Abstract
There is growing concern that exposure to air pollutants during pregnancy affects health outcomes in the offspring due to alterations in the development of immune and other homeostatic processes. To assess the risks of maternal inhalation exposure to ozone (O3), timed pregnant BALB/c mice were exposed to different concentrations of O3 (0, 0.4, 0.8, and 1.2 ppm) for 4 h/day for 10 days during gestation (GD9–GD18), and pulmonary inflammation and immune responses were assessed in the offspring at 6 weeks-of-age. Maternal O3 exposure reduced the number of productive dams by 25% at the highest O3 concentration (1.2 ppm) and decreased the rate of weight gain in the offspring. Delayed-type hypersensitivity responses to bovine serum albumin were suppressed in the female offspring by maternal exposure to the two highest concentrations of O3, whereas humoral immune responses to sheep red blood cells were not altered in either sex. Maternal exposure to 1.2 ppm O3 increased lactate dehydrogenase (LDH) activity in bronchoalveolar lavage fluid (BALF) of the offspring but did not affect the number of inflammatory cells or levels of total protein, IFN-γ, IL-17, and IL-4 cytokines in BALF, or CD4+, CD8+, CD25+, and TCRβ+CD1d+ T-cells in the spleen. Offspring born from air-exposed dams sensitized early in life (postnatal day [PND] 3) to ovalbumin (OVA) antigen and then challenged as adults developed eosinophilia, elevated levels of LDH activity and total protein in BALF, and increased pulmonary responsiveness to methacholine, compared with animals sensitized at PND42. Maternal O3 exposure in the 1.2 ppm O3 group decreased BALF eosinophilia and serum OVA-specific IgE in the female offspring sensitized early in life but did not affect development of allergic airway inflammation by offspring sensitized late in life. In summary, maternal exposure to O3 affected reproductive outcome and produced modest decreases in immune function and indicators of allergic lung disease in surviving offspring.
Introduction
Exposure to air pollution is a worldwide public health issue. In addition to direct effects in both healthy and susceptible individuals, there is increasing concern that prenatal exposure might affect the well-being of the offspring at various stages of development. Epidemiological studies have found air pollution exposure to be associated with numerous developmental health effects ranging from increased infant mortality to low birth weight, reduced ventilatory lung function, and increased incidence of diseases such as asthma (reviewed in Srám et al., Citation2005; Heinrich and Slama, Citation2007; Clark et al., Citation2010). Despite the strong evidence that extreme exposures may cause these effects, it has often been more difficult to quantify the impact of ambient concentrations of air pollution compared with other socioeconomic and environmental influences (Woodruff et al., Citation2009).
The incidence of allergic asthma has risen over the last four decades (Institute of Medicine, Citation2000; Anandan et al., Citation2010) and has been linked to increased urbanization and exposure to airborne pollutants (Delfino et al., Citation1996; Hwang et al., Citation2005). Specifically, asthma rates have been associated with residential highway proximity (Lwebuga-Mukasa et al., Citation2004) and average ozone exposure concentrations (Moore et al., Citation2008). For some time now it has been appreciated that ambient pollutants such as ozone and diesel exhaust (DE) particles can act as adjuvants to enhance allergic sensitization. Early studies by Matsumura (Citation1970) and Osebold et al. (Citation1980) showed that ozone exposure enhanced allergic type immune responses in guinea pigs and mice. Later experiments in mice showed that inhalation of fresh DE or intrapulmonary instillation of DE particles (DEP) caused adjuvant activity resulting in increased sensitization to experimental allergens (Muranaka et al., Citation1986; Takano et al., Citation1998; Takahashi et al., Citation2010), although similar effects were also shown in humans (Diaz-Sanchez et al., Citation1994; Zhang et al., Citation2009). In terms of exposures during pregnancy, it is well-established that in utero exposure to cigarette smoke can have profound effects on reproductive health, childhood obesity, type 2 diabetes, allergic airway symptoms, and cancer (Doherty et al., Citation2009; Tsai et al., Citation2010). It is also clear from numerous case-controlled studies that maternal smoking either during gestation or postpartum is a significant risk factor for developing allergic asthma in children (Weitzman et al., Citation1990; Raherison et al., Citation2007).
Although it is difficult to discern developmental effects of any single air pollutant from complex mixtures in exposed populations, controlled experiments in animals are able to provide dose–response relationships for both individual components and complex mixtures. Early studies on prenatal ozone exposure in rats reported that the number of resorbed fetuses was increased with exposure to 1.26 ppm O3 during mid-gestation (Kavlock et al., Citation1979), and the surviving offspring of dams exposed to 1–1.5 ppm O3 during early or mid-gestation had reduced body weight gain (Kavlock et al., Citation1980). A later report examining pregnant CD-1 mice exposed to 0, 0.4, 0.8, and 1.2 ppm O3 did not find changes in litter size, sex ratio, frequency of stillbirth, and neonatal mortality but did report decreased body weight gain in offspring exposed to the highest concentration (Bignami et al., Citation1994). In addition to altered rate of gain, a diverse range of effects have been reported in the fetal lungs exposed in utero from embryonic days (E)18–21 including edematous mitochondria with disrupted cristae, and damaged membranes and vacuolization in epithelial cell cytoplasm (López et al., Citation2008). Furthermore, decreased levels of cerebellar monoamines in neurotransmitter systems have been reported at postnatal day (PND) 10 following maternal exposure to O3 (Gonzalez-Pina et al., Citation2008).
Maternal exposure to a variety of air pollutants has been shown to up-regulate allergic immune responses and indicators of lung diseases in experimental models. Inhalation exposure to environmental or side stream tobacco smoke enhanced the offspring’s immune responses to allergen (Penn et al., Citation2007) and increased lung resistance (Wu et al., Citation2009). Studies examining prenatal and neonatal inhalation exposures to DE in rats have also shown increases in allergen-specific IgE responses in adults (Watanabe and Ohsawa, Citation2002). Aspiration studies of DEP as well as other more inert materials including titanium dioxide and carbon black at GD14 resulted in enhanced allergic airway inflammation during the preweaning period in murine offspring (Fedulov et al., Citation2008), although ambient particulate matter aspiration in pregnant C57BL6 dams increased airway hyperreactivity and inflammatory cytokines in the offspring postnatally exposed to 1 ppm O3 (Auten et al., Citation2009). Due to the aforementioned studies, we hypothesized that maternal exposure to ozone could alter immune function of the offspring to increase development of allergic airway disease. The reproductive outcome was first assessed in mice exposed to ozone from mid-to-late gestation. Next, some standard immune parameters and immunotoxicological testing was performed in the offspring. We also assessed the development of allergic lung disease in the offspring sensitized either as neonates or at 6 weeks of age.
