p < 0.01.
The cytokine profiles produced after ConA stimulation () consist of three dose groups plus VH control, while the LPS-stimulated experiments () consist only of the 30 mg/kg DBA dose group and VH control. Although 10 cytokines were evaluated for each sample following ConA stimulation (11 following LPS stimulation), emphasis was placed on TH1 and TH2 cytokines following ConA stimulation and on the proinflammatory cytokines following LPS stimulation. Cytokine levels were unaffected after stimulation with ConA, with the exception of IL-1, IL-6, IL-2, IFN-γ, and IL-5, which were each statistically significantly lower than control (decreases of 20, 87, 43, 47, and 46%, respectively) at the high dose level after 24 h (). After 48-h stimulation with ConA, only IL-6 remained significantly decreased (83%) at the high dose. TNF-α levels were not detectable after 24- or 48-h stimulation with ConA, while IL-10 levels were not detectable for any dose group after 24 h and were not detected at the high dose only after 48 h.
Table 2. Supernatant cytokine levels after 24- and 48-h stimulation of splenocytes with ConA following a single pharyngeal aspiration of 1,2:5,6-dibenzanthracene.
Table 3. Supernatant cytokine levels after 24- and 48-h stimulation of splenocytes with lipopolysaccharide (LPS) following a single pharyngeal aspiration of 1,2:5,6-dibenzanthracene.
After stimulation with LPS for 24 h, pro-inflammatory cytokine levels in DBA-exposed splenocyte cultures were not statistically significantly different from control (). However, IL-2, IL-12, IL-5, IL-10, and GM-CSF were each statistically significantly lower than control (decreases of 47, 23, 29, 72, and 66%, respectively) at the high dose. These significant decreases persisted at 48 h for IL-10 only (71%). In addition, IL-6 and IFN-γ were statistically significantly different from control (decreases of 27 and 51%, respectively) at 48 h but not at 24 h. IL-12 was not evaluated at the 48-h timepoint, while IL-4, TNF-α, and IL-17 levels were not detectable after 24- or 48-h stimulation with LPS.
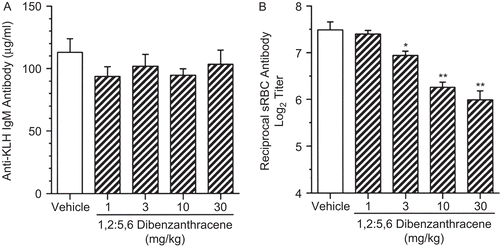
DBA exposure had no effect on the production of KLH-specific IgM (). However, DBA exposure at 3, 10, and 30 mg/kg produced statistically significant decreases of 7, 16, and 20%, respectively, in the serum antibody titers to SRBC as evaluated by ELISA ().
Figure 9. The effects of a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA) on serum antigen-specific IgM antibody levels. Mice received either DBA (1 to 30 mg/kg) or vehicle (VH) in a single pa and then were sensitized by iv tail vein injection within 2 h with either (A) 2 mg keyhole limpet hemocyanin (KLH) antigen or (B) 7.5 × 107 sheep red blood cells (SRBC). Five days later, blood was collected by cardiac puncture to analyze by enzyme-linked immunosorbent assay (ELISA). In (A), the amount of antigen-specific IgM antibody was expressed as mean IgM (μg/mL), while in (B), results are expressed as log2 (titer), where the titer is defined as the reciprocal of the dilution giving an optical density (OD) of 0.5. Values represent the mean (± SE) derived from n = 6, 7, or 8 animals per group; *p < 0.05, **p < 0.01.
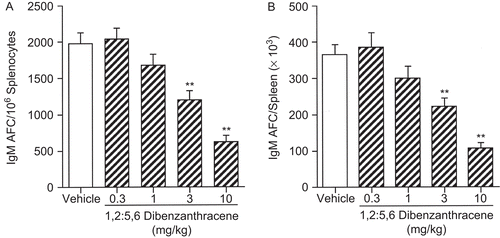
To determine a No Observable Adverse Effect Level (NOAEL) in the AFC assay, doses of 0.3, 1, 3, 10, and 30 mg/ kg DBA were evaluated in a series of experiments, each conducted on the peak day for the AFC response (Day 4) as indicated by Temple et al. (Citation1993). Repeated studies consistently showed significant suppression at 3 mg/kg DBA and intermittent statistically significant decreases at 1 mg/kg DBA, while no effects were observed at 0.3 mg/kg DBA. Therefore, the NOAEL was determined to be 0.3 mg/kg DBA. illustrates the results of two combined experiments in this series, with significant decreases in specific activity (IgM AFC/106 splenocytes; left panel) of 39 and 69% being observed at 3 and 10 mg/kg DBA, respectively.
