Abstract
In the first part of a series of studies to account for perfluorooctane sulfonate (PFOS)-induced sheep red blood cell (SRBC)-specific immunoglobulin M (IgM) antibody suppression in mice, a survey of clinical and immunotoxicological endpoints was examined. Adult female B6C3F1 mice were exposed orally for 28 days to a total administered dose (TAD) of 0, 0.1, 0.5, 1, or 5 mg PFOS/kg. Uterus wet weight was significantly decreased compared with control at the 5 mg/kg dose. No indications of wasting syndrome, malnutrition, alteration of thyroid homeostasis, or signs of overt toxicity were observed. Numbers of splenic CD19+/CD21−, CD19+/CD21+, B220+/CD40+, CD4+/CD154−, CD4+/CD154+, and MHC-II+ cells were not altered. Additionally, ex vivo interleukin-4 (IL-4), IL-5, and IL-6 production by in vitro anti-CD3- or phorbol myristate acetate-stimulated CD4+ T-cells was not affected. Ex vivo IL-6 production by B-cells was significantly increased by in vitro stimulation with either anti-CD40 or lipopolysaccharide. Increased IL-6 production by B-cells was the most sensitive endpoint assessed resulting in alterations at the lowest dose tested (0.1 mg/kg TAD) following anti-CD40 stimulation. Further studies are required to characterize effects on inflammatory markers such as IL-6 at environmentally relevant concentrations of PFOS and to determine the key events associated with PFOS-induced IgM suppression to address potential human health risks.
Introduction
Perfluoroalkyl acids (PFAAs) are manmade fluorinated chemicals that have been widely used in industrial and commercial applications for the past 50 years (Lehmler, Citation2005). Properties of PFAAs include chemical and thermal stability, oleophobicity and hydrophobicity, and resistance to acidic and alkaline conditions (Kissa, Citation2001). Although these properties contribute to their usage in a wide variety of consumer and industrial items (Moody and Field, Citation2000; Kissa, Citation2001; OECD, Citation2002; Lau et al., Citation2007), the stability of these fluorinated compounds also make them extremely resistant to degradation. Consequently, PFAAs are persistent and ubiquitous chemicals in the environment (water, sediment, air, dust) and widespread exposure in wildlife and humans has been documented (Giesy and Kannan Citation2001; Lau et al., Citation2007; Olsen et al., Citation2007). This is an increasing public concern as perfluorooctane sulfonate (PFOS) has a half-life of 5.4 years in humans concentrating primarily in the blood and liver (Johnson et al., Citation1984; Seacat et al., Citation2003; Olsen et al., Citation2007). The United States Environmental Protection Agency and the Centers for Disease Control have, therefore, included PFOS and other perfluorinated compounds in national biomonitoring programs to track the presence of these chemicals in humans and record associated adverse events (Calafat et al., Citation2007a,Citationb).
Health effects due to low-level PFOS exposure and corresponding mechanisms of toxicity are largely not understood. Much of what is known is attributed to animal studies using PFOS concentrations much higher than those reported in wildlife and humans. At these levels, PFOS interferes with liver physiology by causing hepatomegaly in the rat, mouse and cynomolgus monkey with induction of lipidemia and peroxisomal fatty acid β-oxidation, decreases in serum cholesterol, increases in serum alanine aminotransferase (ALT), and wasting syndrome (Luebker et al., Citation2002; OECD, Citation2002; Seacat et al., Citation2002, Citation2003; Thibodeaux et al., Citation2003). Several studies have also demonstrated that gestational exposure to PFOS causes developmental immunological, endocrine (thyroid hormones), and reproductive effects including increased incidence of prenatal mortality, low birth weights, structural defects, developmental delays, and long-term suppressed humoral immune responses (Case et al., Citation2001; Luebker et al., Citation2002; Grasty et al., Citation2003; Lau et al., Citation2003; Thibodeaux et al., Citation2003; Fuentes et al., Citation2006; Keil et al., Citation2008).
Emerging data suggest that the immune system may be a target for PFAAs in both adult and developmental rodent immunotoxicology studies (Nelson et al., Citation1992; Yang et al., Citation2000, Citation2001, Citation2002a,Citationb; Peden-Adams et al., Citation2007, Citation2008; DeWitt et al., Citation2008, Citation2009a; Keil et al., Citation2008; Lefebvre et al., Citation2008; Loveless et al., Citation2008; Guruge et al., Citation2009). Moreover, a field study found significant associations between infectious diseases and elevated exposures to PFOS in sea otters from coastal California suggesting possible immune effects of PFOS and related perfluorochemicals in this species (Kannan et al., Citation2006), while a recent report indicates a 21-day exposure to 25 μg/kg/day PFOS (0.525 mg/kg total) increases susceptibility to the influenza A virus in female B6C3F1 mice (Guruge et al., Citation2009). Decreased antibody production in rodent models seems to be one of the most sensitive immunological targets for PFAAs (Nelson et al., Citation1992; Yang et al., Citation2002b; Peden-Adams et al., Citation2007, Citation2008; DeWitt et al., Citation2008; Keil et al., Citation2008; Dong et al., Citation2009; Zheng et al., Citation2009). For example, Peden-Adams et al. (Citation2008) reported a deficit in sheep red blood cell (SRBC)-specific immunoglobulin M (IgM) production following PFOS exposure in adult male and female mice (beginning at 0.05 and 0.5 mg/kg total dose for duration of 28 days, respectively). Therefore, to date, the reported lowest observed adverse effect level (LOAEL) for PFOS on immune function in adult animals is 0.05 and 0.5 mg/kg total exposure over 28 days in males and females, respectively (i.e., 1.66 and 16.6 μg/kg/day in males and females, respectively). PFOS serum concentrations at these dose levels following 28 days of exposure were 91.5 ± 22.2 ng/g and 666 ± 108 ng/g (mean ± SD), respectively (Peden-Adams et al., Citation2008). This LOAEL is supported for the female B6C3F1 mouse model by Guruge et al. (Citation2009). Statistically, significant increases in susceptibility to influenza A-induced mortality were noted at 0.525 mg/kg total dose (plasma level = 670 ± 47 ng/g; Guruge et al., Citation2009). These LOAEL blood levels are lower than reported mean blood levels from occupationally exposed humans and fall in the upper range of concentrations reported for the general population (Peden-Adams et al., Citation2008; Guruge et al., Citation2009).
Oral reference doses based on the no-observed adverse effect level (NOAEL) for male and female B6C3F1 mice reported by Peden-Adams et al. (Citation2008), and 1–3 uncertainty factors (10, 10 × 10, or 10 × 10 × 10) for interspecies extrapolation, intraspecies differences, and/or protection of children’s health (Faustman and Omenn, Citation2001) range from 0.3 to 0.0002 μg/kg/day. Margin of exposure (MOE) calculations for PFOS, based on the PFOS serum concentration at these same NOAELs, reveal MOE values for both adult males and females below 10 (). MOE values for females based on plasma PFOS concentration (189 ng PFOS/mL plasma) at the NOAEL from Guruge et al. (Citation2009) range from 5.2 to 16.6 μg/kg/day. MOE values <100 indicate the need for further testing and suggest possible risk (Faustman and Omenn, Citation2001). Risk estimates, however, can be improved with mode of action and mechanism of action information that allow adjustment of uncertainty factors (Faustman and Omenn, Citation2001; Andersen et al., Citation2008). Therefore, to better determine risks associated with PFOS exposure, mode of action and mechanism of action studies are required.
Table 1. Perfluorooctane sulfonate (PFOS) margin of exposure (MOE) values based on the no-observed adverse effect levels (NOAEL) for adult male and female mice reported in Peden-Adams et al. (Citation2008).
The current study represents a survey of the effects of PFOS on clinical health indicators, histopathology, thyroid hormones, and key immune parameters important to SRBC-specific IgM production to provide a better understanding of general effects related to PFOS exposure at environmentally relevant concentrations (Olsen et al., Citation2003a,Citationb, Citation2005; Houde et al., Citation2005; Keller et al., Citation2005; Peden-Adams et al., Citation2008). To demonstrate that general systemic toxicity or decreased cell numbers available for immunological responses was not a confounding factor in the immunological evaluations presented here or in previous reports, numbers of B-cells (CD19/CD21) and MHC-II+ cells were evaluated over a broader range of PFOS exposures. Clinical markers of health (i.e., serum clinical chemistry and hematology) and histopathology were assessed over the complete range of exposures to verify that no overt toxicity had occurred at these environmentally-relevant PFOS exposure concentrations. Additionally, exposure was verified by determination of PFOS serum and liver concentrations.
The current study also represents the first part of a series of experiments to determine key events and processes, associated with PFOS-induced SRBC-specific IgM suppression. Research findings indicate that B-cell CD40 control of interleukin-6 (IL-6) production is critical to IgM secretion and is mediated, in part, by c-Jun and nuclear factor-κB (NF-κB) (Delerive et al., Citation1999; Baccam et al., Citation2003; Bishop and Hostager, Citation2003). c-Jun and NF-κB each are modulated by peroxisome proliferator-activated receptor-α (PPARα) agonists like PFOS (Andersen et al., Citation2008; Cunard et al., Citation2002a; Dewitt et al., Citation2009b). Therefore, it was hypothesized that PFOS may cause suppression of B-cell IL-6 production following CD40–CD154 (CD40–CD40L) interactions.