Materials and methods
Animals
Timed-pregnant BALB/c mice (10-12-weeks-of-age) were purchased from Charles River Laboratories (Raleigh, NC) and delivered to the animal facility on gestational day (GD) 3. Mice were housed individually in polycarbonate cages with hardwood chip bedding (NEPCO, Warrensburg, NY) maintained at 22.3 ± 1°C and 50 ± 10% humidity in filtered animal housing racks and provided access to mouse chow (5POO Prolab RMH3000, PMI Nutrition International, Richmond, IN) and water ad libitum. All procedures employed in this study were approved in advance by the Institutional Animal Care and Use Committee of the National Health and Environmental Effects Research Laboratory, US EPA.
Generation of O3 and exposures
Ozone was generated from oxygen by a silent arc discharge generator (OREC, Phoenix, AZ), and its entry into the Rochester style “Hinners” chambers was controlled by a mass flow controller. The O3 concentrations in the chambers were monitored by API Model 400 photometric O3 analyzers. Timed pregnant mice were randomly divided into four groups of 20 each. Pregnant animals were placed in individual stainless steel wire exposure cages and were exposed to HEPA-filtered room air or 0.4, 0.8, or 1.2 ppm of O3 for 4 h/day for 10 consecutive days, from GD9 to GD18. The entire experiment was repeated two additional times to provide enough mice of each gender for the various postnatal tests. In summary, the reproductive outcome and effect on induction of allergic lung disease was measured in all experiments. Pulmonary inflammatory parameters in the offspring were measured in Experiments I and III, and the effect of O3 on antibody titers and delayed-type hypersensitivity (DTH) responses in the offspring was assessed in Experiment I.
Evaluation of reproductive success
The percentage of dams delivering litters was calculated by dividing the number of the dams giving birth by the total number of the dams per group for each experiment. Mice gave birth between GD20 and GD21. Litter size was expressed as the average number of live offspring per treatment group. The offspring were weaned on PND21. Each dam’s litter was weighed together at PND1, PND3, PND7, PND14, and PND21, and the average pup weight was calculated by dividing the weight by total litter number. The offspring were individually weighed before euthanasia at PND42. One day after expected parturition, the remaining females that had not given birth were euthanized and their uteri were visually inspected for size, thickness, blood supply, resorption sites, and presence of dead embryos.
Delayed-type hypersensitivity assessment
The DTH response was assessed as described by Rooney et al. (Citation2003). In brief, purified bovine serum albumin (BSA) (Fraction V; Sigma, St. Louis, MO) at a concentration of 2 mg/mL in sterile normal saline was emulsified in Freund’s complete adjuvant (CFA; Difco, Detroit, MI) at a 1:1 ratio. Six-week-old male and female offspring were anesthetized with isoflurane (VetEquip, Inc., Pleasanton, CA) and sensitized with 0.05 mL BSA–CFA by subcutaneous injection into the caudal tail-fold. Seven days later, animals were re-anesthetized and challenged by injecting 0.05 mL of heat-aggregated BSA into the right rear footpad. The left rear footpad was injected with the same volume of normal saline as a control. Twenty-four hours after challenge, footpad thickness was determined by triplicate measurements in anesthetized animals with an electronic caliper (model shop at the US EPA, Durham, NC). The degree of edema, calculated by the difference between saline- and BSA-injected footpad thicknesses, was then used to assess the degree of DTH responses.
Sheep red blood cell-specific antibody responses
Six-week-old male and female offspring were intravenously injected in the tail with 2 × 107 sheep red blood cell (SRBC; Rockland Laboratories, Gilbertsville, PA) in 0.2 mL of sterile normal saline and blood was collected 5 days later from the facial vein for assessment of SRBC-specific IgM in the serum. An intravenous booster immunization with the same number of SRBC was given to all mice 2 weeks after the primary immunization, and blood was collected 5 days after the secondary immunization for determination of IgG. Relative serum titers of IgM and IgG antibodies were measured by enzyme-linked immunosorbent assay (ELISA). Absorbance was read and processed on a SpectraMax 250-plate reader at 410 nm with SOFTmax PRO software (Molecular Devices Inc., Sunnyvale, CA) as log2 antibody titers from the log–log curve of optical density versus dilution. All ELISA procedures were optimized for differences in SRBC membrane preparations, and standards were assessed as described by DeWitt et al. (Citation2005).
Toxicity assessment and allergen sensitization
Groups of male and female mice (n = 3–6 of each sex/ exposure concentration) were euthanized on PND42 for assessment of immune and inflammatory parameters including protein, inflammatory cells, and T-cell populations in the spleen. Additional groups of male and female mice (n = 5–8 of each sex/exposure concentration) were separated for immunotoxicity testing. Further groups of male and female mice (n = 3–6 of each sex/exposure concentration) were lightly anesthetized with isoflurane and intranasally instilled with chicken ovalbumin (OVA, Fraction V; Sigma) in normal saline (Hospira Inc., Lake Forest, IL) on a weight-to-weight basis (5 µg/g) or normal saline alone (5 or 50 µL) for 2 days either on PND2 and PND3, or on PND42 and PND43 (). This procedure was then repeated at challenge with 10 µg of OVA in 40 µL normal saline or normal saline on PND54, PND55, and PND56. One day after the final OVA challenge, pulmonary function, immune responses, and lung inflammation were assessed.
Figure 1. Experimental regime. Timed-pregnant BALB/c dams were exposed to either O3 or air from gestational day (GD) 9 to GD18 for 4 h/day. The offspring were assessed at postnatal day (PND) 42 before sensitization, or following sensitization with ovalbumin (OVA) at (A) PND2 and PND3, or at (B) PND42 and PND43 with both groups challenged at PND54, PND55, and PND56. Bovine serum albumin (BSA) or sheep red blood cell (SRBC) were injected into the separate groups of the offspring at PND42 to evaluate systemic responses.
