Abstract
Cobalt-chromium (Co-Cr) alloy metal-on-metal hip resurfacing is increasingly common among younger more active patients suffering from osteoarthritis. Recent reports have increased awareness of metal ions leaching from metallic articulations; this ion exposure may have adverse effects on the immune system. As previous studies reported alterations in lymphocyte number and function in patients with Co-Cr implants, we investigated effects of clinically relevant concentrations of Cr6+ and Co2+ on primary human lymphocytes in vitro. Here, both resting and activated (anti-CD3 ± anti-CD28 antibodies) primary human lymphocytes were exposed to Cr6+ or Co2+ (0.1–100 µM). Following 24 or 48 h of exposure, cell viability, proliferation, cytokine [interferon-γ (IFNγ and interleukin-2 (IL-2)] release, and apoptosis (with and without pre-treatment of cells with a caspase-3 inhibitor) were assessed. Exposure to 10 and 100 µM Cr6+ significantly decreased cell viability and increased apoptosis in both resting and activated lymphocytes. Cell proliferation and cytokine release were also significantly reduced in activated lymphocytes following exposure. The exposure of resting lymphocytes to 100 µM Co2+ resulted in significant decreases in cell viability accompanied by a significant increase in apoptosis. Activated lymphocytes also showed this response after exposure to 100 µM Co2+; in fact, activated cells were significantly more sensitive to Co2+ toxicity. Exposure to 10 µM Co2+ led to significant decreases in cell proliferation and cytokine release, but no significant increase in apoptosis, in activated cells. The results indicate that exposure to high concentrations of metal ions initiate apoptosis that results in decreased lymphocyte proliferation. IL-2 release is inhibited by both metal ions at concentrations that are not overtly toxic. However, metal ion concentrations not directly cytotoxic to lymphocytes may affect events at a molecular level, thereby impeding lymphocyte proliferation. Hence, this may contribute to altered immune system function in patients with Co-Cr implants.
Introduction
Over the last two decades, metal-on-metal (MOM) resurfacing hip replacement has become an increasingly common surgical procedure in the management of osteoarthritis. In particular, it has become an attractive alternative to total hip replacement (THR) for younger, more active patients (Boardman et al., Citation2006). There are a number of reported benefits; it preserves femoral bone, reduces risk of dislocation, produces better femoral loading and joint mechanics, reduces stress-shielding and has better wear characteristics. These advantages appear to be supported from early clinical reports that show a low rate of failure following MOM resurfacing in patients with high-level occupational and leisure activities (Kwon et al., Citation2009).
Despite the encouraging clinical follow-up reports, there is growing concern regarding high levels of metal ions released from cobalt-chromium (Co-Cr) alloy (ISO 5832-4 ASTM F-75 and ISO 5832-12 ASTM F-1537) MOM articulations. These implants can release metal ions, metal complexes, or particulate metal following electrochemical or mechanical corrosion (Savarino et al., Citation1999). They mainly release chromium (Cr6+) and cobalt (Co2+) ions (Merritt and Brown, Citation1995; Shettlemore and Bundy, Citation1999). Circulating levels of the other metallic constituent, molybdenum, do not increase after hip resurfacing arthroplasty (Witzleb et al., Citation2006; Antoniou et al., Citation2008). However, patients with a Co-Cr alloy MOM implant have higher levels of circulating Cr and Co ions than both control subjects and patients who do not have a metal component in their prostheses (Back et al., Citation2005; Daniel et al., Citation2007; Vendittoli et al., Citation2007; Afolaranmi et al., Citation2008; Hart et al., Citation2009; Langton et al., Citation2009). Concentrations of > 5 µM have been measured in whole blood. In addition, concentrations of Co and Cr ions in hip aspirate (> 10 µM) have been found to be higher than circulating levels (De Smet et al., Citation2008; Kwon et al., Citation2009). Furthermore, post-mortem studies have shown extremely high levels of metals (> 100 µM) in organs and tissues after traditional metal hip arthroplasty, for example in the liver, lymph nodes, and spleen (Langkamer et al., Citation1992; Case et al., Citation1994).
The high levels of metal ions uniformly measured in MOM resurfacing patients has led to concerns about the long-term effects of metal ion exposure, particularly the immunological effects. A periprosthetic soft-tissue reaction, termed ALVAL (aseptic lymphocytic vasculitis-associated lesion) has been reported following MOM THR (Davies et al., Citation2005; Willert et al., Citation2005). ALVAL has been described as lymphocytic aggregates around the hip capsule. Similar intense lymphocyte infiltrates have been reported in soft-tissue masses, described as pseudo-tumors, following MOM resurfacing arthroplasty (Boardman et al., Citation2006; Counsell et al., Citation2008; Pandit et al., Citation2008). In addition to lymphocyte infiltration, patients with pseudo-tumors show local soft-tissue damage and the outcome of revision surgery following a pseudo-tumor is poor (Grammatopolous et al., Citation2009). It is postulated that this immunologically mediated reaction is triggered by high metal ion concentration/metallic wear debris locally, which leads to lymphocyte activation and appears in the form of a delayed type hypersensitivity (Willert et al., Citation2005; Counsell et al., Citation2008). Further reports also suggest T-lymphocyte mediated metal sensitivity is related to the presence of MOM implants (Goodman, Citation2007; Hallab et al., Citation2008). Hallab et al. (Citation2008) showed an increase in proliferation of lymphocytes from MOM THR patients when challenged with metal ions. The results from the study also described a greater release of TH1 (T-helper Type 1) inflammatory cytokines from these cells, indicating a sensitisation to metal ions.