A single exposure concentration of PFOS above and below the LOAEL was assessed in the testing of this hypothesis. Examination of B-cell CD40 and T-cell CD154 expression along with secretion of cytokines (IL-4, IL-5, and IL-6) from T-helper cells was also included as these parameters are associated with SRBC-specific IgM production. Because lymphocyte cytokine production following in vivo activation 5 days prior to assessment (when SRBC are injected in this model) may drastically differ from responses of resting cells to first time activation (Cook, J., Personal Communication), a critical first step in this process was evaluation of these immune parameters in resting lymphocytes (i.e., isolated lymphocytes that were exposed to PFOS in vivo but not activated with antigen prior to ex vivo activation and assessment). This was conducted to ascertain effects of PFOS exposure on the selected parameters without the confounding consequences of pre-immunization. Second, this approach provides a baseline of effects in non-challenged animals (i.e., not pre-immunized) that can be compared with future studies in animals that are challenged with antigen, thereby allowing for more accurate conclusions describing the effects of PFOS on primary antibody responses.
Without understanding the effects on baseline expression and functionality, an interpretation of mode of action (i.e., the description of key events and processes, starting with interaction of an agent with the cell, through functional and anatomical changes that result in modulated health endpoints) following activation studies only (i.e., those where animals are challenged in vivo with antigen) would be difficult. Although comparable studies with and without antigen (i.e., SRBC) challenge are required to fully assess the mode of action of PFOS-induced SRBC-specific IgM production, the current study presents the first part of these studies focusing on resting cells (i.e., not challenged with antigen in vivo).
Materials and methods
Chemicals, antibodies, and supplies
Unless otherwise stated, all chemicals and reagents were purchased from Sigma (St. Louis, MO). Perfluorooctane sulfonic acid potassium salt (stated purity >98%) used for animal treatments was obtained from Fluka (via Sigma, CAS No. 2795-39-3). PerCP hamster anti-mouse CD3e, R-phycoerythrin (R-PE)-conjugated rat anti-mouse CD45R/B220 monoclonal antibody (Mab), R-PE-conjugated hamster anti-mouse CD154 (CD40 Ligand, gp39) Mab, fluorescein isothiocyanate (FITC)-conjugated mouse anti-mouse I-AP Mab, R-PE-conjugated rat anti-mouse CD21/CD35 (CR2/CR1, CD21a/CD21b) Mab, and FITC-conjugated rat anti-mouse CD40 Mab were obtained from BD Biosciences (San Jose, CA). Rat anti-mouse CD4 and rat anti-mouse CD19 were purchased from Caltag Laboratories (Burlingame, CA).
Mouse IL-4, IL-5, and IL-6 enzyme-linked immunosorbent assay (ELISA) sets, assay diluent, coating buffer, wash concentrate, stop solution, and substrate reagents A and B were obtained from BD Biosciences. Phosphate-buffered saline (with or without Ca2+ and Mg+), RPMI-1640 medium (with l-glutamine and sodium bicarbonate), and N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) were purchased from Cellgro (Mediatech, Herndon, VA). Nonessential amino acids (10 mM 100×), sodium pyruvate (100 mM), and antibiotic/antimycotic (100×) were obtained from Invitrogen (Gibco brand; Carlsbad, CA). Isoflurane (AErrane) was obtained from Baxter Pharmaceutical Products Inc. (Deerfield, IL). Coat-A-Count Total T3 and Total T4 kits were purchased from Siemens Medical Solutions Diagnostics (Deerfield, IL; formerly Diagnostic Products Corporation, Los Angeles, CA). Cell sorting kits were obtained from Miltenyi Biotec (Auburn, CA) including columns, magnetic beads, and magnets. Ethylenediaminetetraacetic acid (EDTA) microtainers were purchased from Becton Dickinson (Franklin Lakes, NJ). Fetal bovine serum was purchased from Gemini Bio-Products (West Sacramento, CA). Flat bottom 96-well plates, other disposables, and Thermo Betasil® C18 columns were obtained from Fisher Scientific (Atlanta, GA). Potassium salt of PFOS (>95%) for analytical determination of serum and liver concentrations was provided by the 3M Company (St. Paul, MN) and 13C4-PFOS was from Wellington Laboratories (>98%, Guelph, ONT, Canada). All solvents were of high-performance liquid chromatography (HPLC) grade and all reagents were of ACS grade (J. T. Baker, Phillipsburg, NJ).
Animal care
Mice were housed in plastic shoebox cages on corncob bedding with micro-isolator lids in a HEPA filtered ventilated rack system and administered food (TekLab Sterilizable Rodent Diet, formula no. 8656; Harlan-Teklab, Madison, WI) and water ad libitum. Five 7–8-week-old female B6C3F1 mice (Charles River Laboratories, Wilmington, MA) were acclimated to the conditions of the treatment room (12 h light/dark cycle, 22 ± 2°C, 60–65% relative humidity) for 1 week before dosing began. Bedding, food, and water were changed twice a week and mice were observed daily. Although male B6C3F1 mice have been shown to be more sensitive to PFOS exposure than females (Peden-Adams et al., Citation2008), the female was chosen for these experiments because it is the standard model suggested for use by the National Toxicology Program’s immunotoxicity testing guidelines (Luster et al., Citation1988, Citation1992, Citation1993).
Animal dosing
PFOS was administered via oral gavage in Milli-Q water containing 0.5% Tween 20 (Lau et al., Citation2003). Control mice received Milli-Q water containing 0.5% Tween 20 only. Mice were dosed daily for 28 days (0, 3.31, 16.6, 33.1, or 166 μg/kg/day) to yield a total administered dose (TAD) of 0, 0.1, 0.5, 1, or 5 mg/kg. The daily doses above reflect only the concentration of the PFOS ion separate from the potassium salt. When comparing doses or concentrations reported in other studies, it is often unknown whether the potassium mass was removed. When it is not removed, the PFOS concentration is overestimated by 7.3%. When rounded to a single significant digit, the TAD is identical for PFOS potassium salt or the PFOS ion; thus, these doses are used throughout the paper for simplicity (Peden-Adams et al., Citation2008). All procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and conducted in an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility.
Endpoint assessment
Measured endpoints, as described below, were assessed at various concentrations. Body and organ mass, numbers of B-cells (CD19/CD20), and MHC-II+ cells, along with clinical markers of health (i.e., serum clinical chemistry and hematology), histopathology, and PFOS serum and liver concentrations were evaluated over the complete range of PFOS exposures. These endpoints were measured at all concentrations: (1) to verify no systemic toxicity had occurred over the dose range assessed by Peden-Adams et al. (Citation2008) and (2) to provide comparable data for comparison with marine mammal studies being conducted by this lab (Peden-Adams et al., Citation2009a). Due to limited availability of serum and tissues, clinical chemistry, hematology, thyroid hormones, and PFOS concentrations were assessed in only one of each of three experimental trials. Splenic histology was assessed in one of the three trials and was performed from a small section that was removed from the whole spleen after spleen mass was determined, leaving the remaining spleen available for immune assessments. Therefore, for immunophenotype data only two trials were assessed as absolute numbers of cells, while all three were assessed for percentage of cell types from 10,000 events.
Due to limitations associated with cell sorting (i.e., numbers of samples that could be sorted at one time and time associated with sorting), cytokine production (B- and T-cell) was assessed only at 0, 0.1, and 1 mg/kg TAD. These PFOS concentrations reflect the doses directly below and above the LOAEL (0.5 mg/kg TAD) for immune alterations reported in this model (adult female B6C3F1 mice) by Peden-Adams et al. (Citation2008). For consistency, cell surface marker analysis for B220/CD40 and CD4/CD154 (endpoints directly associated with the hypothesis and cytokine parameters) was also determined only at 0, 0.1, and 1 mg/kg TAD.
Body and organ mass and spleen cellularity
Body mass change over the 28-day period was calculated as initial mass subtracted from final mass. Twenty-four hours after the final exposure, mice were euthanized with CO2 and organs collected. All balances were calibrated prior to use. Organ mass was normalized for body weight and reported as the somatic index [(organ weight/body weight) × 100]. Organs for histopathology assessment were placed in tissue cassettes and stored in formalin until analysis. Spleens were aseptically processed into single cell suspensions using sterile frosted microscope slides in a sterile hood. Viable cell counts for sample dilutions and total cellularity determinations were obtained via trypan blue exclusion using a hemocytometer following red blood cell (RBC) lysis. Due to the removal of part of the spleen for histology in one of the three trials, only two trials were able to determine total spleen cellularity.
Cell surface marker analysis
For cell surface marker analysis (e.g. CD19/CD21, B220/CD40, CD4/CD154, and MHC-II), a volume of the cell sample was removed to yield 1 × 107 cells/mL in 1 mL of complete RPMI-1640 (RPMI-1640, 10% fetal bovine serum, 1% antibiotic/antimycotic). This was performed prior to cell isolation (see below) for cytokine production. Surface marker determinations were conducted by flow cytometry, as previously described (Peden-Adams et al., Citation2007), following manufacturers’ directions. Non-stained cells and isotypic antibody controls were used to establish gates. Data are represented as absolute number of cells and were determined by multiplying the percentage of gated cells by the total number of viable cells obtained via trypan blue counts.