Bronchoalveolar lavage cytology
Following euthanasia and cardiac puncture for blood collection, the chest wall was opened and the left main stem bronchus was isolated, clamped, and the trachea cannulated. The lung left lobe was excised and frozen at −70°C. Bronchoalveolar lavage fluid (BALF) from the right lung lobes was collected using 3 × 0.6 mL volumes of warmed Hank’s balanced salt solution (HBSS) and centrifuged at 1200 rpm for 5 min. Total cells were counted using a Coulter counter (Coulter Corp., Miami, FL). Differential cell counts were performed on 200 stained (Wright-Giemsa; Fisher Scientific LLC, Kalamazoo, MI) cells prepared on glass slides using a Cytospin centrifuge (Shandon Inc., Pittsburgh, PA). Total cell counts obtained by the Coulter counter were used to calculate the total cell count for each cell type.
Biochemical assays
BALF was analyzed for lactate dehydrogenase (LDH) activity and total protein to detect lung injury and edema, respectively, using a commercially prepared kit and controls and Pierce Commassie Plus Protein Assay Reagent (Sigma). The assays were modified for use on the KONELAB 30 clinical chemistry Spectrophotometer analyzer (Thermo Clinical Labsystems, Espoo, Finland).
Flow cytometry analysis
Single cell suspensions of the spleen from each animal were prepared by pressing the tissue through a 70-µm cell strainer in 1% FCS–HBSS buffer. Red blood cells (RBC) were lysed with 2 mL of 1X RBC lysis solution (10X solution contained 42 g ammonium chloride, 5 g potassium bicarbonate, and 0.185 g EDTA in 500 mL distilled water). Cells were incubated on ice with labeled antibodies to CD45 PE-cy7, CD25 PE, CD4 APC, CD3 FITC, CD8 PE, TCRβ APC, and their appropriate isotype controls (BD Pharmingen, San Diego, CA) and CD1d PE tetramer PBS57 loaded and unloaded as control (MHC tetramer Core Facility, NIAID, Atlanta, GA) in PBS containing 1% BSA/0.1% sodium azide for 30 min. A total of 10,000 events were collected using a Becton Dickenson model LSR-II flow cytometer and the data were analyzed using CellQuest (Becton Dickinson Co., San Jose, CA) or FlowJo software (Tree Star Inc., San Carlos, CA). CD45+ cells were further gated for CD3+CD4+ or CD3+CD8+ subpopulations. The populations of CD3+CD25+, CD4+CD25+, and TCRβ+CD1d+ in the spleen were also quantified.
Cytokine quantitation
The concentrations of interleukin (IL)-4, interferon (IFN)-γ, and IL-17 in BALF were measured with ELISA using cytoset kits (Biosource, Carlsbad, CA). In brief, 96-well plates were coated with appropriate antibodies and then blocked with blocking buffer. Standard antibodies and samples were added and washed. Plates were incubated with appropriate biotinylated antibodies and visualized with horseradish peroxidase (HRP)–streptavidin and tetramethylbenzene (TMB) substrate. The result was read at 650 nm using a SpectraMax reader and SOFTmax PRO software (Molecular Devices Inc.). The sensitivity of detection was 15.6 pg/mL for IL-4 and IFN-γ and 7 pg/mL for IL-17.
Pulmonary responsiveness to methacholine
One day after the last OVA challenge in allergic offspring and at PND42 in non-allergic offspring, pulmonary function changes to aerosolized methacholine (MCh; Sigma) in un-anesthetized and un-restrained mice were assessed in a 12-chamber whole-body plethysmography system (Buxco Electronics, Sharon, CT). After measuring baseline parameters for 10 min, an aerosol of saline or MCh in increasing concentrations (6–25 mg/mL) was nebulized through an inlet of the chamber. Airflow was 6 L/min. For each animal, the data were expressed as percentage of enhanced pause (Penh) over baseline values.
Antigen-specific IgE antibody
Microtiter wells were coated with 10 µg/mL OVA in carbonate buffer (pH 9.6). Non-specific binding was blocked with 1% PBS buffer containing 0.05% Tween-20 and 2% BSA. Sera were applied neat, and purified monoclonal mouse anti-OVA IgE antibody (Serotec, Kidlington, Oxford) was used as standard. The antigen–antibody complex was detected with rat anti-mouse biotinylated IgE antibody (PharMingen, San Diego, CA). The binding was visualized using streptavidin–HRP (Zymed, San Francisco, CA) and TMB substrate (Dako Co., Carpinteria, CA). The plates were read on a Thermomax® Plate Reader (Molecular Devices Co., Menlo Park, CA) after 20 min at a wavelength of 650 nm and analyzed using SOFTmax PRO® version 5.2 (Molecular Devices Inc.).
Statistical analysis
The percentage of successful pregnancies was analyzed for differences among the exposure groups using a Fisher’s exact test. Single measurement responses were analyzed using a two- or multiple-way analysis of variance (ANOVA) model. The factors were gender and exposure, and when appropriate, sensitization time. Pairwise comparisons were performed as subtests of the overall ANOVA for the individual exposure experiments and then on the pooled data from the three experiments when appropriate. This decision was made after a statistical test of compatibility of exposure responses. If the ANOVA requirements of normality and homoskedasticity were not satisfied, the data were transformed. Subsequent to the transformation, the data were checked for requirement compliance and if acceptable, the analysis proceeded. If, however, the transformed data failed to meet the requirements, a distribution-free method of analysis was employed. Data from repeated measurements of the same statistical unit were analyzed in two steps. First, a multivariate repeated measures analysis was performed to establish the existence of differences among the various treatment and gender combinations. Second, and subsequent to a significant finding, pairwise comparisons were made as subtests of the overall multivariate analysis of variance for repeated measures. The level of significance was set at 0.05. No adjustments were made for multiple testing, as this controls the Type II error rate. We recognized that this will tend to increase the Type I error rate. The statistics were generated using SAS statistical software (SAS V9.2, SAS Institute, Cary, NC).