It is reported that metal ions released from implants may lead to genotoxic and suppressive effects on the immune system (Case, Citation2001; Ladon et al., Citation2004; Hart et al., Citation2006), as well as an adaptive immune response. These reports have described chromosomal aberrations and translocations in lymphocytes of patients following MOM hip arthroplasty. MOM hip resurfacing has also been associated with a decrease in the number of circulating peripheral lymphocytes (Savarino et al., Citation1999; Hart et al., Citation2006, 2009). There is a close link between Cr and Co ion levels and the reduction in absolute number of circulating lymphocytes, in particular CD8+ cytotoxic T-lymphocytes. This reduction in circulating lymphocytes could possibly be due to cells being diverted out of the circulation toward the implant. However, it is more likely to be caused by metal ions impeding antigen presentation to the lymphocytes and, consequently, reducing their expansion (Hart et al., Citation2009).
The presence of lymphocytes in perivascular infiltrates and pseudo-tumors around the MOM implant suggests the immunotoxicity of Cr and Co may be mediated via lymphocytes, especially T-lymphocytes. It is possible that high local metal ion concentrations facilitate a T-lymphocyte mediated inflammatory response resulting in the destruction seen around the prostheses (Davies et al., Citation2005; Willert et al., Citation2005; Boardman et al., Citation2006; Counsell et al., Citation2008; Kwon et al., Citation2009). T-lymphocytes are normally quiescent cells that are activated via recognition of a cognate antigen (Signal 1) and costimulatory molecules (Signal 2) that are generated through interactions with antigen-presenting cells. It is generally presumed that metal ions will facilitate activation and sensitization, however as mentioned above they may also be cytotoxic and suppressive. This is likely to depend on the concentration of metal ions present (Kwon et al., Citation2009). Therefore, the aim of this study was to investigate the effects of clinically recorded concentrations of Cr and Co ions on peripheral human lymphocytes.
Materials and methods
Metal ion solution preparation
Co-Cr stock solutions were prepared by dissolving chromium oxide and cobalt chloride (both Alfa Aesar, Johnson Matthey Co., Lancashire, UK) in distilled water and filter-sterilizing (0.22 µm filter) under sterile conditions. The stock solutions were then used to prepare the required concentrations of Cr6+ and Co2+ (0.1, 1, 10, and 100 µM) in complete RPMI-1640 (Lonza, Slough, UK). Complete RPMI-1640 contained 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 mU penicillin/ml, and 100 mg streptomycin/ml.
Human lymphocyte isolation
Peripheral blood mononuclear cells (PBMCs) were isolated under sterile conditions from ≈ 60 ml of Buffy Coat (Scottish Blood Transfusion Service, Glasgow, UK) by density gradient centrifugation using Histopaque-1077 (Sigma-Aldrich, Cambridge, UK). The PBMC were then washed twice with RPMI-1640 and lymphocyte enrichment was then performed as previously described (Martín-Romero et al., Citation2000). Briefly, PBMC (2.5 × 106 cells/ml) were incubated in a 75-cm2 culture flask (TPP, Trasadingen, Switzerland) with complete RPMI-1640 for 1 h at 37°C in a 5% CO2 chamber. The medium with the non-adherent cell suspension was then transferred to another culture flask and incubated for an additional 1 h to further deplete the numbers of any monocytes present in the population.
Lymphocyte culture for cell viability and apoptosis analysis
In order to assess cell viability and apoptosis, lymphocytes were also exposed to Cr6+ and Co2+ in resting and activated states. For the analyses using resting lymphocytes, the lymphocytes were cultured (0.1 × 106 cells/well) in 96-well round-bottom plates with 0.1, 1, 10, and 100 µM of Cr6+ or Co2+ in complete RPMI-1640 for 24 and 48 h at 37°C under 5% CO2. For analyses using anti-CD3-activated cells, lymphocytes were cultured (0.1 × 106 cells/well) in 96-well round-bottom plates (100 µl/well), and incubated with 0.1, 1, 10, or 100 µM of Cr6+ or Co2+ for 24 and 48 h in complete RPMI-1640 that was supplemented with 0.1 µg/ml soluble anti-CD3 (clone HIT3a; eBioscience, Hatfield, UK) at 37°C under 5% CO2.
Pre-treatment of lymphocytes with caspase-3 inhibitor
To evaluate any potential role that caspase-3 might have in any observed effects of Cr6+ or Co2+ on the lymphocytes, both resting and anti-CD3 activated lymphocytes were pre-treated with 50 µM of the caspase-3 inhibitor Z-Asp (OCH3)-Glu (OCH3)-Val-Asp (OCH3)-FMK (Z-DEVD-FMK) (R&D Systems, Abingdon, UK) for 24 h (Vasant et al., Citation2003) prior to their exposure to Cr6+ or Co2+ as described above.
Cell viability assay
At each culture endpoint, cell viability was assessed by the neutral red (NR) assay. The culture plate was centrifuged at 300 × g for 5 min and supernatant aspirated. The cell pellet was suspended in NR solution (100 µl) and then incubated for 3 h at 37°C. Following incubation, the plate was centrifuged at 300 × g for 5 min and washed once with 200 µl of phosphate-buffered saline (PBS). NR de-stain solution (100 µl) was then added to each tube and left for at least 30 min on an orbital shaker until all of the pellet present had been dissolved and a homogeneous color was obtained in each tube. The absorbance of the sample was then measured at 540 nm using a Bio-Rad Model 450 microplate reader (Bio-Rad, Hertfordshire, UK). This method provides an objective quantitative measurement of cell viability and permits analyses of the relationship between cell survival and metal ion concentration under varying conditions.