B- and T-helper cell isolation and stimulation for assessment of cytokine production
To determine the effects of PFOS exposure on B- and T-helper cell cytokine production in relation to IgM suppression, B-cells and CD4+ T-cells were separated from spleen cell solutions by magnetic beads (according to manufacturers’ directions). Cells were then released from the beads by removal of the magnets and diluted to 2 × 106 cell/mL in supplemented RPMI-1640 (RPMI-1640, 10% fetal bovine serum, 1% nonessential amino acids, 1% sodium pyruvate, 10 mM HEPES, 1% antibiotic/antimycotic, and 10 µM 2-mercaptoethanol, pH 7.4). Cells were stimulated, as a modification of Li et al. (Citation1996), with either lipopolysaccharide (LPS; B-cells) or phorbol myristate acetate (PMA; T-cells), respectively. To determine if specific cell-signaling cascades might be altered, cells were additionally stimulated separately with either anti-CD40 (B-cells) or anti-CD3 (T-cells) using a modification of Szocinski et al. (Citation2002) and Ulrich (Citation1999).
Flat bottom 96-well ELISA plates were disinfected with 75% isopropyl alcohol and left to air-dry in a laminar flow hood overnight. They were then coated in triplicate with 100 μL of 10 μg/mL anti-CD3 solution for T-cells, 10 μg/mL anti-CD40 solution for B-cells, or 100 μL supplemented RPMI-1640 (e.g. control wells), covered with sterile lids, and incubated overnight at 4–6°C the day before samples were collected. Plates were then washed twice with 200 μL supplemented RPMI-1640 inside a laminar flow hood prior to addition of sample. Supplemented RPMI-1640 (100 μL) and cell solutions (100 μL) for each experimental sample were then added to appropriate wells in coated plates. B- and T-cell samples were also incubated with LPS (10 μg/mL) or PMA (1.25 μg/mL), respectively, on separate sterile 96-well cell culture plates in triplicate. All plates were covered with sterile lids and incubated at 37°C for 72 h as a combined modification of several studies (Baccam and Bishop, Citation1999; Klaus et al., Citation1999; Ulrich, 1999; Noe et al., Citation2000; Liu et al., Citation2001; Szocinski et al., Citation2002). Supernatant was collected following centrifugation of the plates (390 × g, 4 min) and stored in Eppendorf tubes at −80°C until sample analysis.
Cytokine ELISAs
Ex vivo cytokine production (IL-4, IL-5, and IL-6) was assessed in cell culture supernatant following stimulation as described above. Sandwich ELISA kits were utilized and all procedures were performed according to manufacturer’s instructions. One-hundred microliters of culture media from the stimulated cells were plated per sample without dilution. Plates were read on a spectrophotometer at 450 nm. Absorbance units for samples were related to a standard curve and results are reported as pg/mL.
Serum chemistry and hematology
Following isofluorane anesthetization 24 h after the final exposure, whole blood was collected via the retro-orbital sinus into an EDTA microtainer or a non-heparinized Eppendorf tube for hematology and serum chemistry, respectively. Immediately following blood collection, mice were euthanized with CO2. Blood collected without anticoagulant was permitted to clot for 1 h. After clot formation, the blood sample was centrifuged for 10 min using a microcentrifuge at 1350 × g, and serum was transferred into an Eppendorf tube. Samples were kept cool until processed and shipped on frozen gel packs (wrapped and insulated to prevent freezing but to allow for sample to remain cool during shipping) to the Cornell University Veterinary Diagnostic Laboratory (Ithaca, NY) for analysis.
Complete blood cell counts (CBC) (white blood cells, RBCs, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, RBC distribution width, mean platelet volume, and platelets) were determined using an automated analyzer (Bayer ADVIA 120; Bayer Diagnostics, Tarrytown, NY). Differential leukocyte counts (neutrophils, lymphocytes, eosinophils, and monocytes), as part of the CBC, were performed by microscopic examination of modified Wright-Giemsa stained blood smears (Bayer Healthcare, Tarrytown, NY). Serum chemistry analytes (glucose, uric acid, total protein, calcium, phosphate, ALT, aspartate aminotransferase, amylase, total bilirubin, cholesterol, triglycerides, and creatinine phosphokinase) were measured with an automated analyzer (Roche Hitachi 917, Indianapolis, IN). Sodium, potassium, chloride, bicarbonate, iron, anion gap, creatinine, albumin, globulin, albumin/globulin, magnesium, γ-glutamyltransferase, and total iron-binding capacity are not reported due to low serum volumes resulting in small sample sizes (i.e., 1–3) for some treatment groups.
Thyroid hormones
Following anesthetization with isoflurane 24 h after the final exposure, blood was collected from the retro-orbital sinus into non-heparinized Eppendorf tubes for analysis of total triiodothyronine (T3) and total thyroxine (T4). Immediately following blood collection, mice were euthanized with CO2. Blood was permitted to clot for 1 h. After clot formation, the blood sample was centrifuged for 10 min using a microcentrifuge (1350 × g) and serum was transferred into an Eppendorf tube. Samples were kept cool until processed and shipped (as described above) to the Cornell University Veterinary Diagnostic Laboratory. For total T3 and total T4 measurements, the Coat-A-Count Total T3 and Total T4 procedures were used. Validation of thyroid radioimmunoassay was conducted according to procedures described in Reimers et al. (Citation1981). All results for samples analyzed were within assay curve quality control ranges and met or exceeded established assay criteria for precision and percent coefficient of variation.
Histopathology
Spleen, thymus, liver, adrenal, lung, brain, uterus, and kidney were placed in 10% neutral buffered formalin, routinely processed, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin for examination by light microscopy. A veterinary pathologist assessed the slides and treatment groups were blinded during the process.
Serum and liver PFOS analysis
Serum and liver samples were collected in polypropylene tubes for PFOS determination and frozen at −80°C. Samples were shipped on dry ice to the Wadsworth Center and analysis of PFOS levels by HPLC-MS/MS was conducted following the method described in Kannan et al. (Citation2001). Plasma (1 mL) samples were extracted with 2 mL of 0.25 M sodium carbonate buffer and 1 mL of 0.5 M tetrabutylammonium hydrogen sulfate solution (adjusted to pH 10) after spiking 5 ng of internal standard (13C4-PFOS). For the extraction of liver samples, a small amount of liver tissue (about 1 g) was homogenized with 5 mL of Milli-Q water. One milliliter of the homogenate was then transferred into a tube and extracted as described above. Analytes were detected and quantified using an Agilent 1100 series HPLC coupled with an Applied Biosystems API 2000 electrospray triple-quadrupole mass spectrometer (ESI-MS/MS; Applied Biosystems, Foster City, CA). Ten microliters of the extract were injected onto a 100 × 2.1 mm (5 µm) Thermo Betasil® C18 column. The mobile phase was 2 mM ammonium acetate/methanol starting at 10% methanol, at a flow rate of 300 µL/min. The gradient increased to 100% methanol at 10 min and was held for 2 min, and then reversed back to 10% methanol. The MS/MS was operated in electrospray negative ion mode. Target compounds were monitored by multiple reaction monitoring (MRM). The MRM transitions were 499→99 and 503→99 for PFOS and 13C4-PFOS, respectively. A midpoint calibration standard was injected after every 10 samples to check for the instrumental response and drift. Calibration standards were injected daily before and after the analysis.
The quantitation of PFOS in liver and blood samples was performed using quadratic regression fit analysis weighted by 1/x of the extracted calibration curve. The limit of quantitation (LOQ) was determined as the lowest acceptable standard in the calibration curve, defined as a standard within ±30% of the theoretical value with a peak area twice as great as the analyte peak area in blanks. LOQ was 1 ng/mL in plasma samples, and 1 ng/g in liver samples. Recoveries of the internal standard spiked into samples ranged from 67% to 73%. The recovery of PFOS in liver was 86% (range 82–90%) and in serum is 81% (range 80–82%). Blanks were analyzed by passing water and reagents through the entire analytical procedure.
Statistical analysis
Data were tested for normality (Shapiro–Wilks W-test) and homogeneity (Bartlett’s test for unequal variances) and, if needed, appropriate transformations were made. Using JMP 4.0.2 (SAS Institute Inc., Cary, NC), a one-way ANOVA was performed to determine differences among doses for each endpoint in which the standard error used a pooled estimate of error variance. When significant differences were detected by the F-test (P < 0.05), Dunnett’s t-test was applied to compare treatment groups with the control group. Uterine wet weight data were additionally tested by Kendall’s Tau for relationship analysis (P < 0.05). All variables were assessed in at least two separate experimental trials unless otherwise noted in the figure/table legends and methods sections. Data from a single experiment are typically shown, as results were similar between experimental trials. However, due to low cell recovery following isolation of CD4+ T-cells some samples did not contain sufficient cells for analysis. This resulted in low sample sizes in some of the treatment levels in the experimental trials conducted for T-cell cytokine analysis. Therefore, the T-cell cytokine data was tested for Trial by Treatment (Trial*Treatment) and Trial within Treatment [Trial (Treatment)] interaction. Since no interaction was observed, data from the T-cell cytokine experiments were combined. Because this approach was required for the T-cell cytokine results, it was also applied to the B-cell cytokine data.