Results
The effects of O3 inhalation on reproductive outcome and body weight of the offspring
The reproductive statistics for the control (air-exposed) animals were consistent with information from accredited breeders that report that BALB/c mice have 44.4% non-productive matings (http://phenome.jax.org) and that the average first litter size is 5 (http://www.criver.com/SiteCollectionDocuments/rm_rm_n_techbul_1982.pdf). The average percentage of successful pregnancies for the three experiments was 58% in the air exposure group, 45% (P > 0.05) in the 0.4 and 0.8 ppm exposure groups, and 33% (P < 0.005) in the 1.2 ppm O3 group. The litter size and sex ratios for each exposure group were not different than those of the air animals (). Each litter was weighed together at select timepoints until PND21 and then individual offspring were weighed up to PND42. Offspring born to the 1.2 ppm O3 exposed dams weighed less than the air exposure group. This was significant 1 day after birth, as well as on PND3 and PND7 with the effect persisting in the males at PND42 (). The changes in birth weight were not due to altered gender ratio or litter size in any of the groups.
Table 1. Effect of maternal exposure to ozone on reproductive outcomes.
Table 2. Effect of maternal exposure to ozone on rate of weight gain in the offspring.
Inflammation in non-allergic offspring following maternal O3 exposure
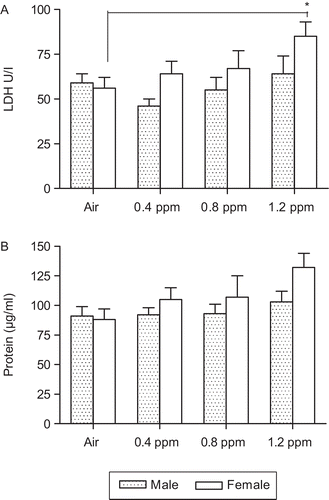
We measured a number of parameters to assess inflammatory processes in non-allergic offspring from Experiments I and III. No differences were observed between groups in the number of macrophages, lymphocytes, neutrophils, and eosinophils in BALF or in pulmonary responsiveness to MCh (data not shown) at PND42. There were no differences in BALF immunomodulatory cytokines including IFN-γ, IL-17, and IL-4, and the percentages of splenic CD45+CD3+CD4+, CD45+CD3+CD8+, CD3+CD25+, and CD4+CD25+ T-cells and TCRβ+CD1d+ invariant natural killer T (iNKT) cell populations across gender or exposure groups (). The level of LDH activity in BALF was significantly elevated in females in the 1.2 ppm O3 group (). Protein levels also appeared to be increased in this group, although the value did not reach statistical significance ().
Figure 2. Splenic T-cell populations and bronchoalveolar lavage fluid (BALF) cytokines in non-allergic offspring born from dams exposed to O3. A representative data of a total 10,000 events of the spleen cells screened by FACS with appropriate antibodies for (A) CD4+ T and (B) iNKT cells, and (C) IFN-γ and (D) IL-17 measured by enzyme-linked immunosorbent assay (ELISA) in BALF at postnatal day (PND) 42 has been shown. Data are the mean (±SEM) of 3–6 offspring for each sex per group in Experiments I (n = 3) and III (n = 6).
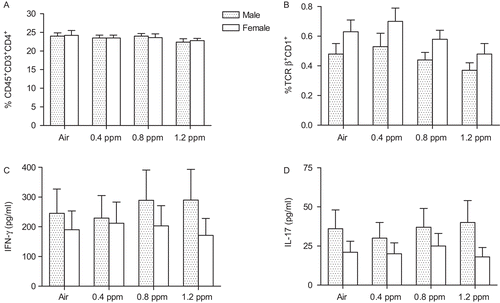
Figure 3. Inflammatory responses in non-allergic offspring born from dams exposed to O3. Bronchoalveolar lavage fluid (BALF) was assessed for (A) lactate dehydrogenase (LDH) and (B) protein at postnatal day (PND) 42. Data are the mean (±SEM) of 3–7 offspring for each sex per group in Experiments I (n = 3–6) and III (n = 6–7). *P < 0.05; significantly different from air group.
Effect of maternal O3 inhalation on immune function
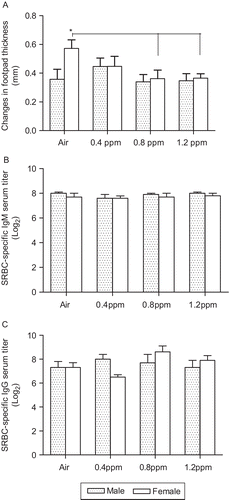
In order to determine the effects of O3 inhalation exposure on systemic immunotoxicity, we assessed DTH responses and SRBC-specific IgM and IgG titers in the offspring in Experiment I. Footpad swelling (DTH reactivity) in the females was higher than males (P < 0.05), and this was significantly reduced (P < 0.05) in the female offspring with the 0.8 and 1.2 ppm O3 exposure concentrations (). After the challenge with BSA, three mice died in the air group, one each died in the 0.4 and 0.8 ppm groups, and no mice died in the 1.2 ppm O3 group. All offspring produced similar titers of IgM () and IgG antibodies () following a booster injection of SRBC regardless of sex or treatment.
Figure 4. Systemic immunotoxicity in the offspring born from dams exposed to O3. (A) DTH was determined by difference in thickness between saline- and bovine serum albumin (BSA)-injected footpads. Data are the mean (±SEM) of 5–6 offspring for each sex in a group, in Experiment I only. *P < 0.05, significantly different from air group. Enzyme-linked immunosorbent assay (ELISA) was used to determine the levels of sheep red blood cell (SRBC)-specific (B) IgM and (C) IgG in the sera. Data are the mean (±SEM) of 6–8 offspring per sex in Experiment I only.
Pulmonary inflammation and IgE antibodies following sensitization and challenge with OVA
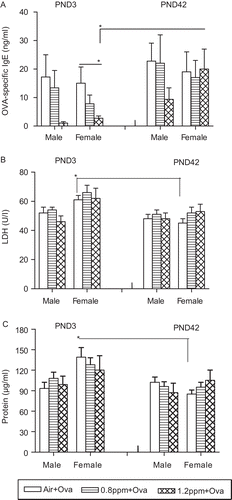
One of the objectives of this study was to determine whether the offspring of O3-exposed dams would develop stronger allergic sensitization resulting in more severe asthma-like inflammation. This effect was tested in mice sensitized either as neonates (PND3) or as adults (PND42) with all animals being challenged on three consecutive days at PND54, PND55, and PND56 prior to assessment. Due to low number of pups in the 0.4 ppm O3 group, there were not enough offspring available to be sensitized at <PND3 in Experiments II and III. Therefore, this exposure group was excluded from the entire analysis of allergic responses. shows that the air-exposed female offspring sensitized at PND3 had greater numbers of eosinophils than the males (P < 0.05) and also had more eosinophils than females sensitized at PND42. Maternal exposure to 1.2 ppm O3 significantly decreased the total cells, macrophages, lymphocytes, and eosinophils in the early-sensitized females. In the late-sensitized females, prenatal exposure to either concentration of O3 significantly reduced the neutrophil numbers compared with air controls (). The levels of OVA-specific IgE antibodies in the sera of early-sensitized females prenatally exposed to 1.2 ppm of O3 were significantly lower than both the air controls and late-sensitized females from the 1.2 ppm O3 group (). In a similar fashion to the eosinophils, the LDH activity and total protein levels were significantly increased in early-sensitized females born from air-exposed dams compared with late-sensitized females. No other significant differences in these parameters were observed with maternal exposure to O3 ( and ).