Lymphocyte proliferation
Lymphocytes were cultured (0.1 × 106 cells/well) in 96-well round-bottom plates (TPP) with anti-CD3 (clone HIT3a; 0.1 µg/ml) ± soluble anti-CD28 (clone CD28.2; 0.1 µg/ml) (eBioscience) and incubated with 0.1, 1, 10, or 100 µM of Cr6+ or Co2+ in complete RPMI-1640 for 48 h at 37°C (under 5% CO2). T-lymphocyte proliferation was then determined using a non-isotopic BrdU Cell Proliferation Assay kit (Merck Chemicals, Nottingham, UK). Briefly, 10 µl of BrdU was added to the wells during the final 4 h of exposure. The cells were then fixed, permeabilized, and their DNA denatured using supplied fixative/denaturing solution. Detector anti-BrdU monoclonal antibody (100 µl) was then added and the samples incubated for 1 h. The wells were then rinsed with wash buffer and 100 µl horseradish peroxidase-conjugated goat anti-mouse antibody then added. Following 30 min incubation, the wells were washed with buffer before 100 µl tetramethylbenzidine was added and the samples incubated a final 15 min in the dark. Finally, 100 µl stop solution was added to each well and the absorbance in each well was then measured using a Thermo Scientific Multiskan Ascent spectrophotometer plate reader (Thermo Scientific, Hampshire, UK) at dual wavelengths of 450–590 nm.
Measurement of cytokine release using ELISA
Cytokine release from the lymphocytes was measured by collecting the supernatant following 48-h culturing in the presence of metal ions, as described above. The concentrations of interferon-γ (IFNγ) and interleukin-2 (IL-2) in the culture media were determined from aliquots of cell-free isolates using Ready-Set-Go ELISA kits (eBioscience) in accordance with the manufacturer’s instructions. Each of the kits had a sensitivity level of 4 pg/ml.
Flow cytometric analysis of apoptosis
At the end of each exposure period, lymphocytes were collected by centrifugation and washed twice with 100 µl fluorescence-activated cell sorting (FACS) buffer (1× PBS containing 2% FCS and 0.05% sodium azide). The cells were then resuspended in 100 µl 1x annexin binding buffer held in 12 × 75 mm polystyrene tubes (BD Bioscience, Oxford, UK). Aliquots (5 µl) of phycoerythrin annexin V and 7-aminoactinomycin D (both from BD Bioscience) were then added to each tube, the cells were vortexed, and the samples were then incubated at room temperature for 15 min in the dark. Thereafter, 200 µl of 1× annexin binding buffer and FACS flow were added to each tube and the samples placed into a FACSCanto flow cytometer (BD Bioscience) for analysis. Lymphocytes were identified by their characteristic forward and side-scatter patterns; a minimum of 20,000 events in the target area was recorded for each sample. All data were analyzed using FlowJo software (Treestar, Ashland, OR).
Statistics
Statistical analyses were carried out by a one-way analysis of variance, followed by a Dunnett’s multiple comparison test and a two-sample t-test. Significance was assigned where p values were found to be < 0.05.
Results
Effects of metal ions on cell viability
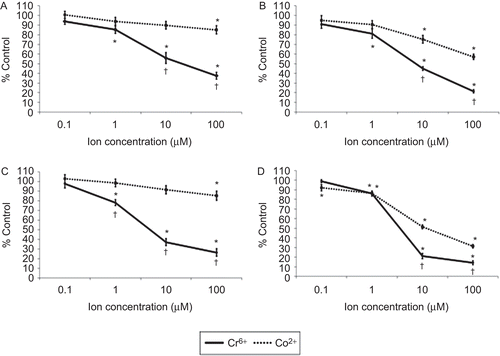
The viability of resting and anti-CD3-activated lymphocytes following exposure to Cr6+ or Co2+ for 24 and 48 h is shown in . The results show that 1, 10, and 100 µM Cr6+ caused a significant (p < 0.05) decrease in the viability of resting and anti-CD3-activated lymphocytes at both timepoints. No effect on cell viability was observed following exposure to 0.1 µM Cr6+. Exposure of resting lymphocytes to Co2+ ions induced a significant decrease in cell viability only at a concentration of 100 µM; this effect was seen at 24 and 48 h where cell viability was reduced to 85.10 (± 4.26) % and 85.28 (± 4.86) % (mean ± SE, n = 18), respectively. However, viability of activated lymphocytes at 24 h was decreased significantly after exposure to 10 and 100 µM Co2+, whereas at 48 h it was reduced after exposure to any of the concentrations of Co2+ ( and ). These results also show that Cr6+ caused a greater decrease in cell viability than Co2+.
Figure 1. Cell viability of lymphocytes following 24 (upper) and 48 (lower) h of exposure to varying concentrations of Cr6+ and Co2+ ions, as measured by neutral red (NR) assay. (A and C) Resting and (B and D) anti-CD3-activated lymphocytes. A value of 100% indicates unexposed control cells; results are means ± SE (n = 18). *Significantly different from control values (at p < 0.05) by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. †Significantly different from Co2+ values (at p < 0.05) by two-sample t-test.
Effects of metal ions on lymphocyte proliferation
The presence of Cr6+ ions significantly reduced anti-CD3-induced proliferation at all concentrations (). This reduction was substantial in lymphocytes exposed to 10 and 100 µM Cr6+ where the proliferation was < 20% of control values. In the presence of both Signals 1 and 2 (anti-CD3 + anti-CD28) stimuli, a substantial significant decrease in proliferation was observed at concentrations of 10 and 100 µMCr6+.
Figure 2. T-lymphocyte proliferation following anti-CD3 ± anti-CD28 activation and exposure to varying concentrations of Cr6+ and Co2+ ions for 48 h. A value of 100% indicates unexposed control cells; results are means ± SE (n = 12). *Significantly different from control values (at p < 0.05) by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test.