Results
Body mass, organ mass and histopathology, and immune organ cellularity
There were no significant changes in body mass of animals exposed to PFOS as compared with controls (data not shown). Uterine mass was significantly decreased by 49% at the 5 mg/kg treatment as compared with control (). There were no other significant differences in mass for any of the remaining organs (e.g. adrenal, spleen, thymus, lung, liver, kidney, and brain) investigated following PFOS treatment (data not shown). Spleen cellularity was also not significantly altered by PFOS treatment (data not shown).
Figure 1. Uterine wet weight normalized for body mass {gonadal somatic index (GSI) = [uterus mass/body mass] × 100} in adult female B6C3F1 mice following oral exposure to perfluorooctane sulfonate (PFOS) for 28 days. Data are presented as mean ± SEM. Sample size for each treatment group is 5. Uterine weight was assessed in only one experimental trial. TAD = Total administered dose (daily doses are 1/28 of the TAD). *Indicates significant difference from control (P ≤ 0.05) with Dunnett’s comparison with control. Additionally, a significant decreasing relationship was observed with increasing PFOS dose (Tau b = −0.4; Prob>|Tau b| < 0.05).
![Figure 1. Uterine wet weight normalized for body mass {gonadal somatic index (GSI) = [uterus mass/body mass] × 100} in adult female B6C3F1 mice following oral exposure to perfluorooctane sulfonate (PFOS) for 28 days. Data are presented as mean ± SEM. Sample size for each treatment group is 5. Uterine weight was assessed in only one experimental trial. TAD = Total administered dose (daily doses are 1/28 of the TAD). *Indicates significant difference from control (P ≤ 0.05) with Dunnett’s comparison with control. Additionally, a significant decreasing relationship was observed with increasing PFOS dose (Tau b = −0.4; Prob>|Tau b| < 0.05).](/cms/asset/012e4f50-0f64-43b3-83e2-7bb4bcd819b4/iimt_a_527868_f0001_b.gif)
Histopathologic findings were generally unremarkable for most of the tissues examined (spleen, lung, thymus, liver, adrenal, uterus, and kidney; data not shown). No histopathologic differences were found between control and experimental groups with the exception of a mild, multifocal, microgliosis in brain tissue in one of the five mice in both the 1 and 5 mg PFOS/kg TAD treatment groups (data not shown).
Serum chemistry and hematology
Although serum glucose in the 5 mg/kg TAD dose group was increased 1.3-fold over control () and serum cholesterol levels in these mice were decreased by 27% compared with control, these changes were not statistically significant with Dunnett’s comparison with control (). No hematology parameters were altered with relation to PFOS dose (). Lastly, total T4 and total T3 serum levels were not affected when compared with control ().
Table 2. Hematology, serum clinical chemistry, and thyroid hormones in adult female B6C3F1 mice treated orally with perfluorooctane sulfonate (PFOS) for 28 days.
Cell surface marker analysis
Expression of cell surface molecules, CD19/CD21 and B220/CD40, were assessed in B-cells. There were no significant differences in absolute numbers of B-cells expressing CD19+/CD21−, CD19+/CD21+, B220+/CD40−, or B220+/CD40+ cells as compared with control ( and ). Additionally, the numbers of MHC-II+ cells were not altered by PFOS exposure (). Increased expression and subsequent ligation with CD40 on B-cells by CD154 on CD4+ T-helper cells is important in T-dependent antibody production; thus, the expression of CD154 on CD4+ cells was also determined. Absolute numbers of CD4+/CD154+ T-helper cells were not altered by PFOS treatment (), nor were numbers of CD4+/CD154− or CD4−/CD154+ cells. No changes in percentages of any cell type were observed (data not shown).
Table 3. Absolute numbers of cells expressing CD19/CD21 or MHC-II in adult female B6C3F1 mice treated orally with perfluorooctane sulfonate (PFOS) for 28 days.
Table 4. Absolute numbers of cells expressing CD4/CD154 or B220/CD40 in adult female B6C3F1 mice treated orally with perfluorooctane sulfonate (PFOS) for 28 days.
Cytokine production following in vitro stimulation
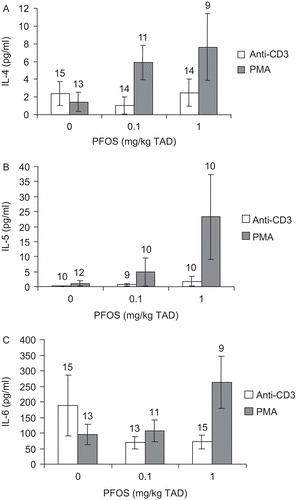
Ex vivo IL-6 production by in vitro anti-CD40-stimulated B-cells was increased ~6-fold over controls at 0.1 and 1 mg/kg TAD (specifically 6.1- and 6.4-fold, respectively; ). Additionally, in vitro LPS stimulation resulted in increased ex vivo IL-6 production by B-cells exposed in vivo to 1 mg PFOS/kg TAD (1.5-fold; ). In vivo PFOS-exposed CD4+ T-cells were stimulated in vitro with either anti-CD3 or PMA and ex vivo production of IL-4, IL-5, and IL-6 were also assessed. Ex vivo production of IL-4, IL-5, or IL-6 by T-cells following either anti-CD3 or PMA stimulation in vitro was not significantly altered by PFOS treatment ().
Figure 2. Interleukin-6 (IL-6) concentrations in culture supernatant from stimulated splenic B-cells obtained from adult female B6C3F1 mice following oral exposure to perfluorooctane sulfonate (PFOS) for 28 days. Splenocytes were stimulated with (A) 10 μg/mL anti-CD40 or (B) 10 μg/mL lipopolysaccharide (LPS). Data are presented as mean ± SEM. TAD = Total administered dose (daily doses are 1/28 of the TAD). Sample size for each treatment group is 10. This experiment was conducted twice. Experimental trials were tested for trial by treatment and trial within treatment interaction and then combined. *Indicates significant difference from control (P ≤ 0.05) with Dunnett’s comparison with control.
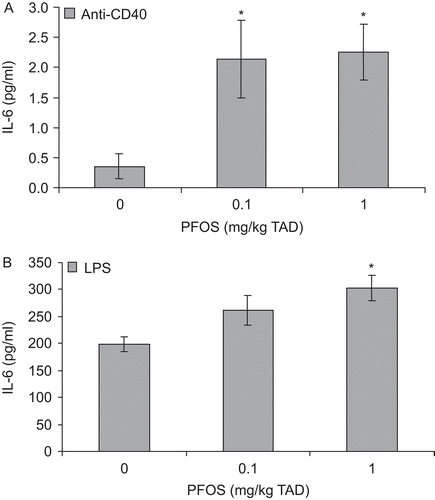
Figure 3. Cytokine concentrations in culture supernatant from stimulated splenic CD4+ T-cells obtained from adult female B6C3F1 mice following oral exposure to perfluorooctane sulfonate (PFOS) for 28 days. (A) Interleukin-4 (IL-4), (B) IL-5, and (C) IL-6. Data are presented as mean ± SEM. TAD = Total administered dose (daily doses are 1/28 of the TAD). This experiment was conducted three times. Due to low CD4+ T-cell counts following cell isolation, experimental trials were tested for “trial by treatment” and “trial within treatment” interaction and then combined. Sample size appears above the error bar. Cells were stimulated with 10 μg/mL anti-CD3 or 1.25 μg/mL PMA.
Liver and serum PFOS concentrations
Concentrations of PFOS in the serum and livers collected on Day 29 (24 h after the final exposure) were determined. The measured serum and liver concentrations are presented in . Liver-to-serum PFOS ratios for all treatments averaged 3:1 ().
Table 5. PFOS concentrations in serum and liver in adult female B6C3F1 mice treated orally with perfluorooctane sulfonate (PFOS) for 28 days.
Discussion
Several laboratories have assessed clinical and immunological effects attributed to perfluorinated compounds in mice (Nelson et al., Citation1992; Yang et al., Citation2000, Citation2001, Citation2002a,Citationb; Peden-Adams et al., Citation2007, Citation2008, Citation2009a,Citationb; DeWitt et al., Citation2008, Citation2009a; Fang et al., Citation2008, Citation2009; Keil et al., Citation2008; Lefebvre et al., Citation2008; Loveless et al., Citation2008; Dong et al., Citation2009; Guruge et al., Citation2009; Qazi et al., Citation2009a,Citationb; Son et al., Citation2009; Zheng et al., Citation2009), but few rodent studies have examined these effects across exposure concentrations that include environmentally relevant concentrations (Lefebvre et al., Citation2008; Peden-Adams et al., Citation2008; Dong et al., Citation2009; Guruge et al., Citation2009). PFOS causes suppression of SRBC-specific IgM production at 0.05 and 0.5 mg/kg TAD over 28 days in male and female B6C3F1 mice, respectively (Peden-Adams et al., Citation2008). This deficit in immune function has been reported at serum PFOS concentrations comparable with documented blood levels from both humans and wildlife (Olsen et al., Citation2003a,Citationb, Citation2005; Houde et al., Citation2005; Keller et al., Citation2005; Peden-Adams et al., Citation2008). Decreases in the plaque-forming cell (PFC) response in the B6C3F1 model are considered predictive of immunotoxicity and decreased host resistance (Luster et al., Citation1992, Citation1993; Selgrade, Citation1999) and alterations in murine immune function have been linked to human health risks (Selgrade, Citation2007). In fact, decreases in the PFC response are suggested to be predicative of susceptibility to influenza virus (Burleson and Burleson, Citation2008) and it was recently shown that 0.525 mg PFOS/kg TAD in female B6C3F1 mice causes increased susceptibility to the influenza A virus (Guruge et al., Citation2009). Moreover, MOE values for PFOS, based on serum PFOS concentrations at the NOAEL for immune parameters reported by Peden-Adams et al. (Citation2008), suggest possible human health risk (). At this time, the mode/mechanism of PFOS-induced SRBC-specific IgM suppression is not known and could affect human health risk assessment depending on the sensitivity of humans as compared with mice (Andersen et al., Citation2008; DeWitt et al., Citation2009b). Information on the mode/mechanism of action would permit adjustment of the uncertainty factors and allow better estimations of risk (Faustman and Omenn, Citation2001; Andersen et al., Citation2008). Thus, a series of studies were begun to assess various possible modes of action of PFOS on IgM production.