Figure 10. IgM AFC response following a single pharyngeal aspiration (pa) of 1,2:5,6-dibenzanthracene (DBA). Mice received either DBA (0.3, 1, 3, or 10 mg/kg) or vehicle (VH) in a single pa 4 days prior to sacrifice and were sensitized i.v. with 7.5 × 107 sheep red blood cells (SRBC) via tail vein within 2 h. The results are combined from two studies, expressed as (A) IgM AFC/106 splenocytes (specific activity) and (B) IgM AFC/spleen (×103; total activity). Values represent the mean (± SE) derived from n = 6, 7, or 8 animals per group in each study; **p < 0.01.
Discussion
In three of the four proliferation assays conducted [αCD3, F(ab′)2, and LPS], lymphocyte proliferation was unaffected, suggesting that DBA alters the functional ability of lymphocytes to a greater extent than proliferative ability. The effect of DBA exposure on ConA-mediated splenocyte proliferation differed from the lack of effect in the other proliferation assays conducted. Although the αCD3 T-lymphocyte proliferation assay showed no reduction in proliferation at doses up to 30 mg/kg, T-lymphocytes stimulated with ConA showed a significant reduction in proliferation at the high dose. While the αCD3 antibody binds to the ϵ chain of the CD3 portion of the T-cell receptor (TCR) complex (Bluestone et al., Citation1987), it has been postulated that ConA binds to carbohydrate residues on the γ and σ chains of the TCR itself (Premack and Gardner, Citation1992) thus directly interacting with and aggregating TCRs to stimulate activation (Palacios, Citation1982). Therefore, there may be distinct differences between ConA and αCD3 in TCR activation, aggregation, and the consequences of these events that may account for the results obtained in these experiments. IL-2 is requisite for T-lymphocyte proliferation, and as both ConA-stimulated T-lymphocyte proliferation and IL-2 production are reduced after DBA exposure, the DBA-mediated decrease in IL-2 may well be the mechanism for suppressed T-lymphocyte proliferation after ConA stimulation.
While no effects were observed on αCD3-stimulated T-lymphocyte proliferation or the MLR response, DBA exposure produced a significant decrease in CTL activity at the 30 mg/kg dose level. The αCD3 response is purely proliferative in nature, the MLR response additionally requires recognition of allogeneic spleen cells prior to proliferation, and as a further complication, the CTL response requires recognition of foreign cells, proliferation, and formation of effector cells capable of a cytotoxic response against the foreign cell type. The DTH assay is an entirely in vivo extension of the previously discussed ex vivo cell-mediated assays, although the CTL assay primarily assesses CD8+ T-lymphocyte activity, while the DTH assay involves CD4+ T-lymphocytes. Suppression of the DTH response was observed at 3 mg/kg and demonstrated the sensitivity of the DTH response to the immunotoxic effects of DBA. Additionally, cytokine expression patterns demonstrate that when examined overall, the TH1 and proinflammatory cytokine profiles parallel the CMI functional assays results, with suppression observed at 30 mg/kg DBA.
B-Lymphocyte proliferation was unaffected when stimulated by either LPS or F(ab′)2 with IL-4, and there was also no effect on total polyclonal IgM production in splenocytes after LPS stimulation. However, dramatic immunosuppression was observed in the IgM AFC assay (see ). Interestingly, there was a significant decrease in total polyclonal IgG production in splenocytes at 3 and 30 mg/kg. IL-10 contributes to the production of IgG4 (Borish and Steinke, Citation2003), IgG3, and IgG1 (Banchereau et al., Citation1994), and total IgG (Hummelshoj et al., Citation2006) and was significantly decreased at 30 mg/kg DBA after LPS stimulation, which may explain the observed decrease in polyclonal total IgG. IL-4 and IFN-γ induce isotype switching from IgM to IgG1 and IgG2a or IgG3, respectively (Purkerson and Isakson, Citation1992) with co-stimulatory factors such as T-lymphocyte contact, IL-5, or LPS. Therefore the decrease in IL-5 and IFN-γ along with the absence of detectable IL-4 mitigates the decrease observed in total polyclonal IgG, but does not completely account for the obtained results. Similar findings were discussed by Nikasinovic et al. (Citation2004) in a review of the effects of diesel exhaust particles, which are known to contain PAHs.