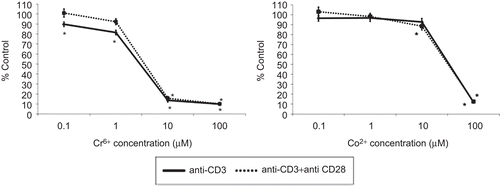
Exposure to 100 µM Co2+ significantly reduced Signal 1- and Signals 1 and 2-stimulated cell proliferation. Signals 1 and 2-induced proliferation was also slightly but significantly reduced to 88.56 (± 3.87) % (n = 12) following exposure to 10 µM Co2+. No other concentration of Co2+ significantly inhibited Signal 1- or Signals 1 and 2-stimulated cell proliferation. The results shown in indicate that metal ion exposure has a similar effect on proliferation of Signal 1- and Signals 1 and 2-activated lymphocytes. No significant differences were measured between Signal 1- and Signals 1 and 2-activated lymphocytes when comparing the effects of metal ions on proliferation.
Effects of metal ions on cytokine release
As shown in , metal ions significantly decreased IL-2 release from stimulated lymphocytes. Both Cr6+ and Co2+ion exposure significantly inhibited IL-2 production, even following exposure to the lowest concentrations of each. also indicates that Signals 1 and 2-stimulated lymphocyte production of IL-2 was more susceptible to inhibition by the metal ions than was Signal 1-stimulated production.
Figure 3. IL-2 release from anti-CD3 ± anti-CD28 activated lymphocytes exposed to varying concentrations of Cr6+ and Co2+ ions for 48 h. Results are means ± SE (n = 3). *Significantly different from control values (at p < 0.05) by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test.
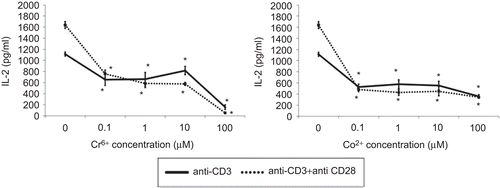
Figure 4. IFNγ release from anti-CD3 ± anti-CD28 activated lymphocytes exposed to varying concentrations of Cr6+ and Co2+ ions for 48 h. Results are means ± SE (n = 3). *Significantly different from control values (at p < 0.05) by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test.
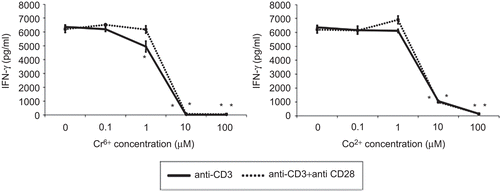
IFNγ production by Signal 1- and Signals 1 and 2-stimulated lymphocytes was significantly inhibited following exposure to 10 and 100 µM Cr6+ (). No significant difference was observed following exposure of stimulated lymphocytes to 0.1 or 1 µM Cr6+. Co2+ ions also inhibited IFNγ release by Signal 1- and Signals 1 and 2-stimulated lymphocytes. Significant inhibition was seen following exposure to concentrations of 10 and 100 µM. Neither Cr6+ nor Co2+ significantly inhibited IFNγ release from Signal 1- or Signals 1 and 2-stimulated lymphocytes at concentrations of 0.1 and 1 µM.
Flow cytometric analysis of apoptosis
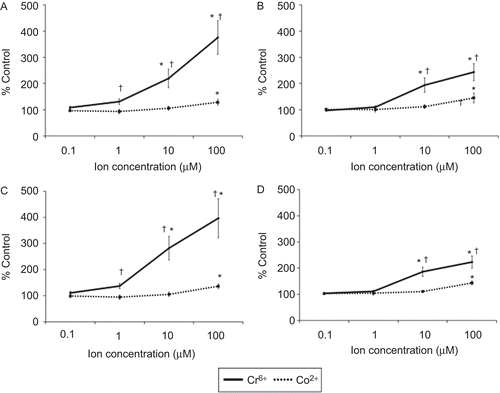
Exposure of resting lymphocytes to chromium ions led to a significant increase of total apoptosis (early plus late) at 10 and 100 µM ( and ). Resting lymphocytes exposed to 100 µM Co2+ show a significant increase in apoptosis at 24 and 48 h. Co2+ concentrations that were < 100 µM did not cause an increase in apoptosis compared to control values. Following anti-CD3 activation, lymphocytes exposed to 10 or 100 µM Cr6+ for 24 and 48 h underwent significantly more apoptosis than unexposed cells ( and ). Treatment of anti-CD3-activated lymphocytes with 100 µM Co2+ led to an increase of apoptosis to 145.42 (± 18.45) % at 24 h and 143.57 (± 7.08) % at 48 h. As with resting lymphocytes, exposure to lower concentrations of Co2+ ions did not lead to any significant increase in the proportion of apoptotic cells. Furthermore, the extent of necrotic cell death was small, even at the highest test concentration (as shown in top left quadrants of ). also illustrates how Cr6+ exposure led to increased levels of apoptosis (compared to those due to Co2+) in both resting and activated lymphocytes.
Figure 5. Resting and anti-CD3 activated lymphocytes stained with annexin V and 7-AAD following exposure to 100 µM Cr6+ and 100 µM Co2+ for 24 h. Viable cells were annexin V− and 7-AAD−. Cells in early stages of apoptosis were annexin V+ but 7-AAD−, whereas cells in late stages of apoptosis were both annexin V+ and 7-AAD+.
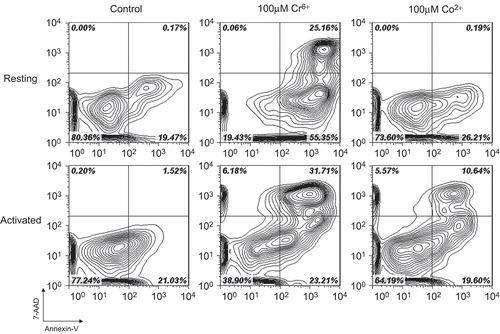
Figure 6. Total apoptosis in lymphocytes following 24 (upper) and 48 (lower) h of exposure to Cr6+ and Co2+. (A and C) Resting and (B and D) anti-CD3-activated lymphocytes. Results are mean (± SE; n = 6) proportion of lymphocytes expressing annexin V. A value of 100% indicates baseline apoptosis in unexposed control cells. *Significantly different from control values (at p < 0.05) by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. †Significantly different from Co2+ values (at p < 0.05) by two-sample t-test.