It has often been reported that exposure to PFOS causes a wasting syndrome and a significant drop in body weight (OECD, Citation2002; Qazi et al., Citation2009b). To demonstrate that overt, nutritional, or general systemic toxicity was not a confounding factor in the immunological evaluations for these animals at these environmentally relevant PFOS concentrations, body and organ weights, clinical serum chemistries, hematology, and histopathology were assessed. These negative results establish that the animals do not exhibit signs of overt toxicity. Therefore, the immune alterations reported here and in previous studies at these PFOS exposure concentrations (Peden-Adams et al., Citation2008) are not likely attributed to systemic toxicity as no indication of this was observed in the clinical, histopathology, organ mass, or body mass data. There was a general lack of histological alterations, with the exception of brain lesions in one animal in each of the highest two doses that may or may not reflect experimental treatment. Brain asymmetry and neurological endpoints were not assessed in the current study, but PFOS has been shown to alter neurological endpoints in adult rodents (Austin et al., Citation2003; Fuentes et al., Citation2007) and to increase the frequency of brain asymmetry in in ovo-exposed chicks (Peden-Adams et al., Citation2009b).
Due to known interactions of the hypothalamus–pituitary–thyroid axis with humoral immunological function (Gupta et al., Citation1983; Nandakumar et al., Citation2008), total serum T4 and T3 levels were evaluated in this study. Recent studies report that PFOS exposure (≥5 mg/kg/day) can induce a state of hypothyroidism (Lau et al., Citation2003; Thibodeaux et al., Citation2003). The current results are consistent with the previous studies as no alterations in serum total T3 or T4 levels were observed at the current PFOS concentrations that were <5 mg/kg/day. A recent study by Chang et al. (Citation2008) identified that PFOS may specifically interfere with proteins that bind thyroid hormones such as albumin and thyroxine-binding globulin, thereby, leading to a false hypothyroidic profile. Based on technical challenges associated with identifying free vs. bound hormone combined with minimal amounts of mouse serum, thyroid-binding proteins were not measured in this study. Considering that no changes in total thyroid hormone levels were apparent, it is unlikely that thyroid hormones contribute to PFOS-induced humoral immunosuppression.
To verify that alterations in antibody responses previously reported at these PFOS concentrations were not merely attributed to decreases in cell subpopulations, quantitation of splenic T-helper cells, B-cells, and MHC-II+ cells were performed. Numbers of B-cells as determined by CD19+/CD21− (immature), CD19+/CD21+ (mature), B220+/CD40−, and B220+/CD40+ cells were not decreased, suggesting suppression of antibody production is not simply due to decreases in B-cell numbers or types (Tedder et al., Citation1997). This is also consistent with studies evaluating sulfluramid (a perfluorinated pesticide that is metabolized to PFOS), where the numbers of B220+ lymphocytes were not altered with corresponding suppression of the PFC response (Peden-Adams et al., Citation2007). Numbers of cells expressing MHC-II and CD40 surface markers, which are found on antigen-presenting cells, were not altered by PFOS treatment. This is supported by data from Mollenhauer (Citation2008), which indicate no alteration in the percent of splenic F4/80+ cells (macrophages) at these PFOS concentrations. Moreover, data from this study confirm that numbers of CD4+ cells were within normal ranges. This contrasts a previous report from this laboratory where absolute numbers of CD4+ cells were decreased in female B6C3F1 mice at 0.1 mg/kg TAD but not at 1.0 mg/kg TAD using a similar 28-day exposure regime (Peden-Adams et al., Citation2008). This previous observation was from a single experiment, whereas in this study, the experiment was repeated twice for absolute numbers and three times for percent changes with all experiments yielding the same results. The effect previously reported was not dose-responsive and is likely to be a transient effect. Overall, these data indicate that T-helper cells, B-cells, and MHC-II+ cells were not selectively eliminated.
Cell signaling through ligation of CD40 on the B-cell with CD154 on the T-helper cell is an important step in antibody responses (Grewal and Flavell, Citation1998; Schonbeck and Libby, Citation2001; Xu and Song, Citation2004). Binding of CD40 to CD154 aids in moving the resting B-cell into the cell cycle and is essential for B-cell responses to T-dependent antigens, such as SRBC (Lee et al., Citation2003). Numbers of CD4 cells expressing CD154 (CD4+/CD154+) were not altered by PFOS exposures above and below the LOAEL in this rodent model, nor were absolute numbers of B-cells (B220+) expressing CD40 (B220+/CD40+). Therefore, PFOS exposure did not alter expression of CD40 or CD154 in resting B- or T-helper cells indicating no effect on background expression levels.
IL-6 is produced for varied purposes by many cell types including muscle, macrophages, B-cells, and T-cells. It is a marker of inflammation, a necessary component for antibody production, and has roles in the hypothalamic–pituitary–adrenal axis (Boss and Neeck, Citation2000). Moreover, studies have shown its role in autocrine stimulation of B-cells resulting in antibody production (Pérez-Melgosa et al., Citation1999; Contin et al., Citation2003). CD40 ligation on the surface of B-cells with CD154 on helper T-cells is critical for humoral responses to T-dependent antigens and results in B-cell proliferation, differentiation, and IL-6 stimulation of immunoglobulin production (Pérez-Melgosa et al., Citation1999; Contin et al., Citation2003). CD40-mediated control of the IL-6 gene requires NF-κB and activation of c-Jun (AP-1) transcriptional activity (Baccam et al., Citation2003; Bishop and Hostager Citation2003). A PPARα-agonist such as WY14,643 represses c-Jun transcription of the IL-6 promoter through negative regulation of NF-κB and activator protein-1 (AP-1) signaling pathways (Delerive et al., Citation1999; Cunard et al., Citation2002a,Citationb). Taken together, PFOS, a PPARα agonist (Shipley et al., Citation2004), could cause suppression of B-cell IL-6 production, which might explain, in part, the previously reported PFOS-induced SRBC-specific IgM suppression.
Interestingly, these data in in vitro-activated cells (i.e., those not exposed to antigen in vivo) suggest a somewhat different picture from the theory above. Ex vivo B-cell IL-6 production stimulated in vitro through CD40 engagement using anti-CD40 (thereby mimicking ligation with CD154 following antigen challenge and causing activation ex vivo) was increased at both dose levels assessed. Qazi et al. (Citation2009a) report increased serum IL-6 concentrations following a 10-day PFOS exposure (0.02% in diet) in male C57Bl/6 mice, but observed no effect on IL-6 production from mixed splenocyte cultures. Noted differences in ex vivo splenocyte IL-6 production between the current study and that of Qazi et al. (Citation2009a) may be related to strain, gender, dose regime, or methodological (i.e., isolated cell types compared with mixed cell cultures) differences. Further studies are needed to directly assess the effects of PFOS on c-Jun (AP-1), as well as other components suggested to be important to this pathway such as TNF receptor-associated factors (TRAFs) (Bishop and Hostager, Citation2003). Additionally, the role of PPARα in these responses should be determined, as PPARα agonists are generally considered anti-inflammatory and would be expected to cause a decrease in IL-6 production.
One possible indirect mechanism that should be mentioned is alteration of steroid hormone homeostasis. Although no histopathological alterations were detected, in this study PFOS decreased uterine wet weight in a linear manner (Kendall’s Tau b = −0.4, P < 0.05). This is similar to results with sulfluramid (Peden-Adams et al., Citation2007) and with previous reports of decreased uterine mass, although not statistically significant with ANOVA, following PFOS exposure at these same concentrations (Peden-Adams et al., Citation2008). Furthermore, uterine weight was decreased in rats following PFOS exposure (10 mg/kg/day) (Wetzel, Citation1983). The rodent uterotropic response that utilizes uterus wet weight or blotted weight is considered the “gold standard” for assessing the estrogenicity of a compound (Evans et al., Citation1941; Dickerson et al., Citation1995; Okazaki et al., Citation2002). Although the classic experiments for this type of study commonly employ either ovariectomized adults or sexually immature female rats (Kanno et al., Citation2001; Owens and Ashby, Citation2002), assessment of this parameter in intact adult rodents can be indicative of modulation in reproductive or endocrine function suggesting the need for further studies (Okazaki et al., Citation2002). Thus, it may be plausible that PFOS possesses anti-estrogenic effects since decreased uterine mass is a standard marker for anti-estrogenicity (Evans et al., Citation1941; Dickerson et al., Citation1995; Okazaki et al., Citation2002). Whether these observations are due to antagonism of the estrogen receptor or a decrease in circulating estradiol is unclear. PFOS has been shown to decrease aromatase activity in fathead minnows and Xenopus, causing increased plasma 11-ketotestosterone and testosterone, respectively (Ankley et al., Citation2005; Fort et al., Citation2007). In cynomolgus monkeys, however, females did not exhibit a decrease in circulating plasma estradiol levels, yet males did without a corresponding increase in testosterone levels (Seacat et al., Citation2002). Since estradiol can increase IgM production (Kanda and Tamaki, Citation1999) while testosterone can inhibit IgM production (Kanda et al., Citation1996), modulation of aromatase activity and the culminating changes in circulating testosterone and estradiol levels could affect IgM production and should be assessed.