While the overall effects of DBA on humoral immunity are pronounced, specifically the marked effects seen in the response to the T-dependent antigen SRBC, the ability of DBA to suppress the KLH IgM T-dependent response, evaluated by ELISA, was absent. The KLH assay was performed repeatedly to determine if DBA at doses up to 30 mg/kg would produce immunosuppression, but no effects were observed. While the SRBC ELISA was not as sensitive as the AFC assay, a dose-responsive decrease in the amount of SRBC-specific IgM was observed. White et al. (Citation2007) have shown that the decreased sensitivity of the KLH ELISA as compared with the plaque assay is not specific to DBA. This may be due to the fact that the plaque assay uses a particulate antigen (i.e., SRBC), which may mimic a pathogen better than a soluble antigen such as KLH.
Air samples in an Italian coke oven plant evaluated for PAH content were reported to contain 0.079 μg/m3 DBA (Cavallo et al., Citation2008). Assuming an average daily air intake of 28.8 m3/day and an occupational exposure of 8 h/day, 5 days/week, 50 weeks a year for 60 years, the estimated total DBA exposure for these coke oven workers would be 11.4 mg DBA, or ≈0.2 mg/kg for a 60 kg person. DBA yields from mainstream cigarette smoke are reported to range between 0.9 and 7.2 ng/cigarette, while sidestream DBA levels ranged from 4.6 to 34.2 ng/cigarette (Lodovici et al., Citation2004). Others have reported DBA levels as high as 76 ng/cigarette (Smith et al., Citation2000). Using the highest of these values (76 ng/cigarette), a 60 kg person who smokes 2 packs/day (i.e., 40 cigarettes) for 70 years would receive a maximum total DBA exposure of 78 mg, or ∼1.3 mg/kg.
The above calculations are based on a cumulative dose over a lifetime, and it is difficult to compare a lifetime exposure with a single administration. That being said, assuming a 10-fold factor for interspecies differences and a 10-fold factor for differences in sensitivity within the human population, the effect level reported herein (3 mg/kg) translates to 0.03 mg/ kg, which is an exposure level that is exceeded by an order of magnitude or more in the cumulative lifetime exposure estimates discussed earlier.
Comparisons between a single pa and 28-day sc exposure to DBA reveal interesting similarities. Hernandez et al. (Citation2005, Citation2006) demonstrated suppression in the NK and MLR assays at 5 mg/kg/day (140 mg/kg total exposure [TE]) DBA with no effect at 1.58 mg/kg/day (44.2 mg/kg TE) DBA. When total DBA exposure is considered over the dosing period, these previous results are consistent with the present studies showing no effect at doses up to 30 mg/kg DBA. Hernandez (Citation2006) also demonstrated no effect in the αCD3 assay, which is consistent with the results of these studies. In the ConA assay, a single dose administered by pa yields significant suppression at 30 mg/kg DBA, while Hernandez (Citation2006) reports no effect at 793 μg/kg/day (22.2 mg/kg TE) with suppression at 2.5 mg/kg/day (70 mg/kg TE). Finally, the absence of effects on B-lymphocyte proliferation in these studies is also consistent with results obtained by Hernandez et al. (Citation2005, Citation2006).
However, in the more sensitive holistic assays such as DTH and AFC, differences were observed between these two exposure regimens. Hernandez (Citation2006) reported no effect in footpad swelling in the DTH assay at 500 μg/kg/ day (14 mg/kg TE) with significant suppression at 1.58 mg/ kg/day (44.2 mg/kg TE), while we observed significant suppression as low as 3 mg/kg DBA. A difference between the two exposure regimens is further demonstrated by comparing the results of the AFC assays. Hernandez (Citation2006) reported a NOAEL of 158 μg/kg/day (4.4 mg/kg TE) following 28-day sc administration of DBA, while we observed intermittent immunosuppression at 1 mg/kg DBA and a NOAEL of 0.3 mg/kg DBA, an order of magnitude lower.
Although comparisons of the effects between different routes of exposure of a compound or drug are often difficult, such comparisons can prove useful; however, additional questions naturally arise. Specifically, are any observed differences due to differences in the VH utilized? Also, are there any differences in localized retention of the drug or compound at the site of exposure that may affect systemic outcomes? With regard to the first of these questions, it should be noted that the VH utilized by Hernandez et al. for sc administration of DBA was corn oil, and DBA is lipophilic. Therefore, the bioavailability of DBA following sc administration may have been less than the total dose administered, although the magnitude of this reduction is unknown and may or may not be an order of magnitude lower.