Effect of caspase-3 inhibitor pre-treatment on apoptosis
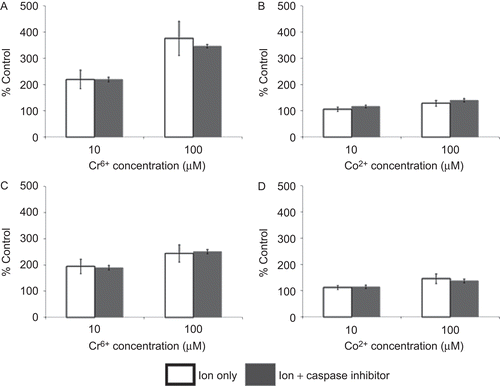
indicates that pre-treatment of lymphocytes with a caspase-3 inhibitor did not reduce the level of apoptosis following 24 h of exposure to 10 and 100 µM of Cr6+ or Co2+. Following pre-treatment of resting or anti-CD3-activated lymphocytes with the Z.Devd.FMK inhibitor, there was no significant difference in the level of metal ion–induced apoptosis when compared to that with cells that were not similarly pre-treated.
Figure 7. Total apoptosis in lymphocytes (pre-treated for 24 h with/without caspase-3 inhibitor (50 µM Z.Devd.FMK)) following 24 h of exposure to Cr6+ or Co2+. (A and C) Resting and (B and D) anti-CD3-activated lymphocytes. Results are mean (± SE; n ≥ 3) proportion of lymphocytes expressing annexin V. A value of 100% indicates baseline apoptosis in unexposed control cells.
Discussion
This study has shown that metal ion exposure decreases the viability of peripheral human lymphocytes. Cr6+ significantly decreased cell viability at concentrations of 1 and 10 µM. These results also indicate that Cr6+ reduces cell viability in resting and CD3-activated lymphocytes to a similar degree, when compared with the respective unexposed controls. The data also showed that Cr6+ exposure inhibited T-lymphocyte proliferation, especially at higher concentrations. However, the results show a significant increase in apoptosis at Cr6+ concentrations that also lead to a significant decrease in cell proliferation and viability. Therefore, it is likely that the cells are becoming apoptotic and unable to divide further rather than any direct impedance of cell activation and proliferation. Cr6+ ions have been shown to induce apoptosis in lung epithelial cells (Russo et al., Citation2005) and fibroblasts (Carlisle et al., Citation2000) as well as in lymphocytes (Vasant et al., Citation2003) and macrophages (Kwon et al., Citation2009). Cr6+ is known to stimulate a number of extrinsic and intrinsic apoptotic pathways (Sargeant and Goswami, Citation2007). Extrinsic pathways usually involve activation of caspases that are cytoplasmic pro-enzymes that initiate and amplify death signals or degrade cellular components (Pulido and Parrish, Citation2003). Intrinsic apoptosis can be initiated following excess reactive oxygen species (ROS) formation during intracellular reduction of Cr6+ (hexavalent chromium) to Cr3+ (trivalent chromium) and the excess ROS disrupt the mitochondrial membrane initiating events which induce apoptosis (Ye et al., Citation1999; Vasant et al., Citation2003; Sargeant and Goswami, Citation2007). This study has shown that the treatment of lymphocytes with a caspase-3 inhibitor does not inhibit the apoptosis induced following metal ion exposure. Although pre-treatment with a caspase-3 inhibitor has previously been shown to impede Cr6+-induced apoptosis in human lymphocytes (Vasant et al., Citation2003), we believe that altering a single pathway may not yield an inhibition in apoptosis due to an involvement of the multiple apoptotic pathways described previously.
The data from this study also indicate that Co2+ exposure may be more toxic to activated T-lymphocytes than to resting lymphocytes. The viability of proliferating lymphocytes is reduced to a greater extent than resting lymphocytes when compared with their respective unexposed control values. These results also indicate that the reduction in cell viability is likely to be due to an increase in apoptosis and/or reduced cell activation and proliferation. Although significant increases in apoptosis are only seen at the highest concentration, there is also a small increase following exposure to 10 µM. It is likely that the highest concentrations of Co2+ ion exposure induce apoptosis (Huk et al., Citation2004), thus inhibiting further T-lymphocyte proliferation. Like Cr6+, Co2+ ions have also been shown to cause apoptosis by initiating intrinsic and extrinsic pathways. However, the greater decrease in viability of activated lymphocytes (at 10 µM Co2+) possibly indicates that Co2+ may be acting through additional mechanisms, which also reduce cell proliferation, rather than simply initiating apoptosis.
The Co2+ ion is a known calcium channel antagonist (Reuter, Citation1983) and we hypothesise that it may compete with calcium (Ca2+) for binding sites associated with both Ca2+channels and intracellular Ca2+-binding proteins. Intracellular Ca2+ flux is essential in T-lymphocyte activation (Wülfing et al., Citation1997). For example, elevated Ca2+ levels result in calcineurin activation that, in turn, leads to increased dephosphorylation of NFAT (nuclear factor of activation of T-cells), a key process in T-lymphocyte activation and proliferation. It may be that Co2+ exposure can result in decreased T-lymphocyte proliferation via means similar to that noted with other Ca2+ channel antagonists (Birx et al., Citation1984; Marx et al., Citation1990). We suggest that this may explain the reduced viability and proliferation measured without the accompanying significant increase in apoptosis following exposure of activated lymphocytes to 10 µM Co2+. This possibly also explains the greater potency of Co2+ to cause loss of viability in activated T-lymphocytes compared with resting lymphocytes.