Table 3. Cell differentials in bronchoalveolar lavage fluid (BALF) of allergic offspring.
Figure 5. Ovalbumin (OVA)-specific IgE and inflammatory responses in allergic offspring of dams exposed to O3 1 day after the last OVA challenge, (A) OVA-specific IgE in the serum was detected by enzyme-linked immunosorbent assay (ELISA). (B) Levels of lactate dehydrogenase (LDH) and (C) protein in bronchoalveolar lavage fluid (BALF) were assessed. All data are the mean (±SEM) of 3–7 offspring for each sex per group in three experiments (offspring numbers per sex in a group were n = 3–5 in Experiment I, n = 5–6 in Experiment II and n = 5–7 in Experiment III). *P < 0.05; significantly different from other groups.
Pulmonary responsiveness to MCh
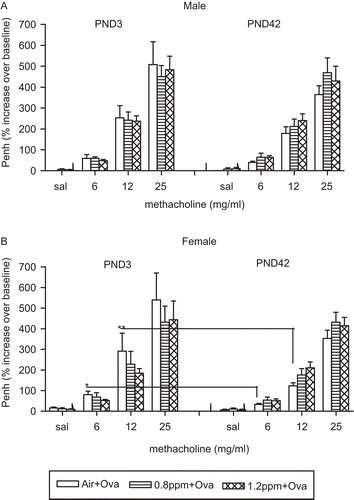
As expected, the increasing concentrations of nebulized MCh caused a stepwise rise in Penh readings over baseline in all animals. No differences were observed in the Penh values with O3 treatment in the male mice sensitized at either timepoint (). In the female mice exposed at the early timepoint, however, the Penh was significantly higher at 6 (P < 0.05) and 12 (P < 0.005) mg/mL concentrations of MCh compared with the later sensitized animals. No differences were noted between animals derived from air- or ozone-exposed dams (). No significant differences in Penh values were observed between groups of non-allergic mice (data not shown).
Figure 6. Effect of maternal O3 exposure on airway responsiveness to methacholine (MCh) in the offspring. One day after the last ovalbumin (OVA) challenge, Penh to MCh was recorded in a 12-chamber Buxco system. Data are the mean (±SEM) of 3–6 offspring for (A) males and (B) females per group in three experiments (offspring numbers per sex in a group were n = 3–5 in Experiment I, n = 5–6 in Experiment II, and n = 5–7 in Experiment III). *P < 0.05 and **P < 0.005; significantly different from other groups.
Discussion and conclusions
There is an accumulating body of evidence from the epidemiology and toxicology literature to suggest that exposure to high concentrations of air pollutants such as cigarette smoke, organic vapors, and environmental toxicants during pregnancy can cause adverse health outcomes in the offspring (Srám et al., Citation2005; Tsai et al., Citation2010). In addition, recent reports have found associations between exposure to lower levels of pollutants such as those occurring at near-road environments and increased risk of developing allergic asthma (Lwebuga-Mukasa et al., Citation2004; Clark et al., Citation2010). The purpose of this experimental study in mice was to determine whether maternal exposure to O3 would affect reproductive outcome, as well as common immune function endpoints, and the development of allergic lung disease in the offspring. Maternal exposure to O3 decreased the percentages of viable pregnancies and reduced the body weight gain in the offspring to the highest concentration of O3. The surviving offspring from the highest exposure group had increased LDH activity in BALF but no significant change in number of pulmonary inflammatory cells, total protein, and cytokines, or T-cell markers in the spleen. Maternal exposure to the two highest doses of O3 suppressed DTH responses in the female offspring, although BALF eosinophilia and serum OVA-specific IgE were decreased by the 1.2 ppm exposure in female offspring sensitized to antigen as neonates. We concluded that maternal exposure to O3 adversely affects reproductive outcome and may cause modest decreases in immune function depending on the timing of antigen sensitization.
Epidemiology studies in humans have shown that O3 exposure in the last trimester is associated with decreased birth weight (Salam et al., Citation2005). The results presented here show that O3 exposure decreased the number of viable pregnancies as well as body weight gain in the surviving offspring. These observations are consistent with previous reports in both rats and mice (Kavlock et al., Citation1979, Citation1980; Bignami et al., Citation1994). Though the mechanism for this is not known, it is clear that many forms of stress, including inhalation of toxicants, can reduce pregnancy outcome and adversely affect weight gain in the pups (reviewed in Stillerman et al., Citation2008).
Exposure to O3 causes lung injury and inflammation, increased airway hyperresponsiveness, and altered immune function (Hollingsworth et al., Citation2007; Williams et al., Citation2007), and it was of interest to determine whether any of these effects, or indeed other alterations, could be transmitted to offspring following maternal exposure. Assessment of the adult offspring exposed to O3 in utero revealed no changes in baseline or MCh-induced lung function. The female offspring from the high O3 exposure group, however, had increased markers of lung injury (LDH activity) indicating that normal lung development was somehow altered. We previously reported that maternal DE exposure increased total protein levels in female offspring as adults (Sharkhuu et al., Citation2010). Quite possibly, these effects occurred at an epigenetic level through mechanisms such as DNA methylation, histone modification, chromatin remodeling, polycomb/thrithorax protein complexes, and micro-RNAs (reviewed by Bollati and Baccarelli, Citation2010; Kabesch et al., Citation2010). In support of this hypothesis, it has recently been demonstrated that epigenetic patterns of asthma-associated genes are altered by air pollutants associated with traffic (Perera et al., Citation2009), and result in phenotype changes in metabolism during prenatal and postnatal life (Gluckman et al, Citation2009).