Regarding the potential for DBA retention at the sites of exposure to contribute to the differences noted earlier, Tomingas and Pott (Citation1976) have suggested that the retention rate of DBA at the site of exposure is significantly longer following sc injection than following it instillation. The implications of this report are unclear, however, because the authors utilized different animal models for the two routes of exposure (hamsters for it exposure and mice for sc exposure). Indeed, Bermudez et al. (Citation2002, Citation2004) have indicated that hamsters are much more efficient than mice at clearing inhaled particles from the lung. Specifically, they have reported that clearance half-times in mice after inhalation of titanium dioxide are similar to the mouse sc exposure half-times as reported by Tomingas and Pott (Citation1976). Therefore, it is not likely that retention at the site of administration has contributed to differences in the immunosuppressive effects of DBA between those reported herein and those reported by Hernandez et al. (Citation2005, Citation2006).
Conclusion
Among the systemic immunosuppressive effects of DBA following a single pa, the holistic assays, i.e., the DTH for CMI and the AFC for humoral immunity, were more sensitive than the ex vivo assays, with a NOAEL of 0.3 mg/kg being observed in the AFC assay. In addition, results suggest that a single pa of DBA produces greater immunosuppressive effects in the holistic assays, on a total exposure basis, than a 28-day sc exposure regimen.
Acknowledgements
Special thanks to Dr. Denise Roesh, Ronnetta Brown, Deborah Musgrove, Colleen McLoughlin, Dr. Wimolnut Auttachoat, and especially Dr. Chris Sheth. Thanks also to Dr. Jane Lewis and Dr. Debbie Koller of Altria Client Services for providing the opportunity for this project to be completed.
Declaration of interests
Dr. Kimber L. White, Jr. is the owner of a company, ImmunoTox®, Inc., that conducts immunotoxicological studies under Good Laboratory Practices (GLP); however, none of the work presented here involved his company. Dr. Donna C. Smith is currently an employee of Altria Client Services, and the studies conducted herein were completed as a part of her doctoral thesis in the Department of Pharmacology and Toxicology at Virginia Commonwealth University. This work was supported in part by the National Institute of Environmental Health Sciences (ES 05454).
References
- Banchereau, J., Briere, F., Liu, Y. J., and Rousset, F. 1994. Molecular control of B-lymphocyte growth and differentiation. Stem Cells 12:278–288.
- Bermudez, E., Mangum, J. B., Asgharian, B., Wong, B. A., Reverdy, E. E., Janszen, D. B., Hext, P. M., Warheit, D. B., and Everitt, J. I. 2002. Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles. Toxicol. Sci. 70:86–97.
- Bermudez, E., Mangum, J. B., Wong, B. A., Asgharian, B., Hext, P. M., Warheit, D. B., and Everitt, J. I. 2004. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol. Sci. 77:347–357.
- Bluestone, J. A., Pardoll, D., Sharrow, S. O., and Fowlkes, B. J.1987. Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature 326:82–84.
- Borish, L. C., and Steinke, J. W. 2003. Cytokines and chemokines. J. Allergy Clin. Immunol. 111:S460–S475.
- Bradley, S. G., Munson, A. E., McCay, J. A., Brown, R. D., Musgrove, D. L., Wilson, S., Stern, M., Luster, M. I., and White, K. L., Jr. 1994. Subchronic 10-day immunotoxicity of polydimethylsiloxane (silicone) fluid, gel, and elastomer and polyurethane disks in female B6C3F1 mice. Drug Chem. Toxicol. 17:175–220.
- Cavallo, D., Ursini, C. L., Pira, E., Romano, C., Maiello, R., Petyx, M., and Iavicoli, S. 2008. Evaluation of DNA damage induction on human pulmonary cells exposed to PAHs from organic extract of PM10 collected in a coke-oven plant. Acta Biomed. 79(S1):97–103.
- Chuang, C. Y., and Chang, C. C. 2007. Urinary 1-hydroxypyrene level relative to vehicle exhaust exposure mediated by metabolic enzyme polymorphisms. J. Occup. Health 49:140–151.
- Davila, D. R., Romero, D. L., and Burchiel, S. W. 1996. Human T-cells are highly sensitive to suppression of mitogenesis by polycyclic aromatic hydrocarbons and this effect is differentially reversed by α-naphthoflavone. Toxicol. Appl. Pharmacol. 139:333–341.
- Driscoll, K. E., Costa, D. L., Henderson, R., Oberdorster, G., Salem, H., and Schlesinger, R. B. 2000. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 55:24–35.