Although Co2+ exposure has been reported to cause cell cycle dysregulation (Glahn et al., Citation2008), we observed no evidence of this here, even using flow cytometric cell cycle analysis of CD3-activated lymphocytes exposed to Co2+ (data not shown). Therefore, Co2+ concentrations that are not directly cytotoxic to lymphocytes may affect events at a molecular level impeding lymphocyte activation and proliferation.
Interestingly, both metal ions inhibited T-lymphocyte proliferation in both Signal 1- and Signals 1 and 2-stimulated cells to a similar degree, when compared to their respective unexposed controls. T-lymphocyte activation initiated via Signal 1 and Signal 2 augments T-lymphocyte proliferation and survival. Costimulation leads to enhanced T-lymphocyte generation and survival by a number of mechanisms, including increased IL-2 production. IL-2 has previously been shown to be the primary T-lymphocyte growth factor and its production is key to their survival (Jenkins, Citation1994; Rüdiger et al., Citation2006). Reduced IL-2 release and availability can lead T-lymphocytes to become unresponsive and apoptotic (Boise et al., Citation1995b). Although the data show that metal ion exposure leads to decreased IL-2 release, the reduction began at concentrations that have a limited effect on lymphocyte viability and proliferation. As a result, it appears that this is not the mechanism by which these metal ions affect cell proliferation or induce apoptosis in lymphocytes. Interestingly, Wang et al. (Citation1996) previously showed that metal ion exposure of phytohemagglutinin-activated lymphocytes led to an IL-2-dependent decrease in cell proliferation. However, the data from and here suggest that IL-2 release was not the prime mechanism through which the Cr and Co ions reduced anti-CD3 (± CD28)-induced lymphocyte proliferation. Further, when the lymphocyte cultures (exposed to metal ions) were supplemented with 50 U recombinant IL-2/ ml, no recovery in cell proliferation was observed (data not shown). As a result, we believe that inhibition of IL-2 release was not directly involved in the mechanism by which these two metal ions affected proliferation or induced apoptosis in lymphocytes.
As well as enhanced IL-2 release, costimulation also upregulates expression of anti-apoptotic molecules and resists pro-apoptotic signaling. Previous research has demonstrated lymphocytes to have enhanced expression of the survival gene BCL-XL (long form of B-cell lymphoma X) following costimulation (Boise et al., Citation1995a; Noel et al., Citation1996). This upregulation correlates with protection from CD95-mediated apoptosis (Lenschow et al., Citation1996; Kerstan et al., Citation2006). The results of this study indicate that the anti-apoptotic benefits of costimulation provide little protection against the cytotoxic effects of these metal ions on lymphocytes. However, activation of T-lymphocytes also sensitizes cells to apoptosis by increasing expression of pro-apoptotic molecules (Brenner et al., Citation2008). For example, Co2+ exposure in vitro can lead to a downregulation of BCL-XL (Pulido and Parrish, Citation2003). The current authors speculate that exposure to these metal ions may affect the equilibrium between anti- and pro-apoptotic protein expression, possibly explaining the lack of apoptotic protection observed following costimulation.
IFNγ release from activated lymphocytes was also inhibited following the metal ion exposures. However, unlike for IL-2, only exposure to 10 and 100 µM of Cr6+ or Co2+ led to a reduction in IFNγ release. These are the same concentrations that substantially inhibited cell proliferation, reduced cell viability, and led to an increase in apoptosis. Therefore, it is likely that these ion concentrations induce activated lymphocytes to become apoptotic and non-viable such that the cells are unable to produce this key cytokine.
Although exposure of lymphocytes to high concentrations of these metal ions leads to increased apoptosis and reduced cytokine release, cell proliferation, and cell viability, the data indicate that Cr6+ is more toxic than Co2+. Here, when compared to unexposed control, Cr6+ reduced cell viability and increased apoptosis to a greater degree than Co2+. This is likely to be due to the difference in mode of toxicity between the ions. Specifically, Cr6+ enters cells and yields reactive intermediates as it is reduced to Cr3+. These intermediates are extremely toxic and, in combination with oxidative stress and damage, lead to a cascade of cellular events that activate the p53 apoptosis regulatory gene, causing the genotoxicity/carcinogenicity associated with Cr6+ (Sargeant and Goswami, Citation2007). Although Co2+ can inhibit DNA repair as well as induce mitochondrial permeability (Pulido and Parrish, Citation2003), it does not produce reactive intermediates; this may explain its inferior cytotoxicity potency.
In addition, exposure of lymphocytes, whether resting or anti-CD3 activated, to Cr6+ and Co2+ in combination (data not shown) does not lead to any additional cytotoxicity when compared to individual ion exposure. This indicates for these two ions, there is no synergistic effect on lymphocytes in vitro.
Conclusion
This study has shown that the metal ions, Cr6+ and Co2+, can induce apoptosis and thus inhibit T-lymphocyte expansion following Signal 1 and Signals 1 and 2 types of activation. This inhibition is seen at concentrations that have been measured in hip aspirate and local lymph nodes in patients with a MOM implant. Co2+ is less potent than Cr6+ and at lower levels does not induce apoptosis, but may still inhibit lymphocyte function. Whilst the precise mechanism of Co or Cr toxicity to lymphocytes was not elucidated, it is clear that it is likely to be multifactorial and that a singular intervention (i.e. treatment with a caspase-3 inhibitor) may not be sufficient to reverse the toxic effects of these metal ions. A number of further experiments must be conducted in order to fully explain the mechanism of metal ion toxicity in human lymphocytes. Also, the current findings show that IL-2 release is inhibited following exposure to these metal ions, even at the lowest concentrations. Although this does not appear to have an affect on T-lymphocyte proliferation in vitro, it still could result in disturbed immune regulation. This may contribute to the adverse effects, such as inflammatory masses, presented following MOM arthroplasty. In conclusion, this study shows that in vitro exposure of lymphocytes to the metal ions Cr6+ or Co2+, at concentrations that have previously been measured in patients with a MOM hip implant, have a significant negative effect on the adaptive immune system.