These results corroborate earlier studies demonstrating that immunological function is sensitive to PFOS exposure (Keil et al., Citation2008; Peden-Adams et al., Citation2008). This study establishes that low-level exposure at environmentally relevant concentrations can modulate lymphocyte cytokine production. Although the current data from resting, in vitro-activated cells indicates that suppression of B-cell IL-6 production may not play a role in the previously reported PFOS-induced IgM suppression, this should be verified in mice challenged with antigen. Assessing these key pathways in isolated resting cells activated in vitro rather than in vivo was, however, a necessary first step in determination of the effects of PFOS on immune function parameters associated with IgM production. Increases in IL-6 secretion could indicate a proclivity toward inflammatory processes, as increased IL-6 is a marker for inflammation in mammals (Bauer, Citation1991; Jaatela, Citation1991). Studies determining the immune effects of PFOS should, therefore, include assessment of inflammatory markers to better understand the overall effects PFOS may have on immunity and health, as well as continue to assess events related to the mode of action of PFOS-induced antibody suppression.
Acknowledgements
The authors would like to thank the following reviewers for their critical review of the manuscript: Drs. Malcolm Meaburn and Mike Twiner and Mr. Jeff Adams. We acknowledge the Medical University of South Carolina (MUSC) and the Hollings Cancer Center for their support of the Flow Cytometry & Cell Sorting Shared Resource Facility. This publication does not constitute an endorsement of any commercial product or intend to be an opinion beyond scientific or other results obtained by the National Oceanic and Atmospheric Administration (NOAA). No reference shall be made to NOAA, or this publication furnished by NOAA, to any advertising or sales promotion that would indicate or imply that NOAA recommends or endorses any proprietary product mentioned herein, or which has as its purpose an interest to cause the advertised product to be used or purchased because of this publication. The authors have no conflict of interest to declare.
Declaration of interest
The authors report no declaration of interest. The project was funded in part by a grant from National Institutes of Environmental Health Sciences 1R03ES014058-01A1 (MMPA) and the National Oceanic and Atmospheric Administration/National Ocean Service/Center for Coastal Environmental Health and Biomolecular Research.
References
- Andersen, M. E., Butenhoff, J. L., Chang, S. C., Farrar, D. G., Kennedy, G. L. Jr Lau, C., Olsen, G.W., Seed, J. and Wallace, K. B. 2008. Perfluoroalkyl acids and related chemistries–toxicokinetics and modes of action. Toxicol. Sci. 102:3–14.
- Ankley, G. T., Kuehl, D. W., Kahl M. D., Jensen, K. M., Linnum, A., Leino, R. L. and Villeneuvet, D. A. 2005. Reproductive and developmental toxicity and bioconcentration of perfluorooctanesulfonate in a partial life-cycle test with the fathead minnow (Pimephales promelas). Environ Toxicol Chem., 24(9):2316–2324.
- Austin, M. E., Kasturi, B. S., Barber, M., Kannan, K., MohanKumar, P. S. and MohanKumar, S. M. 2003. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ. Health Perspect. 111:1485–1489.
- Baccam, M. and Bishop, G. A. 1999. Membrane-bound CD154, but not CD40-specific antibody, mediates NF-kappaB-independent IL-6 production in B-cells. Eur. J. Immunol. 29:3855–3866.
- Baccam, M., Woo, S. Y., Vinson, C. and Bishop, G. A. 2003. CD40-mediated transcriptional regulation of the IL-6 gene in B-lymphocytes: Involvement of NF-kappa B, AP-1, and C/EBP. J. Immunol. 170:3099–3108.
- Bauer, J. 1991. IL-6 in clinical medicine. Ann. Hematol. 62:203–210.
- Bishop, G. A. and Hostager, B. S. 2003. The CD40–CD154 interaction in B-cell-T-cell liaisons. Cytokine Growth Factor Rev. 14:297–309.
- Boss, B. and Neeck, G. 2000. Correlation of IL-6 with the classical humoral disease activity parameters ESR and CRP and with serum cortisol, reflecting the activity of the HPA axis in active rheumatoid arthritis. Z. Rheumatol. 59(Suppl. 2):II/62–II/64.
- Burleson, G. R. and Burleson, F. G. 2008. Testing human biologicals in animal host resistance models. J. Immunotoxicol. 52:3–31.
- Calafat, A. M., Kuklenyik, Z., Reidy, J. A., Caudill, S. P., Tully, J. S. and Needham, L. L. 2007a. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES). Environ. Sci. Technol. 41:2237–2242.
- Calafat, A. M., Wong, L. Y., Kuklenyik, Z., Reidy, J. A. and Needham, L. L. 2007b. Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 115:1596–1602.
- Case, M. T., York, R. G. and Christian, M. S. 2001. Rat and rabbit oral developmental toxicology studies with two perfluorinated compounds. Int. J. Toxicol. 20:101–109.
- Chang, S. C., Thibodeaux, J. R., Eastvold, M. L., Ehresman, D. J., Bjork, J. A., Froehlich, J. W., Lau, C., Singh, R. J., Wallace, K. B. and Butenhoff, J. L. 2008. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology 243:330–339.
- Contin, C., Pitard, V., Delmas, Y., Pelletier, N., Defrance, T., Moreau, J. F., Merville, P. and Dé,chanet-Merville, J. 2003. Potential role of soluble CD40 in the humoral immune response impairment of uraemic patients. Immunology 110:131–140.
- Cunard, R., DiCampli, D., Archer, D. C., Stevenson, J. L., Ricote, M., Glass, C. K. and Kelly, C. J. 2002a. WY14,643, a PPAR alpha ligand, has profound effects on immune responses in vivo. J. Immunol. 169:6806–6812.
- Cunard, R., Ricote, M., DiCampli, D., Archer, D. C., Kahn, D. A., Glass, C. K. and Kelly, C. J. 2002b. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J. Immunol. 168:2795–2802.
- Delerive, P., De Bosscher, K., Besnard, S., Vanden Berghe, W., Peters, J. M., Gonzalez, F. J., Fruchart, J. C., Tedgui, A., Haegeman, G. and Staels, B. 1999. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J. Biol. Chem. 274:32048–32054.
- DeWitt, J. C., Copeland, C. B. and Luebke, R. W. 2009a. Suppression of humoral immunity by perfluorooctanoic acid is independent of elevated serum corticosterone concentration in mice. Toxicol. Sci. 109:106–112.
- DeWitt, J. C., Copeland, C. B., Strynar, M. J. and Luebke, R. W. 2008. Perfluorooctanoic acid-induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ. Health Perspect. 116:644–650.
- DeWitt, J. C., Shnyra, A., Badr, M. Z., Loveless, S. E., Hoban, D., Frame, S. R., Cunard, R., Anderson, S. E., Meade, B. J., Peden-Adams, M. M., Luebke, R. W. and Luster, M. I. 2009b. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit. Rev. Toxicol. 39:76–94.
- Dickerson, R., Keller, L. H. and Safe, S. 1995. Alkyl polychlorinated dibenzofurans and related compounds as antiestrogens in the female rat uterus: Structure–activity studies. Toxicol. Appl. Pharmacol. 135:287–298.
- Dong, G. H., Zhang, Y. H., Zheng, L., Liu, W., Jin, Y. H. and He, Q. C. 2009. Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Arch. Toxicol. 83:805–815.
- Evans, J. S., Varney, R. F. and Koch, F. C. 1941. The mouse uterine wet weight method for the assay of estrogens. Endocrinology 28:747–752.
- Fang, X., Feng, Y., Shi, Z. and Dai, J. 2009. Alterations of cytokines and MAPK signaling pathways are related to the immunotoxic effect of perfluorononanoic acid. Toxicol. Sci. 108:367–376.
- Fang, X., Zhang, L., Feng, Y., Zhao, Y. and Dai, J. 2008. Immunotoxic effects of perfluorononanoic acid on BALB/c mice. Toxicol. Sci. 105:312–321.
- Faustman, E. M. and Omenn, G. S. 2001. Risk assessment. In: Casarett and Doull’s Toxicology: The Basic Science of Poisons (Klaassen, C. D., Ed), New York: McGraw-Hill Companies, Inc, pp. 83–104.
- Fort, D., Rodgers, R. L., Guiney, P. D. and Weeks, J. A. 2007. Effects of Perfluorooctane Sulfonate (PFOS) Exposure on Steroidogensis in Juvenile Xenopus (Silurana) tropicalis. Society of Environmental Toxicology and Chemistry—Abstract Book, 28th Annual Meeting, November 11–15, Milwaukee, WI, pp. 231.