- Galvan, N., Page, T. J., Czuprynski, C. J., and Jeffcoate, C. R. 2006. Benzo(a)pyrene and 7,12-dimethylbenz(a)anthrecene differentially affect bone marrow cells of the lymphoid and myeloid lineages. Toxicol. Appl. Pharmacol. 213:105–116.
- Guo, T. L., McCay, J. A., Brown, R. D., Musgrove, D. L., Butterworth, L., Munson, A. E., Germolec, D. R., and White, K. L., Jr. 2000. Glycidol modulation of the immune responses in female B6C3F1 mice. Drug Chem. Toxicol. 23:433–457.
- Guo, T. L., McCay, J. A., Zhang, L. X., Brown, R. D., You, L., Karrow, N. A., Germolec, D. R., and White, K. L., Jr. 2001. Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J. Nutr. 131:3251–3258.
- Hernandez, D. M. 2006. Immunotoxicological Evaluation of Critical Windows of Development Following Exposure to 1,2:5,6-dibenzanthracene in B6C3F1 Mice. PhD Dissertation, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA.
- Hernandez, D. M., Auttachoat, W., Guo, T. L., and White, K. L. ,Jr. 2005. Evaluation of the immunomodulatory effects of dibenz(a,h)anthracene in adult female B6C3F1 mice. Toxicologist 84:182.
- Hummelshoj, L., Ryder, L. P., and Poulsen, L. K. 2006. The role of the interleukin-10 subfamily members in immunoglobulin production by human B-cells. Scand. J. Immunol. 64:40–47.
- Jerne, N. K., Nordin, A. A., and Henry, C. 1963. The agar plaque technique for recognizing antibody-producing cells. In: Cell-Bound Antibodies (Amos, B., and Koprowski, H., Eds.), Philadelphia: Wistar Institute Press.
- Kawabata, T. T., Babcock, L. S., Gauggel, D. L., Asquith, T. N., Fletcher, E. R., Horn, P. A., Ratajczak, H. V., and Graziano, F. M. 1995. Optimization and validation of an ELISA to measure specific guinea pig IgG1 antibody as an alternative to the in vivo passive cutaneous anaphylaxis assay. Fundam. Appl. Toxicol. 24:238–246.
- Llewellyn, G. C., and White, K. L., Jr. 1998. Benzo(a)pyrene induced suppression of the memory humoral immune response in female B6C3F1 mice. Toxicologist 42:207–208.
- Lodovici, M., Akpan, V., Evangelisti, C., and Dolara, P. 2004. Sidestream tobacco smoke as the main predictor of exposure to polycyclic aromatic hydrocarbons. J. Appl. Toxicol. 24:277–281.
- Nesnow, S., Ross, J. A., Stoner, G. D., and Mass, M. J. 1995. Mechanistic linkage between DNA adducts, mutations in oncogenes and tumorigenesis of carcinogenic environmental polycyclic aromatic hydrocarbons in strain A/J mice. Toxicology 105:403–413.
- Nghiem, D. X., Walterscheid, J. P., Kazimi, N., and Ullrich, S. E. 2002. Ultraviolet radiation-induced immunosuppression of delayed-type hypersensitivity in mice. Methods 28:25–33.
- Nikasinovic, L., Momas, I., and Just, J. 2004. A review of experimental studies on diesel exhaust particles and nasal epithelium alterations. J. Toxicol. Environ. Health Crit. Rev. 7:81–104.
- Okona-Mensah, K. B., Battershill, J., Boobis, A., and Fielder, R. 2005. An approach to investigating the importance of high potency polycyclic aromatic hydrocarbons (PAHs) in the induction of lung cancer by air pollution. Food Chem. Toxicol. 43:1103–1116.
- Palacios, R. 1982. Concanavalin A triggers T-lymphocytes by directly interacting with their receptors for activation. J. Immunol. 128:337–342.
- Parrot, M. C., Kawabata, T. T., and White, K. L., Jr. 1989. Suppression of humoral immunity following dermal exposure to benzo(a)pyrene in B6C3F1 mice. Toxicologist 9:201.
- Premack, B. A., and Gardner, P. 1992. Signal transduction by T-cell receptors: Mobilization of Ca and regulation of Ca-dependent effector molecules. Am. J. Physiol. 263:C1119–C1140.
- Pufulete, M., Battershill, J., Boobis, A., and Fielder, R. 2004. Approaches to carcinogenic risk assessment for polycyclic aromatic hydrocarbons: A UK perspective. Regul. Toxicol. Pharmacol. 40:54–66.