Acknowledgements
M.A. is recipient of a case award from the EPSRC and DePuy International Ltd.
Declaration of interest
The Authors report no conflicts of interest. The Authors are alone responsible for the content and writing of the paper.
References
- Afolaranmi, G. A., Tettey, J., Meek, R. M. and Grant, M. H. 2008. Release of chromium from orthopaedic arthroplasties. Open Orthop. J. 2:10–18.
- Antoniou, J., Zukor, D. J., Mwale, F., Minarik, W., Petit, A. and Huk, O. L. 2008. Metal ion levels in the blood of patients after hip resurfacing: A comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J Bone Joint Surg. Am. 90 Suppl 3:142–148.
- Back, D. L., Young, D. A. and Shimmin, A. J. 2005. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin.Orthop. Relat. Res. 438:177–181.
- Birx, D. L., Berger, M. and Fleisher, T. A. 1984. The interference of T-cell activation by calcium channel blocking agents. J. Immunol. 133:2904–2909.
- Boardman, D. R., Middleton, F. R. and Kavanagh, T. G. 2006. A benign psoas mass following metal-on-metal resurfacing of the hip. J. Bone Joint Surg. Br. 88:402–404.
- Boise, L. H., Minn, A. J., Noel, P. J., June, C. H., Accavitti, M. A., Lindsten, T. and Thompson, C. B. 1995a. CD28 costimulation can promote T-cell survival by enhancing the expression of Bcl-XL. Immunity 3:87–98.
- Boise, L. H., Noel, P. J. and Thompson, C. B. 1995b. CD28 and apoptosis. Curr. Opin. Immunol. 7:620–625.
- Brenner, D., Krammer, P. H. and Arnold, R. 2008. Concepts of activated T-cell death. Crit. Rev. Oncol. Hematol. 66:52–64.
- Carlisle, D. L., Pritchard, D. E., Singh, J., Owens, B. M., Blankenship, L. J., Orenstein, J. M. and Patierno, S. R. 2000. Apoptosis and p53 induction in human lung fibroblasts exposed to chromium (VI): Effect of ascorbate and tocopherol. Toxicol. Sci. 55:60–68.
- Case, C. P. 2001. Chromosomal changes after surgery for joint replacement. J. Bone Joint Surg. Br. 83:1093–1095.
- Case, C. P., Langkamer, V. G., James, C., Palmer, M. R., Kemp, A. J., Heap, P. F. and Solomon, L. 1994. Widespread dissemination of metal debris from implants. J. Bone Joint Surg. Br. 76:701–712.
- Counsell, A., Heasley, R., Arumilli, B. and Paul, A. 2008. A groin mass caused by metal particle debris after hip resurfacing. Acta. Orthop. Belg. 74:870–874.
- Daniel, J., Ziaee, H., Pradhan, C., Pynsent, P. B. and McMinn, D. J. 2007. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: Four-year results of a prospective longitudinal study. J. Bone Joint Surg. Br. 89:169–173.
- Davies, A. P., Willert, H. G., Campbell, P. A., Learmonth, I. D. and Case, C. P. 2005. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J. Bone Joint Surg. Am. 87:18–27.
- De Smet, K., De Haan, R., Calistri, A., Campbell, P. A., Ebramzadeh, E., Pattyn, C. and Gill, H. S. 2008. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J. Bone Joint Surg. Am. 90 Suppl 4:202–208.
- Glahn, F., Schmidt-Heck, W., Zellmer, S., Guthke, R., Wiese, J., Golka, K., Hergenröder, R., Degen, G. H., Lehmann, T., Hermes, M., Schormann, W., Brulport, M., Bauer, A., Bedawy, E., Gebhardt, R., Hengstler, J. G. and Foth, H. 2008. Cadmium, cobalt and lead cause stress response, cell cycle deregulation and increased steroid as well as xenobiotic metabolism in primary normal human bronchial epithelial cells which is coordinated by at least nine transcription factors. Arch. Toxicol. 82:513–524.
- Goodman, S. B. 2007. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 28:5044–5048.
- Grammatopolous, G., Pandit, H., Kwon, Y. M., Gundle, R., McLardy-Smith, P., Beard, D. J., Murray, D. W. and Gill, H. S. 2009. Hip resurfacings revised for inflammatory pseudo-tumour have a poor outcome. J. Bone Joint Surg. Br. 91:1019–1024.
- Hallab, N. J., Caicedo, M., Finnegan, A. and Jacobs, J. J. 2008. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J. Orthop. Surg. Res. 3:6.
- Hart, A. J., Hester, T., Sinclair, K., Powell, J. J., Goodship, A. E., Pele, L., Fersht, N. L. and Skinner, J. 2006. The association between metal ions from hip resurfacing and reduced T-cell counts. J. Bone Joint Surg. Br. 88:449–454.
- Hart, A. J., Skinner, J. A., Winship, P., Faria, N., Kulinskaya, E., Webster, D., Muirhead-Allwood, S., Aldam, C. H., Anwar, H. and Powell, J. J. 2009. Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8+ T-cell lymphopenia. J. Bone Joint Surg. Br. 91:835–842.
- Huk, O. L., Catelas, I., Mwale, F., Antoniou, J., Zukor, D. J. and Petit, A. 2004. Induction of apoptosis and necrosis by metal ions in vitro. J. Arthroplasty. 19:84–87.
- Jenkins, M. K. 1994. The ups and downs of T-cell costimulation. Immunity 1:443–446.