- Fuentes, S., Colomina, M. T., Rodriguez, J., Vicens, P. and Domingo, J. L. 2006. Interactions in developmental toxicology: Concurrent exposure to perfluorooctane sulfonate (PFOS) and stress in pregnant mice. Toxicol. Lett. 164:81–89.
- Fuentes, S., Colomina, M. T., Vicens, P., Franco-Pons, N. and Domingo, J. L. 2007. Concurrent exposure to perfluorooctane sulfonate and restraint stress during pregnancy in mice: Effects on postnatal development and behavior of the offspring. Toxicol. Sci. 98:589–598.
- Giesy, J. P. and Kannan, K. 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 35:1339–1342.
- Grasty, R. C., Wolf, D. C., Grey, B. E., Lau, C. S. and Rogers, J. M. 2003. Prenatal window of susceptibility to perfluorooctane sulfonate-induced neonatal mortality in the Sprague-Dawley rat. Birth Defects Res. B Dev. Reprod. Toxicol. 68:465–471.
- Grewal, I. S. and Flavell, R. A. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111–135.
- Gupta, M. K., Chiang, T. and Deodhar, S. D. 1983. Effect of thyroxine on immune response in C57Bl/6J mice. Acta Endocrinol. 103:76–80.
- Guruge, K. S., Hikono, H., Shimada, N., Murakami, K., Hasegawa, J., Yeung, L. W., Yamanaka, N. and Yamashita, N. 2009. Effect of perfluorooctane sulfonate (PFOS) on influenza A virus-induced mortality in female B6C3F1 mice. J. Toxicol. Sci. 34:687–691.
- Houde, M., Wells, R. S., Fair, P. A., Bossart, G. D., Hohn, A. A., Rowles, T. K., Sweeney, J. C., Solomon, K. R. and Muir, D. C. 2005. Polyfluoroalkyl compounds in free-ranging bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean. Environ. Sci. Technol. 39:6591–6598.
- Jaattela, M. 1991. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab. Invest. 64(6), 724–742.
- Jin, Y., Saito, N., Harada, K. H., Inoue, K. and Koizumi, A. 2007. Historical trends in human serum levels of perfluorooctanoate and perfluorooctane sulfonate in Shenyang, China. Tohoku J. Exp. Med. 212:63–70.
- Johnson, J. D., Gibson, S. J. and Ober, R. E. 1984. Cholestyramine-enhanced fecal elimination of carbon-14 in rats after administration of ammonium [14C]perfluorooctanoate or potassium [14C]-perfluorooctanesulfonate. Fundam. Appl. Toxicol. 4:972–976.
- Kanda N., Tsuchida T. and Tamaki K. 1996. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin Exp Immunol., 106(2):410–415.
- Kanda N. and Tamaki K. 1999. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol. 103(2 Pt 1):282–288.
- Kannan, K., Franson, J. C., Bowerman, W. W., Hansen, K. J., Jones, P. D. and Giesy, J. P. 2001. Perfluorooctane sulfonate in fish-eating water birds including bald eagles and albatrosses. Environ. Sci. Technol. 35:3065–3070.
- Kannan, K., Perrotta, E. and Thomas, N. J. 2006. Association between perfluorinated compounds and pathological conditions in southern sea otters. Environ. Sci. Technol. 40:4943–4948.
- Kanno, J., Onyon, L., Haseman, J., Fenner-Crisp, P., Ashby, J. and Owens, W. Organisation for Economic Co-operation and Development. 2001. The OECD program to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: phase 1. Environ. Health Perspect. 109:785–794.
- Keil, D. E., McGuinn, W. D., Dudley, A. C., EuDaly, J. G., Gilkeson, G. S. and Peden-Adams, M. M. 2009. N, N,-Diethyl-m-toluamide (DEET) suppresses humoral immunological function in B6C3F1 mice. Toxicol. Sci. 108:110–123.
- Keil, D. E., Mehlmann, T., Butterworth, L. and Peden-Adams, M. M. 2008. Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol. Sci. 103:77–85.
- Keller, J. M., Kannan, K., Taniyasu, S., Yamashita, N., Day, R. D., Arendt, M. D., Segars, A. L. and Kucklick, J. R. 2005. Perfluorinated compounds in the plasma of loggerhead and Kemp’s ridley sea turtles from the southeastern coast of the United States. Environ. Sci. Technol. 39:9101–9108.
- Kissa, E. (Ed.). 2001. Fluorinated Surfactants and Repellents, 2nd Ed., New York: Marcel Keller.
- Klaus, G. G., Holman, M., Johnson-Léger, C., Christenson, J. R. and Kehry, M. R. 1999. Interaction of B-cells with activated T-cells reduces the threshold for CD40-mediated B-cell activation. Int. Immunol. 11:71–79.
- Lau, C., Anitole, K., Hodes, C., Lai, D., Pfahles-Hutchens, A. and Seed, J. 2007. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 99:366–394.
- Lau, C., Thibodeaux, J. R., Hanson, R. G., Rogers, J. M., Grey, B. E., Stanton, M. E., Butenhoff, J. L. and Stevenson, L. A. 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: Post-natal evaluation. Toxicol. Sci. 74:382–392.
- Lee, B. O., Moyron-Quiroz, J., Rangel-Moreno, J., Kusser, K. L., Hartson, L., Sprague, F., Lund, F. E. and Randall, T. D. 2003. CD40, but not CD154, expression on B-cells is necessary for optimal primary B-cell responses. J. Immunol. 171:5707–5717.
- Lefebvre, D. E., Curran, I., Armstrong, C., Coady, L., Parenteau, M., Liston, V., Barker, M., Aziz, S., Rutherford, K., Bellon-Gagnon, P., Shenton, J., Mehta, R. and Bondy, G. 2008. Immunomodulatory effects of dietary potassium perfluorooctane sulfonate (PFOS) exposure in adult Sprague-Dawley rats. J. Toxicol. Environ. Health Part A 71:1516–1525.
- Lehmler, H. J. 2005. Synthesis of environmentally relevant fluorinated surfactants—a review. Chemosphere 58:1471–1496.
- Li, Y. Y., Baccam, M., Waters, S. B., Pessin, J. E., Bishop, G. A. and Koretzky, G. A. 1996. CD40 ligation results in protein kinase C-independent activation of ERK and JNK in resting murine splenic B-cells. J. Immunol. 157:1440–1447.
- Liu, J., Na, S., Glasebrook, A., Fox, N., Solenberg, P. J., Zhang, Q., Song, H. Y. and Yang, D. D. 2001. Enhanced CD4+ T-cell proliferation and TH2 cytokine production in DR6-deficient mice. Immunity 15:23–34.
- Loveless, S. E., Hoban, D., Sykes, G., Frame, S. R. and Everds, N. E. 2008. Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate. Toxicol. Sci. 105:86–96.
- Luebker, D. J., Hansen, K. J., Bass, N. M., Butenhoff, J. L. and Seacat, A. M. 2002. Interactions of fluorochemicals with rat liver fatty acid-binding protein. Toxicology 176:175–185.
- Luster, M. I., Munson, A. E., Thomas, P. T., Holsapple, M. P., Fenters, J. D., White, K. L. Jr, Lauer, L. D., Germolec, D. R., Rosenthal, G. J. and Dean, J. H. 1988. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program’s guidelines for immunotoxicity evaluation in mice. Fundam. Appl. Toxicol. 10:2–19.
- Luster, M. I., Portier, C., Pait, D. G., Rosenthal, G. J., Germolec, D. R., Corsini, E., Blaylock, B. L., Pollock, P., Kouchi, Y. and Craig, W. 1993. Risk assessment in immunotoxicology. II. Relationships between immune and host resistance tests. Fundam. Appl. Toxicol. 21:71–82.
- Luster, M. I., Portier, C., Pait, D. G., White, K. L. Jr Gennings, C., Munson, A. E., and Rosenthal, G. J. 1992. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 18:200–210.
- Mollenhauer, M. A. 2008. Determination of the Role of PPARα in Inflammatory Processes Following Perfluorooctane Sulfonate Exposure in a Murine Model. Dissertation, Medical University of South Carolina.
- Moody, C. A. and Field, J. A. 2000. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 34:3864–3870.
- Nandakumar, D. N., Koner, B. C., Vinayagamoorthi, R., Nanda, N., Negi, V. S., Goswami, K., Bobby, Z. and Hamide, A. 2008. Activation of NF-kappaB in lymphocytes and increase in serum immunoglobulin in hyperthyroidism: Possible role of oxidative stress. Immunobiology 213:409–415.
- Nelson, D. L. Jr, Frazier, D. E. Ericson, J. E., Tarr, M. J. and Mathes, L. E. 1992. The effects of perfluorodecanoic acid (PFDA) on humoral, cellular, and innate immunity in Fischer 344 rats. Immunopharmacol. Immunotoxicol. 14:925–938.
- Noe, S. N., Newton, C., Widen, R., Friedman, H. and Klein, T. W. 2000. Anti-CD40, anti-CD3, and IL-2 stimulation induce contrasting changes in CB1 mRNA expression in mouse splenocytes. J. Neuroimmunol. 110:161–167.