- Purkerson, J., and Isakson, P. 1992. A two-signal model for regulation of immunoglobulin isotype switching. FASEB J. 6:3245–3252.
- Rao, G. V., Tinkle, S., Weissman, D. N., Antonini, J. M., Kashon, M. L., Salmen, R., Battelli, L. A., Willard, P. A., Hoover, M. D., and Hubbs, A. F. 2003. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J. Toxicol. Environ. Health 66:1441–1452.
- Reynolds, C. W., and Herberman, R. B. 1981. In vitro augmentation of rat natural killer (NK) cell activity. J. Immunol. 126:1581–1585.
- Smith, C. J., Perfetti, T. A., Rumple, M. A., Rodgman, A., and Doolittle, D. J. 2000. “IARC Group 2A Carcinogens”; reported in cigarette mainstream smoke. Food Chem. Toxicol. 38:371–383.
- Talaska, G., Roh, J., and Zhou, Q. 1996. Molecular biomarkers of occupational lung cancer. Yonsei Med. J. 37:1–18.
- Temple, L., Kawabata, T. T., Munson, A. E., and White, K. L., Jr. 1993. Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 21:412–419.
- Tomingas, R., and Pott, F. 1976. An animal experimental study on the retention rate of polycyclic aromatic hydrocarbons in the lung and in the subcutaneous tissue. Zentralbl. Bakteriol. Orig. B 162:18–26.
- Ullrich, S. E. 1999. Dermal application of JP-8 jet fuel induces immune suppression. Toxicol. Sci. 52:61–67.
- Ullrich, S. E., and Lyons, H. J. 2000. Mechanisms involved in the immunotoxicity induced by dermal application of JP-8 jet fuel. Toxicol. Sci. 58:290–298.
- Uno, S., Dalton, T. P., Derkenne, S., Curran, C. P., Miller, M. L., Shertzer, H. G., and Nebert, D. W. 2004. Oral exposure to benzo(a)pyrene in the mouse: Detoxification by inducible cytochrome P450 is more important than metabolic activation. Mol. Pharmacol. 65:1225–1237.
- Uno, S., Dalton, T. P., Dragin, N., Curran, C. P., Derkenne, S., Miller, M. L., Shertzer, H. G., Gonzalez, F. J., and Nebert, D. W. 2006. Oral benzo(a)pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxification, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol. Pharmacol. 69:1103–1114.
- Uno, S., Dragin, N., Miller, M. L., Dalton, T. P., Gonzalez, F. J., and Nebert, D. W. 2008. Basal and inducible CYP1 mRNA quantitation and protein localization throughout the mouse gastrointestinal tract. Free Rad. Biol. Med. 44:570–583.
- Weston, B. J.1967. Effect of route of administration of immunosuppression by DMBA in CBA mice. Nature 215:1497–1498.
- White, K. L., Lysy, H. H., and Holsapple, M. P. 1985. Immunosuppression by polycyclic aromatic hydrocarbons: A structure–activity relationship in B6C3F1 and DBA/2 mice. Immunopharmocology 9:155–164.
- White, K. L., Musgrove, D. L., and Brown, R. D. 2010. The sheep erythrocyte T-dependent antibody response (TDAR). Methods Mol. Biol. 598:173–184.
- White, K. L., Jr., Sheth, C. M., and Peachee, V. L. 2007. Comparison of primary immune responses to SRBC and KLH in rodents. J. Immunotoxicol. 4:153–158.
- Wilson, S. D., McCay, J. A., Butterworth, L. F., Munson, A. E., and White, K. L., Jr. 2001. Correlation of suppressed natural killer cell activity with altered host resistance models in B6C3F1 mice. Toxicol. Appl. Pharmacol. 177:208–218.
- Wojdani, A., and Alfred, L. J. 1984. Alterations in cell-mediated immune functions induced in mouse splenic lymphocytes by polycyclic aromatic hydrocarbons. Cancer Res. 44:942–945.
- Wojdani, A., Attarzadeh, M., Wolde-Tsadik, G., and Alfred, L. J. 1984. Immunocytotoxicity effects of polycyclic aromatic hydrocarbons on mouse lymphocytes. Toxicology 31:181–189.
- Woolhiser, M. R., Munson, A. E., and Meade, B. J. 2000. Immunological responses of mice following administration of natural rubber latex proteins by different routes of exposure. Toxicol. Sci. 55:343–351.