Prenatal or early life exposures have been associated with a variety of forms of immune dysregulation including reduced innate immunity and altered T-cell function in association with an array of neurological, endocrine, reproductive, metabolic, respiratory, allergic, and autoimmune diseases (reviewed in Dietert et al., Citation2010). In the present study, maternal exposure to O3 did not change humoral immune responses to SRBC. In contrast, however, the DTH responses were suppressed in the female offspring from the 0.8 or 1.2 ppm groups. Similar effects have been reported in mice exposed prenatally to nicotine where the offspring exhibited a >2-fold decrease in DTH responses in association with lower numbers of lymphoid progenitors and mature lymphocytes in the bone marrow (Serobyan et al., Citation2005). Compromised DTH responses following in utero exposure to 2,3,7,8-TCDD have been associated with increases in thymic γσTCR+ cell levels and a decreased number of γσTCR+CD4−CD8− cells (Gehrs and Smialowicz, 1999). Although direct exposure to O3 is known to decrease CD4+ and CD28+ T-cell populations, NK cell function, and IL-2 cytokine secretion in the spleen (Feng et al., Citation2006), these effects were not evident in the offspring studied here. The DTH regime resulted in some fatalities in the air-exposed control mice possibly as a result of using BALB/c mice as opposed to the B6C3F1 strain traditionally used for this protocol. Fewer mice in the lower dose exposure groups and none in the high O3-treated animals died. Similar effects have been noted for the immunosuppressive agent, chlordane, which suppressed DTH reactions in association with increased survival to influenza infection (Menna et al., Citation1985).
The ability of the immune system to respond appropriately to antigenic stimuli, and the pathology associated with immunological diseases changes throughout life from infancy to old age. It is known that allergic sensitization more readily occurs in younger animals, because the activation of pulmonary T-cells is attenuated in older mice (Gelfand et al., Citation2004). Female mice in the current study that were sensitized on PND3 developed more pronounced airway hyper-responsivity (AHR), eosinophilia, and increased markers of pulmonary injury and edema in the BALF than offspring sensitized on PND42. This outcome is consistent with published reports suggesting that allergic sensitization early in life induces prominent allergic airway inflammation than sensitization later in life (Gelfand et al., Citation2004). Furthermore, rodents prenatally exposed to air pollutants such as DE (Watanabe and Ohsawa, Citation2002) and residual oil fly ash (Hamada et al., Citation2007) are more sensitive to allergen in early life and developed stronger allergen-specific IgE responses than animals derived from control instilled dams. In contrast to these findings, the study reported here found that maternal exposure to the highest O3 level dampened markers of allergic lung disease in the offspring sensitized soon after birth and to a lesser extent in offspring sensitized at 6 weeks of age.
In support of the overall finding for reduced allergic sensitization, we have previously reported that maternal exposure to DE suppresses similar features of allergic lung inflammation including immediate airway responsiveness to allergen and induction of tissue eosinophils in a dose- and gender-specific manner (Sharkhuu et al., Citation2010). Furthermore, a recent study has likewise demonstrated that in utero exposure to DE reduced IgE production to Aspergillus antigen sensitization and decreased the extent of pulmonary eosinophilia (Lin et al., Citation2010). From these studies, it would appear that inhalation of air pollutants such as DE or ozone during pregnancy suppresses aspects of immune function and development of immune-mediated disease in the offspring. One possible mechanism by which this may occur is through the action of arachidonic acid metabolites that are known to be immunosuppressive particularly during pregnancy (Papadogiannakis et al., Citation1985; Parhar et al., Citation1989). Both O3 and diesel exposures increase prostaglandin E2 (PGE2) levels in the lung fluid of mice (Canning et al., Citation1991; Ahn et al., Citation2008), and blocking this activity with indomethacin in the case of O3 has reversed health effects such as reduced phagocytosis (Canning et al., Citation1991) and increased susceptibility to infection (Gilmour et al., Citation1993). However, maternal exposure to cigarette smoke components by inhalation increases allergic sensitization in both experimental animals (Penn et al., Citation2007; Wu et al., Citation2009) and in humans (Strachan and Cook, Citation1998; Gilliland et al., Citation2006), and this may be due to a different profile of mediators produced in the lung. To better understand how these inhalation exposures to common air pollutants produce contrasting effects through maternal exposure, a comparative study is warranted using the efficient sensitization regimes to identify chemical components and biomarkers that differentially modulate fetal immune development.
Acknowledgements
The project was funded by US EPA through the Oak Ridge Universities Association, TN. The authors wish to thank E. Lappi, P. Evansky, W. Zhu, E. Boykin, M. Daniels, S. Cho, G. McGee, J. Richards, J. Lehmann, C. Copeland, W. Williams, and D. Andrew for technical assistance. They appreciate the careful reviews by Drs. Marsha Ward and Janice Dye. This article has been reviewed by the US Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names or commercial products constitute endorsement or recommendation for use.
Declaration of interest
The authors report no declarations of interest.
References
- Ahn, E. K., Yoon, H. K., Jee, B. K., Ko, H. J., Lee, K. H., Kim, H. J., and Lim, Y. 2008. COX-2 expression and inflammatory effects by diesel exhaust particles in vitro and in vivo. Toxicol Lett. 176:178–187.
- Anandan, C., Nurmatov, U., van Schayck, O. C., and Sheikh, A. 2010. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 65:152–167.
- Auten, R. L., Potts, E. N., Mason, S. N., Fischer, B., Huang, Y., and Foster, W. M. 2009. Maternal exposure to particulate matter increases postnatal ozone-induced airway hyperreactivity in juvenile mice. Am J Respir Crit Care Med. 180:1218–1226.
- Bignami, G., Musi, B., Dell’Omo, G., Laviola, G., and Alleva, E. 1994. Limited effects of ozone exposure during pregnancy on physical and neurobehavioral development of CD-1 mice. Toxicol Appl Pharmacol. 129:264–271.
- Bollati, V., and Baccarelli, A. 2010. Environmental epigenetics. Heredity. 105:105–112.
- Canning, B. J., Hmieleski, R. R., Spannhake, E. W., and Jakab, G. J. 1991. Ozone reduces murine alveolar and peritoneal macrophage phagocytosis: The role of prostanoids. Am J Physiol. 261:L277–L282.