- Kerstan, A., Armbruster, N., Leverkus, M. and Hünig, T. 2006. Cyclosporin A abolishes CD28-mediated resistance to CD95-induced apoptosis via superinduction of caspase-3. J. Immunol. 177:7689–7697.
- Kwon, Y. M., Xia, Z., Glyn-Jones, S., Beard, D., Gill, H. S. and Murray, D. W. 2009. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed. Mater. 4:025018.
- Ladon, D., Doherty, A., Newson, R., Turner, J., Bhamra, M. and Case, C. P. 2004. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J. Arthroplasty. 19:78–83.
- Langkamer, V. G., Case, C. P., Heap, P., Taylor, A., Collins, C., Pearse, M. and Solomon, L. 1992. Systemic distribution of wear debris after hip replacement. A cause for concern? J. Bone Joint Surg. Br. 74:831–839.
- Langton, D. J., Sprowson, A. P., Joyce, T. J., Reed, M., Carluke, I., Partington, P. and Nargol, A. V. 2009. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J. Bone Joint Surg. Br. 91:1287–1295.
- Lenschow, D. J., Walunas, T. L. and Bluestone, J. A. 1996. CD28/B7 system of T-cell co-stimulation. Annu. Rev. Immunol. 14:233–258.
- Martín-Romero, C., Santos-Alvarez, J., Goberna, R. and Sánchez-Margalet, V. 2000. Human leptin enhances activation and proliferation of human circulating T-lymphocytes. Cell. Immunol. 199:15–24.
- Marx, M., Weber, M., Merkel, F., Meyer zum Büschenfelde, K. H. and Köhler, H. 1990. Additive effects of calcium antagonists on cyclosporin A-induced inhibition of T-cell proliferation. Nephrol. Dial. Transplant. 5:1038–1044.
- Merritt, K. and Brown, S. A. 1995. Release of hexavalent chromium from corrosion of stainless steel and cobalt-chromium alloys. J. Biomed. Mater. Res. 29:627–633.
- Noel, P. J., Boise, L. H., Green, J. M. and Thompson, C. B. 1996. CD28 costimulation prevents cell death during primary T-cell activation. J. Immunol. 157:636–642.
- Pandit, H., Glyn-Jones, S., McLardy-Smith, P., Gundle, R., Whitwell, D., Gibbons, C. L., Ostlere, S., Athanasou, N., Gill, H. S. and Murray, D. W. 2008. Pseudo-tumours associated with metal-on-metal hip resurfacings. J. Bone Joint Surg. Br. 90:847–851.
- Pulido, M. D. and Parrish, A. R. 2003. Metal-induced apoptosis: mechanisms. Mutat. Res. 533:227–241.
- Reuter, H. 1983. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature 301:569–574.
- Rüdiger, A., Dirk, B., Mareike, B., Christian, R. F. and Peter, H. K. 2006. How T-lymphocytes switch between life and death. Eur. J. Immunol. 36:1654–1658.
- Russo, P., Catassi, A., Cesario, A., Imperatori, A., Rotolo, N., Fini, M., Granone, P. and Dominioni, L. 2005. Molecular mechanisms of hexavalent chromium-induced apoptosis in human bronchoalveolar cells. Am. J. Respir. Cell Mol. Biol. 33:589–600.
- Sargeant, A. and Goswami, T. 2007. Hip implants. Paper VI - Ion concentrations. Mat. Design. 28:155–171.
- Savarino, L., Granchi, D., Ciapetti, G., Stea, S., Donati, M. E., Zinghi, G., Fontanesi, G., Rotini, R. and Montanaro, L. 1999. Effects of metal ions on white blood cells of patients with failed total joint arthroplasties. J. Biomed. Mater. Res. 47:543–550.
- Shettlemore, M. G. and Bundy, K. J. 1999. Toxicity measurement of orthopedic implant alloy degradation products using a bioluminescent bacterial assay. J Biomed. Mater. Res. 45:395–403.
- Vasant, C., Rajaram, R. and Ramasami, T. 2003. Apoptosis of lymphocytes induced by chromium(VI/V) is through ROS-mediated activation of Src-family kinases and caspase-3. Free Radic. Biol. Med. 35:1082–1100.
- Vendittoli, P. A., Mottard, S., Roy, A. G., Dupont, C. and Lavigne, M. 2007. Chromium and cobalt ion release following the Durom high carbon content, forged metal-on-metal surface replacement of the hip. J. Bone Joint Surg. Br. 89:441–448.
- Wang, J. Y., Tsukayama, D. T., Wicklund, B. H. and Gustilo, R. B. 1996. Inhibition of T- and B-cell proliferation by titanium, cobalt, and chromium: Role of IL-2 and IL-6. J. Biomed. Mater. Res. 32:655–661.
- Willert, H. G., Buchhorn, G. H., Fayyazi, A., Flury, R., Windler, M., Köster, G. and Lohmann, C. H. 2005. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J. Bone Joint Surg. Am. 87:28–36.
- Witzleb, W. C., Ziegler, J., Krummenauer, F., Neumeister, V. and Guenther, K. P. 2006. Exposure to chromium, cobalt and molybdenum from metal-on-metal total hip replacement and hip resurfacing arthroplasty. Acta. Orthop. 77:697–705.
- Wülfing, C., Rabinowitz, J. D., Beeson, C., Sjaastad, M. D., McConnell, H. M. and Davis, M. M. 1997. Kinetics and extent of T-cell activation as measured with the calcium signal. J. Exp. Med. 185:1815–1825.
- Ye, J., Wang, S., Leonard, S. S., Sun, Y., Butterworth, L., Antonini, J., Ding, M., Rojanasakul, Y., Vallyathan, V., Castranova, V. and Shi, X. 1999. Role of reactive oxygen species and p53 in chromium(VI)-induced apoptosis. J. Biol. Chem. 274:34974–34980.