- OECD. 2002. Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and its Salts. ENV/JM/RD(2002)17/Final. http://www.oecd.org/document/58/0,2340,en_2649_34379_2384378_1_1_1_1,00.html. Accessed May 12, 2004
- Okazaki, K., Imazawa, T., Nakamura, H., Furukawa, F., Nishikawa, A. and Hirose, M. 2002. A repeated 28-day oral dose toxicity study of 17alpha-methyltestosterone in rats, based on the ‘enhanced OECD Test Guideline 407’ for screening the endocrine-disrupting chemicals. Arch. Toxicol. 75:635–642.
- Olsen, G. W., Burris, J. M., Burlew, M. M. and Mandel, J. H. 2003a. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J. Occup. Environ. Med. 45:260–270.
- Olsen, G. W., Burris, J. M., Ehresman, D. J., Froehlich, J. W., Seacat, A. M., Butenhoff, J. L. and Zobel, L. R. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 115:1298–1305.
- Olsen, G. W., Church, T. R., Miller, J. P., Burris, J. M., Hansen, K. J., Lundberg, J. K., Armitage, J. B., Herron, R. M., Medhdizadehkashi, Z., Nobiletti, J. B., O’Neill, E. M., Mandel, J. H. and Zobel, L. R. 2003b. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ. Health Perspect. 111:1892–1901.
- Olsen, G. W., Huang, H. Y., Helzlsouer, K. J., Hansen, K. J., Butenhoff, J. L. and Mandel, J. H. 2005. Historical comparison of perfluorooctanesulfonate, perfluorooctanoate, and other fluorochemicals in human blood. Environ. Health Perspect. 113:539–545.
- Olsen, G. W., Mair, D. C., Church, T. R., Ellefson, M. E., Reagen, W. K., Boyd, T. M., Herron, R. M., Medhdizadehkashi, Z., Nobiletti, J. B., Rios, J. A., Butenhoff, J. L. and Zobel, L. R. 2008. Decline in perfluorooctanesulfonate and other polyfluoroalkyl chemicals in American Red Cross adult blood donors, 2000–2006. Environ. Sci. Technol. 42:4989–4995.
- Owens, J. W. and Ashby, J. 2002. Critical review and evaluation of the uterotrophic bioassay for the identification of possible estrogen agonists and antagonists: In support of the validation of the OECD uterotrophic protocols for the laboratory rodent. Organisation for Economic Co-operation and Development. Crit. Rev. Toxicol. 32:445–520.
- Peden-Adams, M. M., EuDaly, J. G., Dabra, S., EuDaly, A., Heesemann, L., Smythe, J. and Keil, D. E. 2007. Suppression of humoral immunity following exposure to the perfluorinated insecticide sulfluramid. J. Toxicol. Environ. Health Part A. 70:1130–1141.
- Peden-Adams, M. M., Keil, D. E., Romano, T., Mollenhauer, M. A. M., Fort, D. J., Guiney, P. D., Houde, M., Kannan, K., Muir, D. C., Rice, C. D., Stuckey, J., Segars, A. L., Scott, T., Talent, L., Bossart, G. D., Fair, P. A. and Keller, J. M. 2009a. Health effects of perfluorinated compounds—what are the wildlife telling us? Reprod. Toxicol. 27:414–415.
- Peden-Adams, M. M., Keller, J. M., Eudaly, J. G., Berger, J., Gilkeson, G. S. and Keil, D. E. 2008. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci. 104:144–154.
- Peden-Adams, M. M., Stuckey, J. E., Gaworecki, K. M., Berger-Ritchie, J., Bryant, K., Jodice, P. G., Scott, T. R., Ferrario, J. B., Guan, B., Vigo, C., Boone, J. S., McGuinn, W. D., DeWitt, J. C. and Keil, D. E. 2009b. Developmental toxicity in white leghorn chickens following in ovo exposure to perfluorooctane sulfonate (PFOS). Reprod. Toxicol. 27:307–318.
- Pérez-Melgosa, M., Hollenbaugh, D. and Wilson, C. B. 1999. Cutting edge: CD40 ligand is a limiting factor in the humoral response to T-cell-dependent antigens. J. Immunol. 163:1123–1127.
- Qazi, M. R., Bogdanska, J., Butenhoff, J. L., Nelson, B. D., DePierre, J. W. and Abedi-Valugerdi, M. 2009a. High-dose, short-term exposure of mice to perfluorooctanesulfonate (PFOS) or perfluorooctanoate (PFOA) affects the number of circulating neutrophils differently, but enhances the inflammatory responses of macrophages to lipopolysaccharide (LPS) in a similar fashion. Toxicology 262:207–214.
- Qazi, M. R., Xia, Z., Bogdanska, J., Chang, S. C., Ehresman, D. J., Butenhoff, J. L., Nelson, B. D., DePierre, J. W. and Abedi-Valugerdi, M. 2009b. The atrophy and changes in the cellular compositions of the thymus and spleen observed in mice subjected to short-term exposure to perfluorooctanesulfonate are high-dose phenomena mediated in part by peroxisome proliferator-activated receptor-alpha (PPARalpha). Toxicology 260:68–76.
- Reimers, T. J., Cowan, R. G., Davidson, H. P. and Colby, E. D. 1981. Validation of radioimmunoassay for triiodothyronine, thyroxine, and hydrocortisone (cortisol) in canine, feline, and equine sera. Am. J. Vet. Res. 42:2016–2021.
- Renwick, A. G. 1995. Toxicokinetics. In: General and Applied Toxicology: College Edition (Ballantyne, B., Marrs, T., Turner, P., Eds.), New York: Stockton Press, pp. 111–142.
- Schonbeck, U. and Libby, P. 2001. The CD40/CD154 receptor/ligand dyad. Cell. Mol. Life Sci. 58:4–43.
- Seacat, A. M., Thomford, P. J., Hansen, K. J., Clemen, L. A., Eldridge, S. R., Elcombe, C. R. and Butenhoff, J. L. 2003. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 183:117–131.
- Seacat, A. M., Thomford, P. J., Hansen, K. J., Olsen, G. W., Case, M. T. and Butenhoff, J. L. 2002. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol. Sci. 68:249–264.
- Selgrade, M. K. 2007. Immunotoxicity: The risk is real. Toxicol. Sci. 100:328–332.
- Selgrade, M. K. 1999. Use of immunotoxicity data in health risk assessments: Uncertainties and research to improve the process. Toxicology 133:59–72.
- Shipley, J. M., Hurst, C. H., Tanaka, S. S., DeRoos, F. L., Butenhoff, J. L., Seacat, A. M. and Waxman, D. J. 2004. Trans-activation of PPARalpha and induction of PPARalpha target genes by perfluorooctane-based chemicals. Toxicol. Sci. 80:151–160.
- Son, H. Y., Lee, S., Tak, E. N., Cho, H. S., Shin, H. I., Kim, S. H. and Yang, J. H. 2009. Perfluorooctanoic acid alters T-lymphocyte phenotypes and cytokine expression in mice. Environ. Toxicol. 24:580–588.
- Szocinski, J. L., Khaled, A. R., Hixon, J., Halverson, D., Funakoshi, S., Fanslow, W. C., Boyd, A., Taub, D. D., Durum, S. K., Siegall, C. B., Longo, D. L. and Murphy, W. J. 2002. Activation-induced cell death of aggressive histology lymphomas by CD40 stimulation: Induction of bax. Blood 100:217–223.
- Tedder, T. F., Inaoki, M. and Sato, S. 1997. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity 6:107–118.
- Thibodeaux, J. R., Hanson, R. G., Rogers, J. M., Grey, B. E., Barbee, B. D., Richards, J. H., Butenhoff, J. L., Stevenson, L. A. and Lau, C. 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol. Sci. 74:369–381.
- Ullrich, S. E. 1999. Dermal application of JP-8 jet fuel induces immune suppression. Toxicol Sci., 52(1):61–67.
- Wetzel, L. T. 1983. Rat Teratology Study, T-3351, Final Report. Hazelton Laboratories America, Inc. Project number 154–160. December 19, 1983 (8EHQ-0399-374). Available on USEPA Public docket AR-226 (No. 226-0014).
- Xu, Y. and Song, G. 2004. The role of CD40–CD154 interaction in cell immunoregulation. J. Biomed. Sci. 11:426–438.
- Yang, Q., Abedi-Valugerdi, M., Xie, Y., Zhao, X. Y., Möller, G., Nelson, B. D. and DePierre, J. W. 2002b. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int. Immunopharmacol. 2:389–397.
- Yang, Q., Xie, Y., Alexson, S. E., Nelson, B. D. and DePierre, J. W. 2002a. Involvement of the peroxisome proliferator-activated receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem. Pharmacol. 63:1893–1900.
- Yang, Q., Xie, Y. and Depierre, J. W. 2000. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin. Exp. Immunol. 122:219–226.
- Yang, Q., Xie, Y., Eriksson, A. M., Nelson, B. D. and DePierre, J. W. 2001. Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluoroctanoic acid in mice. Biochem. Pharmacol. 62:1133–1140.
- Zheng, L., Dong, G. H., Jin, Y. H. and He, Q. C. 2009. Immunotoxic changes associated with a 7-day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice. Arch. Toxicol. 83:679–689.