- Clark, N. A., Demers, P. A., Karr, C. J., Koehoorn, M., Lencar, C., Tamburic, L., and Brauer, M. 2010. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 118:284–290.
- Delfino, R. J., Coate, B. D., Zeiger, R. S., Seltzer, J. M., Street, D. H., and Koutrakis, P. 1996. Daily asthma severity in relation to personal ozone exposure and outdoor fungal spores. Am J Respir Crit Care Med. 154:633–641.
- DeWitt, J. C., Copeland, C. B., and Luebke, R. W. 2005. Immune responses in Sprague-Dawley rats exposed to dibutyltin dichloride in drinking water as adults. J Immunotoxicol. 2:151–160.
- Diaz-Sanchez, D., Dotson, A. R., Takenaka, H., and Saxon, A. 1994. Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms. J Clin Invest. 94:1417–1425.
- Dietert, R. R., DeWitt, J. C., Germolec, D. R., and Zelikoff, J. T. 2010. Breaking patterns of environmentally influenced disease for health risk reduction: Immune perspectives. Environ Health Perspect. 118:1091–1099.
- Doherty, S. P., Grabowski, J., Hoffman, C., Ng, S. P., and Zelikoff, J. T. 2009. Early life insult from cigarette smoke may be predictive of chronic diseases later in life. Biomarkers. 14(S1):97–101.
- Fedulov, A. V., Leme, A., Yang, Z., Dahl, M., Lim, R., Mariani, T. J., and Kobzik, L. 2008. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol. 38:57–67.
- Feng, R., He, W., Ochi, H., and Castranova, V. 2006. Ozone exposure impairs antigen-specific immunity but activates IL-7-induced proliferation of CD4−CD8− thymocytes in BALB/c mice. J Toxicol Environ Health Part A. 69:1511–1526.
- Gehrs, B. C., and Smialowicz, R. J. 1999. Persistent suppression of delayed-type hypersensitivity in adult F344 rats after perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology. 134:79–88.
- Gelfand, E. W., Joetham, A., Cui, Z. H., Balhorn, A., Takeda, K., Taube, C., and Dakhama, A. 2004. Induction and maintenance of airway responsiveness to allergen challenge are determined at the age of initial sensitization. J Immunol. 173:1298–1306.
- Gilliland, F. D., Islam, T., Berhane, K., Gauderman, W. J., McConnell, R., Avol, E., and Peters, J. M. 2006. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med. 174:1094–1100.
- Gilmour, M. I., Park, P., Doerfler, D., and Selgrade, M. K. 1993. Factors that influence the suppression of pulmonary antibacterial defenses in mice exposed to ozone. Exp Lung Res. 19:299–314.
- Gluckman, P. D., Hanson, M. A., Buklijas, T., Low, F. M., and Beedle, A. S. 2009. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 5:401–408.
- Gonzalez-Pina, R., Escalante-Membrillo, C., Alfaro-Rodriguez, A., and Gonzalez-Maciel, A. 2008. Prenatal exposure to ozone disrupts cerebellar monoamine contents in newborn rats. Neurochem Res. 33:912–918.
- Hamada, K., Suzaki, Y., Leme, A., Ito, T., Miyamoto, K., Kobzik, L., and Kimura, H. 2007. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health Part A. 70:688–695.
- Heinrich, J., and Slama, R. 2007. Fine particles, a major threat to children. Int J Hyg Environ Health. 210:617–622.
- Hollingsworth, J. W., Maruoka, S., Li, Z., Potts, E. N., Brass, D. M., Garantziotis, S., Fong, A., Foster, W. M., and Schwartz, D. A. 2007. Ambient ozone primes pulmonary innate immunity in mice. J Immunol. 179:4367–4375.
- Hwang, B. F., Lee, Y. L., Lin, Y. C., Jaakkola, J. J., and Guo, Y. L. 2005. Traffic related air pollution as a determinant of asthma among Taiwanese school children. Thorax. 60:467–473.
- Institute of Medicine. 2000. Clearing the Air, Asthma and Indoor Air Exposures. Washington, DC: Institute of Medicine, National Academy Press.
- Kabesch, M., Michel, S., and Tost, J. 2010. Epigenetic mechanisms and the relationship to childhood asthma. Eur Respir J. 36:950–961.
- Kavlock, R., Daston, G., and Grabowski, C. T. 1979. Studies on the developmental toxicity of ozone. I. Prenatal effects. Toxicol Appl Pharmacol. 48:19–28.
- Kavlock, R. J., Meyer, E., and Grabowski, C. T. 1980. Studies on the developmental toxicity of ozone: Postnatal effects. Toxicol Lett. 5:3–9.
- Lin, L., Zhu, H., Quan, C., Grunig, G., Ballaney, M., Jin, X., Perera, F. P., Factor, P. H., Chen, L. C., and Miller, R. L. 2010. Prenatal allergen and diesel exhaust exposure and their effects on allergy in adult offspring mice. Allergy Asthma Clin Immunol. 6:7.
- López, I., Sánchez, I., Bizarro, P., Acevedo, S., Ustarroz, M., and Fortoul, T. 2008. Ultrastructural alterations during embryonic rats’ lung development caused by ozone. J Electron Microsc (Tokyo). 57:19–23.
- Lwebuga-Mukasa, J. S., Oyana, T., Thenappan, A., and Ayirookuzhi, S. J. 2004. Association between traffic volume and health care use for asthma among residents at a U.S.-Canadian border crossing point. J Asthma. 41:289–304.
- Matsumura, Y. 1970. The effects of ozone, nitrogen dioxide, and sulfur dioxide on the experimentally induced allergic respiratory disorder in guinea pigs. I. The effect on sensitization with albumin through the airway. Am Rev Respir Dis. 102:430–437.
- Menna, J. H., Barnett, J. B., and Soderberg, L. S. 1985. Influenza type A virus infection of mice exposed in utero to chlordane: Survival and antibody studies. Toxicol Lett. 24:45–52.
- Moore, K., Neugebauer, R., Lurmann, F., Hall, J., Brajer, V., Alcorn, S., and Tager, I. 2008. Ambient ozone concentrations cause increased hospitalizations for asthma in children: An 18-year study in Southern California. Environ Health Perspect. 116:1063–1070.
- Muranaka, M., Suzuki, S., Koizumi, K., Takafuji, S., Miyamoto, T., Ikemori, R., and Tokiwa, H. 1986. Adjuvant activity of diesel-exhaust particulates for the production of IgE antibody in mice. J Allergy Clin Immunol. 77:616–623.
- Osebold, J. W., Gershwin, L. J., and Zee, Y. C. 1980. Studies on the enhancement of allergic lung sensitization by inhalation of ozone and sulfuric acid aerosol. J Environ Pathol Toxicol. 3:221–234.
- Papadogiannakis, N., Johnsen, S. A., and Olding, L. B. 1985. Strong prostaglandin associated suppression of the proliferation of human maternal lymphocytes by neonatal lymphocytes linked to T- vs T-cell interactions and differential PGE2 sensitivity. Clin Exp Immunol. 61:125–134.
- Parhar, R. S., Yagel, S., and Lala, P. K. 1989. PGE2-mediated immunosuppression by first trimester human decidual cells blocks activation of maternal leukocytes in the decidua with potential anti-trophoblast activity. Cell Immunol. 120:61–74.
- Penn, A. L., Rouse, R. L., Horohov, D. W., Kearney, M. T., Paulsen, D. B., and Lomax, L. 2007. In utero exposure to environmental tobacco smoke potentiates adult responses to allergen in BALB/c mice. Environ Health Perspect. 115:548–555.
- Perera, F., Tang, W. Y., Herbstman, J., Tang, D., Levin, L., Miller, R., and Ho, S. M. 2009. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS ONE. 4:e4488.
- Raherison, C., Pénard-Morand, C., Moreau, D., Caillaud, D., Charpin, D., Kopfersmitt, C., Lavaud, F., Taytard, A., and Annesi-maesano, I. 2007. In utero and childhood exposure to parental tobacco smoke, and allergies in schoolchildren. Respir Med. 101:107–117.
- Rooney, A. A., Matulka, R. A., and Luebke, R. W. 2003. Developmental atrazine exposure suppresses immune function in male, but not female Sprague-Dawley rats. Toxicol Sci. 76:366–375.
- Ruidavets, J. B., Cournot, M., Cassadou, S., Giroux, M., Meybeck, M., and Ferrières, J. 2005. Ozone air pollution is associated with acute myocardial infarction. Circulation. 111:563–569.
- Salam, M. T., Millstein, J., Li, Y. F., Lurmann, F. W., Margolis, H. G., and Gilliland, F. D. 2005. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: Results from the Children’s Health Study. Environ Health Perspect. 113:1638–1644.
- Serobyan, N., Orlovskaya, I., Kozlov, V., and Khaldoyanidi, S. K. 2005. Exposure to nicotine during gestation interferes with the colonization of fetal bone marrow by hematopoietic stem/progenitor cells. Stem Cells Dev. 14:81–91.
- Sharkhuu, T., Doerfler, D. L., Krantz, Q. T., Luebke, R. W., Linak, W. P., and Gilmour, M. I. 2010. Effects of prenatal diesel exhaust inhalation on pulmonary inflammation and development of specific immune responses. Toxicol Lett. 196:12–20.
- Srám, R. J., Binková, B., Dejmek, J., and Bobak, M. 2005. Ambient air pollution and pregnancy outcomes: A review of the literature. Environ Health Perspect. 113:375–382.
- Stillerman, K. P., Mattison, D. R., Giudice, L. C., and Woodruff, T. J. 2008. Environmental exposures and adverse pregnancy outcomes: A review of the science. Reprod Sci. 15:631–650.
- Strachan, D. P., and Cook, D. G. 1998. Health effects of passive smoking. 6. Parental smoking and childhood asthma: Longitudinal and case–control studies. Thorax. 53:204–212.
- Takahashi, G., Tanaka, H., Wakahara, K., Nasu, R., Hashimoto, M., Miyoshi, K., Takano, H., Yamashita, H., Inagaki, N., and Nagai, H. 2010. Effect of diesel exhaust particles on house dust mite-induced airway eosinophilic inflammation and remodeling in mice. J Pharmacol Sci. 112:192–202.
- Takano, H., Ichinose, T., Miyabara, Y., Yoshikawa, T., and Sagai, M. 1998. Diesel exhaust particles enhance airway responsiveness following allergen exposure in mice. Immunopharmacol Immunotoxicol. 20:329–336.
- Tsai, C. H., Huang, J. H., Hwang, B. F., and Lee, Y. L. 2010. Household environmental tobacco smoke and risks of asthma, wheeze and bronchitic symptoms among children in Taiwan. Respir Res. 11:11.
- Watanabe, N., and Ohsawa, M. 2002. Elevated serum immunoglobulin E to Cryptomeria japonica pollen in rats exposed to diesel exhaust during fetal and neonatal periods. BMC Pregnancy Childbirth. 2:2.
- Weitzman, M., Gortmaker, S., Walker, D. K., and Sobol, A. 1990. Maternal smoking and childhood asthma. Pediatrics. 85:505–511.
- Williams, A. S., Issa, R., Leung, S. Y., Nath, P., Ferguson, G. D., Bennett, B. L., Adcock, I. M., and Chung, K. F. 2007. Attenuation of ozone-induced airway inflammation and hyper-responsiveness by c-Jun NH2 terminal kinase inhibitor SP600125. J Pharmacol Exp Ther. 322:351–359.
- Woodruff, T. J., Parker, J. D., Darrow, L. A., Slama, R., Bell, M. L., Choi, H., Glinianaia, S., Hoggatt, K. J., Karr, C. J., Lobdell, D. T., and Wilhelm, M. 2009. Methodological issues in studies of air pollution and reproductive health. Environ Res. 109:311–320.
- Wu, Z. X., Hunter, D. D., Kish, V. L., Benders, K. M., Batchelor, T. P., and Dey, R. D. 2009. Prenatal and early, but not late, postnatal exposure of mice to sidestream tobacco smoke increases airway hyperresponsiveness later in life. Environ Health Perspect. 117:1434–1440.
- Zhang, J. J., McCreanor, J. E., Cullinan, P., Chung, K. F., Ohman-Strickland, P., Han, I. K., Järup, L., and Nieuwenhuijsen, M. J. 2009. Health effects of real-world exposure to diesel exhaust in persons with asthma. Res Rep Health Effects Inst. 5:109–123.
